Abstract—The structure and the physicochemical properties of boron carbide powders obtained by the mechanochemical synthesis of the carbon char and amorphous boron mix by applying the methods of the X-ray phase analysis (XPA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and chemical analysis were investigated. On the basis of the X-ray phase analysis, it was found that full transformation of the original materials (carbon char and amorphous boron (B4C) takes place during the mechanical treatment of the mix for 30–60 min. Judging by the micro-electron diffraction pattern of the B4C obtained by the mechanical synthesis, the product of synthesis was amorphized and it contained some inclusions of the crystal phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Strict requirements are imposed on the absorbing elements (AEL) of modern nuclear reactors; they determine the life cycle of the regulating element such as high efficiency of the neutron absorption, low rate of the absorbing isotope burnup in the process of operation in the reactor, high resistance to the radiation damage, volume stability both at operating temperature and at overheating, and corrosion resistance [1, 2].
Boron carbide, dysprosium hafnate, hafnium diboride (HfB2), and composition B4C–10–20 wt % HfB2 are considered as advanced absorbing materials [3].
Such physical property of boron as the effective thermal-neutron capture cross section is widely used; it determines its application in B4C compounds used as the expendable absorber of the thermal neutrons in the heat-producing elements as well as for biological protection around the core of the nuclear reactor.
It was found that application of a nanostructural ceramic made of boron carbide allows obtaining a set of higher physicochemical properties as compared with a coarse-grained ceramic (microhardness along with the crack resistance and strength) [4, 5].
In case of materials applied in nuclear and space systems, application of boron carbide in the nanocrystalline state will contribute to reduction of the negative impact on materials exposed to radiation—decrease in their swelling and radiation embrittlement [6, 7].
Obtaining materials in the highly dispersive state with a large specific surface according to the standard technology, namely, sintering with subsequent grinding, is practically impossible.
The mechanochemical method is the most advanced one for obtaining highly dispersive nanopowder of boron carbide. Interaction takes place during processing in the mechanical reactor between original substances (mechanochemical synthesis), inducing formation of new phases. Under the optimum conditions of the process implementation, the mechanically synthetized phases are in the ultra-dispersive state with a highly developed surface of the grain boundaries and subgrains with the nano- and microcrystal type of structure; this will allow increasing its density after the vibrocompaction, thereby decreasing the burnup rate by the AEL cross section and slowing down the decrease of the absorbing properties under the influence of neutron irradiation [8–11].
Thus, the product of the mechanochemical synthesis has a given composition and specific structural state. In addition, the mechanochemical synthesis refers to the least energy-intensive and simple methods which may be ascribed to high-rate solid phase reactions [12–16].
The aim of this work is the analysis of the structure and properties of the boron carbide powders obtained by the mechanochemical treatment of carbon char and amorphous boron.
MATERIALS AND METHODS
The carbon char of the PM-15 grade and amorphous boron of the A grade in the stoichiometric ratio were used as the original substances for synthesis.
The mechanochemical synthesis was implemented with an Aktivator 2S planetary ball mill at the rotational rate of the planetary disk of 600–900 rpm, at the rotational rate of drums of 1000–1800 rpm, at the ball mass and charge ratio of (30–45) : 1 in an argon atmosphere at P = 3–5 atm for 5–120 min.
Specific surface Ssp of the original oxides and obtained powders of the boron carbide were determined with the help of a NOVA 1200e analyzer of the specific surface and porosity by the method of the low-temperature adsorption of nitrogen (BET method). The measurement error of the specific surface is 3%; the surface range is from 0.01 to 2000 m2/g.
A general-purpose laser device for measurement of the particle size, model FRITSCH ANALYSETTE 22 MicroTec plus, equipped with the block of dispersion in the liquid media with the measurement ratio from 0.08 to 2000 μm and the measurement accuracy according to ISO 13320 was used for determination of the granulometric composition of the boron carbide obtained by the mechanical activation of carbon char and amorphous boron. The nanoamorphous boron carbide powder obtained by the mechanical synthesis was controlled by a Beckman COULTER no. 5 analyzer of the submicron particles. This device is intended for the determination of the granulometric composition of powders with particle size within 3–3000 nm. The size of the particles is determined by the measurement of the diffusion rate of the particles in liquid. For a solution with the specified viscosity and at constant temperature, the diffusion rate or the diffusion coefficient is inversely proportion to the size of the particles.
