Abstract
In accordance with the techniques developed for the InP–CdTe and InAs–CdTe systems, within the regions of mutual solubility of initial binary compounds (InP and CdTe, InAs and CdTe), the solid solutions (InP)х(CdTe)1 – x and (InAs)х(CdTe)1 – x assayed as substitutional solid solutions with cubic sphalerite structure were obtained. The X-ray, microscopic, and electron microscope investigations and investigations of surface (acid–base) properties are carried out. The concentration dependences of the studied bulk and surface properties and correlations between them are determined. The expediency of their use as orienting points in the search for new efficient materials for semiconductor gas analysis is demonstrated. Practical recommendations on use of the materials obtained in this work (solid solutions with minimum values of pHiso) for development of sensors and transducers for trace amounts of basic gases are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
One of contemporary issues of the current technology, including sensor technology, is the search for and development of new materials providing efficient and stable operation of the corresponding devices and equipment. Here, the search including both expansion of the repertoire of multicomponent semiconductors on the basis of already having proven itself, owing to the unique properties of the binary semiconductor compounds (predominantly of the AIIIBV and AIIBVI types), and the comprehensive research of bulk and especially surface, often defining, properties looks fruitful [1].
In this case, for the forecasted and less labor-consuming search, comparative examination of the selected objects is also useful. Precisely this approach is used in this work.
The solid solutions of systems of the type AIIIBV–AIIBVI (InP–CdTe, InAs–CdTe), distinguished by the binary component InBV (InP, InAs), were the objects of the research.
Considering the distinction between InP and InAs with one another and with the common binary component CdTe and also the special experience of our investigations carried out earlier, one would expect predominantly statistical (smooth) changes and changes with extreme exhibition of the studied properties of the solid solutions with the compositions, respectively, in the InAs–CdTe and InP–CdTe systems. The results discussed below are indicative of confirmation of such announcement.
EXPERIMENTAL
The examined objects were finely divided powders (Ssp = 0.30–0.48 m2/g) of initial binary compounds (InP, InAs, and CdTe) and solid solutions (InP)x(CdTe)1 – x (x = 9, 12, 16, 18, 78, 82, 84, 88 mol %) and (InAs)x(CdTe)1 – x (x = 4, 15, 22, 27, 32, 75, 81, 89, 93 mol %). The techniques (with the proven modes and programs of thermal heating) were developed to obtain the solid solutions according to the possibilities of the method of isothermal diffusion and the known data on the basic bulk physical and physicochemical properties of InP, InAs, and CdTe [1, 2].
The synthesis was implemented in vacuum-sealed quartz ampoules, within the regions of mutual solubility of the initial binary compounds. A mechanical activation was carried out at its initial stage for intensification of the synthesis process.
An inference of termination of synthesis, generation, and structure of the solid solutions was drawn from the results of the X-ray investigations, engaging the results of microscopic and electron microscope investigations.
The products of synthesis are the polycrystalline ingots going after that through size degradation.
The predetermined molar compositions were checked against the elemental compositions determined by the results of electron microscope investigations.
The X-ray investigations were carried out using a D8 Advance diffractometer (Bruker AXS, Germany) in CuKα radiation (λ = 0.15406 nm, T = 293 K) by the large-angle imaging technique [3, 4] with use of a Lynxeye position-sensitive detector and also the database on powder diffraction ICDDIPDF-2 and TOPAS 3.0 software (Bruker) for interpretation of the obtained diffraction patterns and refinement of lattice constants, respectively; the microscopic investigations were performed using KH-8700 (Xirox, Japan) and Micromed POLAR-3 devices with resolving power up to 7000 [5]; the electron microscope investigations were performed using a JSM-5700 scanning electron microscope equipped with a JED-2300 attachment for energy-dispersive analysis [6].
The surface (acid-base) properties were studied by the method of hydrolytic adsorption providing the means for determination of the hydrogen values of the isoelectric state of the surfaces (pHiso) [7]. The essence of the method consists in determination of the pH of the medium in which the ampholite adsorbents split off equal (insignificant) amounts of H+ and OH– ions. The role of ampholite adsorbents was played by the binary components and solid solutions of the InP–CdTe and InAs–CdTe systems with the characteristic isoelectric points corresponding to the minimum of solubility. An average power and proportion between acid and basic sites on the surface of the components can be inferred by the pHiso values.
