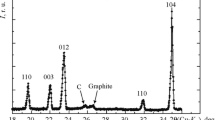
Results are given for a study by scanning electron microscopy of the effect of original powder composition on physicomechanical properties of reaction-bonded boron carbide and during new material structure formation in the B–Si–C system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ceramics based on boron carbide and composites based on it are distinguished by high hardness, chemical inertness, strength at high temperature, semiconductor properties, and a capacity to absorb thermal neutrons. This has given rise to extensive use in machining, radioelectronic, atomic, and other branches of industry. Dense materials based on boron carbide B4C are prepared mainly by high-temperature hot pressing and sintering at 2000 – 2200°C. This is connected with presence within this compound of strong covalent bonds. Processes have been studied to a lesser extent for preparing materials based on boron carbide by siliciding, for example such promising systems as B4C–SiC–C and B4C–C.
The main problem in developing structural and other materials based on these compounds is a requirement for considering a variety of complex physicochemical processes occurring during heat treatment and siliciding workpieces in the range 1400 – 2200°C. In the opinion of the authors in [1–3] the main ones are as follows:
-
chemical reaction of B4C with molten silicon with formation of silicon carbide SiC and also other compounds in the B–Si–C system;
-
direct formation of SiC with presence of carbon in a starting composition;
-
solid solution formation in the B4C–SiC system;
-
eutectic formation in the B4C–SiC system;
-
eutectic melting at above 2070°C;
-
additional phase formation during cooling of heating equipment after completion of the siliciding thermal regime.
Formation of many phases occurs with an increase in molar volume of final reaction products compared with indices of the starting components, and this leads to occurrence of internal stresses in silicided workpieces up to breakdown of their integrity.
In the course of studies it was detected that cooling during reaction bonding of B4C and Si gives shrinkage at a different rate. The linear thermal expansion coefficient (LTEC) of B4C is 5.6 × 10–6 K–1, and for Si it is 4 × 10–6 K–1, and therefore Si experiences greatest stresses, which is due to the high dislocation density [1]. Consequently, in order to develop structural materials in the B4C–SiC–C and B4C–C systems by reaction bonding technology it is necessary to slow down or remove the processes indicated above.
A special method has been proposed in a patent [4] for suppressing or slowing down reaction of B4C with molten Si: preliminary of metallic silicon with boron compounds. Partial equilibrium between molten Si, B4C, and SiC is achieved by saturating a silicon melt with boron in an amount of 8 at.%.
Analysis of contemporary information, presented in various international scientific conferences and on internet sites, shows that currently within Russia development of reaction sintered materials based on B4C is performed by several Russian firms. Among them it is possible to distinguish OAO TsNIIM (St Petersburg) [5], OOO VIRIAL (St Petersburg) [6], OOO NPP ARMOKOM-TsENTR (Khot’kovo, Moscow region) [7], whose materials exhibit good physicomechanical properties and have been successfully approved for various protective structures (Table 1).
Ceramic materials have been studied quite widely overseas, and used extensively by the majority of firms (Ceradyne, Inc., MCT, Saint-Gobain, ETEC, Advanced Ceramics, etc. [8]) as an alternative to using silicon carbide ceramic for manufacturing lighter and more effective means of personal protection and transport technology.
Earlier attempts by the authors to develop reliable protective material based on B4C by reaction sintering have not provided favorable results. This work was carried out with the aim pf preparing ceramic with a fine-grained structure in the B–Si–C system with better physicomechanical properties.
Experimental Procedure
In this work boron carbide and silicon carbide were used of different grain size composition developed by OAO UNIKhIM with Test Plant (Ekaterinburg) and commercial-grade carbon black with a specific surface S sp = 30,000 cm2/g. Powder grain size composition is given in Table 2.
Analysis of starting boron carbide powder showed a high content of the main B4C phase (96 – 96.8%) and presence of admixtures of Fe in an amount 0.3% and B2O3 in an amount of 0.26%, and this corresponds to GOST 26327.
In order to slow down or remove the processes indicated above the siliciding temperature was reduced to 1550°C, and cooling rate in the range from 1550 to 1350°C to 20°C/h.
Boron carbide, silicon carbide, and carbon black powders were mixed by a liquid-phase method. The binder used was phenolformaldehyde polymer oligomer by semidry compaction under a pressure of 700 – 750 MPa for preparation of plates with a size of 70 × 70 × 10 mm. Siliciding was performed in a graphite assembly in a vacuum at 1550°C using semiconductor purity silicon. Excess silicon in the reaction system provided a possibility of occurrence of chemical reactions and filling of residual porosity in specimens of metallic silicon. Specimens were cut from plates that were used in determining density, porosity, studying microstructure and phase composition, measurement of ultimate strength in bending, microhardness, ultrasound propagation rate, and other indices.
Density and porosity were determined by hydrostatic weighing, and standard specimens with a size of 7 × 7 × 70 mm were used in order to determine ultimate strength with three-point bending. Critical stress intensity factor K 1c and microhardness of the main phase according to the Vickers scale were measured by indentation with a load of 200 g in a DuraScan 50 instrument. Microstructure was studied in specimen polished surfaces by means of an Axio Observer MAT optical inversion microscope. Phase composition was determined by x-ray phase analysis (XPA) in a DRON-6 unit (CuK α-radiation, Ni filter).