The poured density was determined by the standard method in accordance with the GOST 19449-94.
The X-ray phase analysis of the obtained compounds was carried out on a DRON-2.0 X-ray diffractometer in the copper radiation (Kα) within the range of the diffraction angles of 2θ from 10° to 130°.
A JEM-2100 analytical electron microscope comprising the system of the computer control with integration of the observation device in the mode of a scanning transmission electron microscope (STEM) and energy-dispersive X-ray spectrometer (JED-2300) was used in order to obtain the electron microscope images and electronograms.
The impurity content was determined by the method of the atomic absorption and emission-spectral analysis.
RESULTS AND DISCUSSION
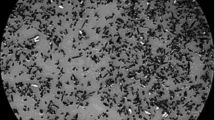
Figure 1 shows the results of determination of the granulometric composition and microstructure of the particles of the boron carbide powder obtained after the mechanical treatment of the amorphous boron and carbon char mix taken in the stoichiometric ratio.
On the basis of data from the analysis of the powder granulometric composition (see Fig. 1) and scanning electron microscopy (SEM), it was found that the boron carbide synthesized by mechanochemical activation is nanosized particles of the non-equiaxial shape with diameters of 50–500 nm combined in agglomerates.
Investigations by the SEM method showed that the dispersive product obtained by the mechanical synthesis is highly agglomerated. The maximum size of agglomerate is about 12.0 μm and consists of spherical particles (Fig. 2). TEM results shown in the Fig. 3 give more qualitative representation of the synthesized product.
The TEM image (Fig. 3) proves that the powder was in the highly agglomerated state and it consisted of mainly ultra-dispersive amorphous particles. A rather large number of particles of spherical shape with size of 20 nm are observed (Figs. 3, 4). The concave and straight-line segments, skewing the perimeter, are seen on some large particles.
Table 1 shows some properties of the boron carbide powder obtained by the mechanical synthesis.
On the basis of the micro-electron diffraction pattern of the powder B4C obtained by the mechanical synthesis, the product of synthesis is amorphized and contains some inclusions of the crystal phase (see Fig. 3).
Data concerning the elemental composition for the spherical particle (see Fig. 4) are given in Table 2.
The statistical data analysis concerning the elemental composition of particles shows that they contain boron and carbon practically in the stoichiometric ratio. An insignificant iron content in the boron carbide is explained by application of steel balls during mechanical synthesis. The presence of oxygen allows assuming that this impurity is found in the form of the oxide compound (see Table 2).
Figure 5 shows the microstructure of the boron carbide sample obtained by the mechanochemical method.
Investigations performed with TEM allowed revealing the structure with the disordered state of atoms (close to amorphous) (region 1) as well as the typical banded structure from the atomic planes (region 2) indicative of the crystalline state, which was shown by the micro-electron diffraction pattern (see Fig. 3) of the powder B4C synthesized by the mechanochemistry.
CONCLUSIONS
(1) As a result of investigations, the possibility to obtain the nano-dispersive boron carbide powder by the mechanochemical treatment of carbon char and amorphous boron was established.
(2) The structure and properties of the boron carbide powders were studied by the methods of the scanning electron microscopy, Raman spectroscopy (RS spectra), TEM, and XPA.
(3) It was shown that full transformation of the original materials (carbon char and amorphous boron) to the boron carbide (B4C) takes place during mechanical treatment of the mix for 30–60 min. The micro-electron diffraction pattern of the B4C powder obtained by the mechanical synthesis has a ring structure with some inclusions of the crystalline phase indicative of the X-ray amorphous phase.
REFERENCES
Sickafus, K.E., Grimes, R.W., Valdez, J.A., Cleave, A., Ming, T., Ishimaru, M., Corish, S.M., Stanek, C.R. and Uberuaga, B.P., Radiation-induced amorphization resistance and radiation tolerance in structurally related oxides, Nat. Mater., 2007, no. 6, pp. 217–223.
Belash, N.N., Kushtym, A.V., Tatarinov, V.R., and Chernov, I.A., Analysis of the development of designs and materials of absorbing rods of control and protection systems with higher performance, Yad. Radiats. Tekhnol., 2007, vol. 7, nos. 3–4, pp. 18–28.