Repeatability and accuracy of the experimental data was checked by the results of parallel measurements with use of the methods of mathematical statistics and processing of the results of quantitative analysis, as well as the Stat-2, Microsoft Excel, and Origin, software programs.
RESULTS AND DISCUSSION
The results of X-ray investigations are indicative of generation of substitutional solid solutions with cubic sphalerite structure in the InP–CdTe and InAs–CdTe systems at the predetermined compositions.
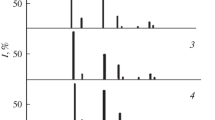
We would like to specifically note the respective relative position and the distribution over the intensities of the main lines on the X-ray diffraction patterns of the binary compounds and the solid solutions, the shift of the lines corresponding to the solid solutions relative to the lines of the binary compounds at their fixed number, the absence of additional lines of unreacted binary compounds, the spread of the main lines (Fig. 1), and the predominantly smooth behavior (obedience of Vegard’s law) of the dependences of the parameter on composition (a), interplanar spacings (dhkl) of the crystal lattices, and also density (ρr) relating to the InAs–CdTe system and (Fig. 2).
Line diagrams of X-ray diffraction patterns of components of InP–CdTe systems (I): (1) InP; (2) (InP)0.88(CdTe)0.12; (3) (InP)0.86 (CdTe)0.14; (4) (InP)0.84(CdTe)0.16; (5) (InP)0.78(CdTe)0.22; (6) (InP)0.18(CdTe)0.82; (7) (InP)0.16(CdTe)0.84; (8) (InP)0.12(CdTe)0.88; (9) (InP)0.09(CdTe)0.91; (10) CdTe and InAs–CdTe; (II): (1) InAs; (2) (InAs)0.89(CdTe)0.11; (3) (InAs)0.81(CdTe)0.19; (4) (InAs)0.75(CdTe)0.25; (5) (InAs)0.32(CdTe)0.68; (6) (InAs)0.27(CdTe)0.73; (7) (InAs)0.22(CdTe)0.78; (8) (InAs)0.15(CdTe)0.85; (9) (InAs)0.04(CdTe)0.96; (10) CdTe.
The noted, with regard to the InP–CdTe system, deviation from Vegard’s law (this refers to the dependence ρr = f(хCdTe)) is most likely caused by proceeding of complex endogenous processes accompanying formation of the solid solutions [8] and, as a consequence, nonuniform distribution of cation-anion complexes, as indicated also by the results of electron microscope investigations. They demonstrate polycrystalline structure of the components of the systems with nonuniform distribution of the crystalline grains (Figs. 3, 4).
We several times expressed, relying upon our own and published data [1, 8–10], our insights into possible deviations of the bulk properties of the semiconductor solid solutions from Vegard’s law.
The average sizes (davg) and average numbers (navg) of predominant particles in the components of the systems, their elemental compositions almost coinciding with the predetermined mole compositions (Tables 1, 2) were also determined at cumulative use of the results of electron microscope and microscopic investigations.
With increase in the cadmium telluride content in the InP–CdTe and InAs–CdTe systems, the trend of increase of navg and, correspondingly, the density ρr determined on the basis of X-ray investigations (Fig. 5) is observed. This must cause the saturating capacity of unsaturated bonds and decrease in the number of coordinatively unsaturated atoms predominantly responsible for Lewis acidic sites, and therefore there is an increase in relative contribution of the Bronsted sites. The expressed assumptions confirmed the results of the direct studies of acid-base properties (see Fig. 5).
According to such results, the values of pHiso of the surfaces of the solid solutions of the InP–CdTe, InAs–CdTe systems stay within the ranges 5.38–6.0 and 5.75–6.27 (at the values of pHiso of binary components InP, InAs, CdTe of 5.9, 5.7, 6.3), growing in the subsequences:
In the same subsequences, in parallel, the density (ρr) and average number of the particles (navg) change: smoothly in the InAs–CdTe system and extremely in the InP–CdTe system (see Fig. 5). In view of this, the closed relationship between bulk (ρr, navg) and surface (pHiso) properties of the components of the systems, the reason for which is inherent in the nature of active (acidic) sites, is traced.
Indeed, as was forecasted above, with change in navg, ρr, coordinative unsaturation of the surface atoms primarily responsible for Lewis sites [1], and, in response, the observed change in acidity of the surfaces (pHiso) change (see Fig. 5).
Moreover the relationship between the bulk and surface properties is traced not only in comparison of the dependences navg = f(хCdTe), ρr = f(xCdTe), and pHiso = f(хCdTe), but also in comparison of absolute values of the designated properties. For example, the values of density of the solid solutions of the InP–CdTe and InAs–CdTe systems vary within the range of 5.1395–5.8734 and 5.8086–6.0467, and the pHiso values are within the range of 5.38–6.0 and 5.75–6.27, confirming the increase in relative contribution of the Bronsted sites (growth of pHiso) with increase in navg, ρr and decrease in coordinative unsaturation of the surface atoms.
Owing to the fact that, by the absolute values pHiso, the surfaces of components of the InP–CdTe and InAs–CdTe systems are classified as slightly acidic (pHiso < 7), it is reasonable to expect their increased sensitivity to basic gases. Confirmation of this assumption is an increase in pHiso at exposures of the basic gas (NH3) (for example, for InAs from 5.7 to 7.7; for CdTe from 6.3 to 8.2) and a decrease in pHiso at exposures of the acid gas (NO2) (for InAs, from 5.7 to 5.0; for CdTe, from 6.3 to 5.0).
In comparison of the concentration dependences of bulk and surface properties of the solid solutions of the InP–CdTe and InAs–CdTe systems, conspicuous are the specific manifestations (in relation to navg, ρr, pHiso) of an extreme factor in the first case and the determinative contribution of a statistical factor in the second case (see Fig. 5). This fact clearly confirms our earlier expressed (for example, in [11]) insights into the influence of degree of discrepancy in the values of energy band gap (ΔE) of the initial binary compounds on the type of dependences of properties of the solid solutions on their composition: at a larger difference, the tendency to statistical (smooth) change; at smaller difference, the tendency to extreme change.
Accordingly, in this case, at a larger difference between the values of ΔE of InAs and CdTe (ΔEInAs = 0.36 eV, ΔECdTe = 1.51 eV), smooth changes in properties of the solid solutions (InAs)х(CdTe)1 – х are observed; at a smaller difference of ΔE of InP and CdTe (ΔEInP = 1.35 eV, ΔECdTe = 1.51 eV), extreme changes in a number of properties of the solid solutions (InP)х(CdTe)1 – х are observed.
In support of the aforementioned, it is reasonable to emphasize that the influence of the degree of discrepancy between the values of the energy band gap (ΔE) of binary components of the systems on the behavior of concentration dependences of properties of the solid solutions (smooth, extreme) was observed also at investigation of the systems of AIIBVI–AIIBVI type—homogeneous substitution systems. The CdSe–CdTe system would be an example.
At a small difference between the values of ΔE of CdSe and CdTe (1.88 and 1.51 eV), such properties of the solid solutions (CdSe)x(CdTe)1 – х as the average number of predominant particles (davg), density (ρr), pH of the isoelectric state of the surfaces (pHiso), the magnitude of adsorption (αCO), catalytic activity (χCO) in the reaction of oxidation of CO, change extremely and mutually (Fig. 6). Here, at placement of a smaller number of particles (falling on the minimum) in a unit volume of the crystal lattice, are a less dense neighborhood, smaller intersaturation of the bonds, higher coordinative unsaturation of the atoms acting mainly as the Lewis acid sites and relatively lower pHiso, larger magnitude of adsorption of the gas interacting with the surface by donor–acceptor mechanism with the participation of coordinatively unsaturated atoms, and higher catalytic activity in the reaction proceeding in accordance with collisional mechanism with preliminary adsorption of CO (on the coordinatively unsaturated atoms).
Determination of correlations between the dependences “bulk property–composition,” “surface property–composition,” and, accordingly, relation between the bulk and surface properties, undoubtedly, is of scientific and practical interest. Their presence enriches the predictabilities of the surface (more specifically, acid-base) properties by already known or more accessibly determined bulk properties and thereby makes it possible to forecast the adsorbents active toward gases of different electron nature, which are materials for semiconductor gas analysis. To put it in other words, a less labor-consuming way of determination of new on-demand materials is established here.
The new materials obtained in this work—the solid solutions of the InP–CdTe and InAs–CdTe systems with slightly acidic surfaces—are recommended (mainly with minimum pHiso) for development of sensors and transducers for trace amounts of basic gases (for example hydrogen nitride).
CONCLUSIONS
On the basis of the method of isothermal diffusion with use of known data on the main bulk physical and physicochemical properties of the initial binary compounds (InP, InAs, CdTe), the techniques of manufacturing of multicomponent diamond-type semiconductors—solid solutions of InP–CdTe and InAs–CdTe systems—were developed.