Risovany, V.D., Zakharov, A.V., Muraleva, E.M., Kosenkov, V.M., and Latypov, R.N., Dysprozium hafnate as absorbing material for control rods, J. Nucl. Mater., 2006, vol. 355, pp. 163–170.
Risovannyi, V.D., Zakharov, A.V., and Muraleva, E.M., New advanced absorbing materials for nuclear reactors with thermal neurons, Vopr. At. Nauki Tekh., Ser.: Fiz. Radiats. Povrezhdenii Radiats. Materialoved., 2005, no. 3, pp. 87–93.
Andrievskii, R.A., Nanocomposites based on refractory compounds: development and prospects, Materialovedenie, 2006, no. 4, pp. 2–10.
Fridman, S.R., Risovany, V.D., et al., Radiation stability of WWER-1000 CPS AR absorber element with boron carbide, Vopr. At. Nauki Tekh., Ser.: Fiz. Radiats. Povrezhdenii Radiats. Materialoved., 2001, no. 2, pp. 84–90.
Nefedova, E., Aleksandrova, E., Grigoryeva, E., and Olevsky, E., Research high-temperature consolidation of nanostructured bimodal materials, Phys. Procedia, 2015, vol. 72, pp. 390–393.
Lukin, E.S., Popova, N.A., Pavlyukova, L.T., and Sannikova, S.N., The use of oxide nanopowders and their compositions in ceramics technology, Konstr. Kompoz. Mater., 2014, no. 3, pp. 28–32.
Moshtaghioun, B.M., Cumbrera-Hernández, F.L., Gómez García, D., de Bernardi-Martín, S., Domínguez Rodríguez, A., Monshi, A., and Abbasi, M.H., Effect of spark plasma sintering parameters on microstructure and room-temperature hardness and toughness of fine-grained boron carbide (B4C), J. Eur. Ceram. Soc., 2013, vol. 33, no. 2, pp. 361–369.
Moshtaghioun, B.M., Gómez García, D., and Domínguez Rodríguez, A., High-temperature deformation of fully-dense finegrained boron carbide ceramics: experimental facts and modeling, Mater. Des., 2015, vol. 88, pp. 287–293.
Khalameida, S.V., New approaches in the mechanochemical synthesis of nanodispersed barium titanate, Nanosyst., Nanomater., Nanotechnol., 2009, vol. 7, no. 3, pp. 911–918.
Xue, J., Wang, J., and Wan, D., Nanosized barium titanate powder by mechanical activation, J. Am. Ceram. Soc., 2000, vol. 83, no. 1, pp. 232–234.
Lyashenko, L.P., Shcherbakova, L.G., Kolbanev, I.V., Knerel’man, E.I., and Davydova, G.I., Mechanism of structure formation in samarium and holmium titanates prepared from mechanically activated oxides, Inorg. Mater., 2007, vol. 43, no. 1, pp. 46–54.
Szafraniak-Wiza, I., Hilczer, B., Talik, E., Pietraszko, A., and Malic, B., Ferroelectric perovskite nanopowders obtained by mechanochemical synthesis, Process. Appl. Ceram., 2010, vol. 4, pp. 99–106.
Kolmakov, A.G., Solntsev, K.A., Vityaz’, P.A., Il’yu-shchenko, A.F., Kheifets, M.L., and Barinov, S.M., Systematic description of nanomaterial structure, Inorg. Mater.: Appl. Res., 2013, vol. 4, no. 4, pp. 313–321.
Lysenkov, A.S., Timoshkin, I.A., Kargin, Yu.F., Titov, D.D., Fedotov, A.Yu., Ashmarin, A.A., and Baranchikov, A.E., Synthesis of aluminum oxynitride (AlON) and study of the properties of ceramics based on it, Inorg. Mater.: Appl. Res., 2016, vol. 7, no. 4, pp. 517–519.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research, grant no. 15‑08‑00231.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Grishina
Rights and permissions
About this article
Cite this article
Eremeeva, Z.V., Myakisheva, L.V., Panov, V.S. et al. Structure and Properties of the Boron Carbide Powder Obtained by the Mechanochemical Synthesis of the Carbon Char and Amorphous Boron Mix. Inorg. Mater. Appl. Res. 10, 49–52 (2019). https://doi.org/10.1134/S2075113319010088
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2075113319010088