The X-ray, microscopic, and electron microscope investigations were implemented. According to their results, the obtained solid solutions are assayed as the substitutional solid solutions with cubic sphalerite structure. The elemental compositions almost coinciding with predetermined molar compositions were determined.
The acid–base properties of the surfaces of components of the InP–CdTe and InAs–CdTe systems were investigated. Their slightly acidic nature and increased activity in relation to the basic gases were demonstrated.
The common factors of changes with composition of the bulk (crystallochemical, structural) and surface (acid-base) properties which are of mainly smooth nature for the solid solutions of the InAs–CdTe system and include extreme exhibition for the solid solutions of the InP–CdTe system were determined. The influence of the degree of discrepancy of InP and InAs with CdTe by the values of energy band gap (ΔЕ) on the type of concentration dependences of properties of the solid solutions was demonstrated.
The correlations between the determined common factors of changes in the bulk and surface properties, the reason for which is inherent in the nature of active (acid-base) sites, were revealed.
The revealed correlations can be used for less labor-consuming determination of new materials of sensor technology (more specifically, for semiconductor gas analysis). The orienting points for search with use of bulk properties of the examined objects are designated.
Specific practical recommendations are provided.
REFERENCES
Kirovskaya, I.A., Fiziko-khimicheskie svoistva binarnykh i mnogokomponentnykh almazopodobnykh poluprovodnikov (Physical and Chemical Properties of Binary and Multicomponent Diamond-Like Semiconductors), Novosibirsk: Sib. Branch Russ. Acad. Sci., 2015.
Morozov, V.N. and Chernov, V.G., Phase equilibria in the InAs–CdTe system, Izv. Akad. Nauk, Ser. Khim., 1979, no. 8, pp. 1324–1329.
Gorelik, S.S., Rastorguev, L.N., and Skakov, Yu.A., Rentgenograficheskii i elektronnoopticheskii analiz (X-Ray and Electron-Optical Analysis), Moscow: Metallurgiya, 1970.
Smyslov, E.F., Express X-ray method of nanocrystal materials lattice parameter determination, Zavod. Lab. Diagn. Mater., 2006, vol. 72, no. 5, pp. 33–35.
Clarke, A.R. and Eberhardt, C.N., Microscopy Techniques for Materials Science, Cambridge: Woodhead, 2002.
Goldstein, J.I., Newbury, D.E., Echlin, P., Joy, D.C., Fiori, C., and Lifshin, E., Scanning Electron Microscopy and X-ray Microanalysis, New York–London: Plenum, 1981.
Maidanovskaya, L.G., On the hydrogen index of the isoelectric state of amphoteric catalysts, in Kataliticheskie reaktsii v zhidkoi faze (Catalytic Reactions in the Liquid Phase), Alma-Ata: Akad. Nauk KazSSR, 1963, pp. 212–217.
West, A.R., Solid State Chemistry and Its Applications, Chichester: Wiley, 1984, part 1.
Morozov, V.N. and Chernov, V.G., Phase equilibria in the InAs–CdTe system, Izv. Akad. Nauk, Ser. Khim., 1979, no. 8, pp. 1324–1329.
Brodovoi, V.A., Vyalyi, N.G., and Knorozok, L.M., Peculiarities of lattice parameter changing of the (InSb)1 – x(CdTe)x solid solutions, Neorg. Mater., 1997, vol. 33, no. 3, pp. 303–304.
Kirovskaya, I.A., Mironova, E.V., Umanskiy, I.Yu., Ekkert, A.O., Ekkert, R.V., Kopylova, E.N., Blesman, A.I., Polonyankin, D.A., and Goncharov, V.B., Semiconductor heterosystem InAs–ZnS. Physical and chemical properties, J. Phys.: Conf. Ser., 2020, vol. 1441, art. ID 012007. https://doi.org/10.1088/1742-6596/1441/1/012007
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Levina
Rights and permissions
About this article
Cite this article
Kirovskaya, I.A., Ekkert, A.O., Kopylova, E.N. et al. Physicochemical Properties of New Semiconductor Materials—Solid Solutions of the InBV–CdTe Systems. Inorg. Mater. Appl. Res. 13, 44–51 (2022). https://doi.org/10.1134/S2075113322010178
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113322010178