Experimental Section
Microscope studies of boron carbide powder showed that B4C has an extended acicular shape (Fig. 1).
Nine compositions were prepared from test powder batches, containing in order to obtain maximum packing density 70% of coarse (F 220, F 240, and F 320) and 20 – 30% fine (6U-13) B4C fractions and 10% SiC or C addition. Specimens were separated into three groups (Table 3). For specimens of group 2 (compositions 4 – 6) there was typically good particle packing density (1.59 – 1.68 g/cm3) and the maximum value was achieved on introducing 10% SiC (specimen 6). It should be noted that in groups 1 and 3 the maximum density values were also achieved with addition of 10% SiC Fig. 2.
Results and Discussion
Macrostructural analysis showed that in many specimens in the area of workpiece contact with molten silicon a defective layer up to 1 mm thick formed, whose microstructure consists of prismatic and irregular morphology with a size of 4 – 50 μm, and also intergranular phase (light area) (Fig. 3). According to XPA data the defective layer consists of a main phase Sicub, β-SiC, and traces of B12(C,Si,B)3 (silicon carbide solid solution in boron carbide). During analysis of physicomechanical properties of specimens it was established that for all of the compositions values of porosity are at one level and do not exceed 0.4% (Table 4). Maximum density values of 2.65 – 2.70 g/cm3 are achieved in specimens containing carbon black.
Specimens of group III are distinguished by maximum strength values. The greatest K 1c value is achieved in specimens of group I. It should be noted that maximum strength values of (for a specimen of composition 7 σben is 350 MPa) and fracture toughness (for a specimen of composition 1 K 1c is 4.5 MPa·m1/2) were typical for specimens containing only B4C fractions. A reduction in grain size to 40 μm (fraction F 320) leads to an increase in strength, and an increase to 60 – 80 μm (fraction F 220) combined with fine faction 6U-13 provides an increase in fracture toughness.
The greatest microhardness is exhibited by group I specimens, manufactured from coarse-grained B4C powder. Introduction of 10% carbon black increases HV to 33 GPa (specimen of composition 2) and introduction of 10% SiC to 34 GPa (specimen of composition 3). USV velocity for specimens of all compositions is within the limits 11,700 – 12,400 m/sec.
A study of microstructure and phase composition was performed on specimens of group II, for which high values of all test indices are typical: strength, microhardness, and fracture toughness.
According to XPA data specimens of group II consist silicon carbide β-SiC (main phase, associated compounds B12(C, Si, B)3 and B12.97C2.88Si0.35, rhombohedral boron carbide of structure B13C and B10C, and a small amount of silicon Sicub.
Studies of specimen polished surfaces by optical microscopy showed that the microstructure is formed by four phases. In studies of a microsection in an optical microscope it is separated with respect to color (Fig. 4): white phase, which does not have clearly defined morphology (free silicon Si); gray phase is grains of different morphology (prismatic indefinite agglomerates) with well-formed boundaries (silicon carbide SiC); dark gray phase in the form of inclusions in grains of gray color or in the form of individual grains (B4C boron carbide), and dark phase (tear-outs, formed during microsection preparation, and pores). In all specimens there is a volume content of phases differing with respect to color characteristics (Table 5).
As studies showed, in specimens of compositions 4 – 6 during siliciding there is no marked grain growth of the primary boron carbide, although presence is revealed of especially coarse grains (up to 100 μm) of extended shape.
In a specimen of composition 4 of pure boron carbide there is weak particle adhesion and absence of fine B4C fraction (see Fig. 4). Presence within specimens of compositions 4 – 6 of a considerable amount of β-SiC and complex compounds B12(C,Si,B)3 and B12.97C2.88Si0.35 confirms that fine B4C powder enters into reaction with silicon with formation of secondary SiC and complex compounds in the S–B–C system, which form at a surface coarse grains of original boron carbide (lighter shell over a grain surface), and this correlates well with previously published data [9].
It should be noted that reaction of B4C with Si with solid solution and silicon carbide formation occurs with a reduction in volume. This leads to a situation that intergranular space is weakly filled with secondary SiC, and in some cases there is formation of intergranular and intragranular porosity.
With introduction of 10% C to an original workpiece material is prepared with a more densely reacted structure (specimen of composition 5, Fig. 5). It is well known that direct formation of silicon carbide from C and Si proceeds with an increase in volume by factor of 2.3 and leads to more complete filling of pore channels and formation of an interconnected structure [4]. A specimen of composition 5 (see Table 5) has the least volume content of free silicon (white phase).
Within a specimen of composition 6 with 10% SiC there is formation of secondary silicon carbide both on coarse B4C grains (light rim on born carbide grains), and also on fine SiC grains (accumulation of light gray grain between coarse grains). This leads to occurrence of a volumetric structure (Fig. 6). A considerable content of free silicon (see Table 5) points to a situation that there is inadequate carbon in order to form a volumetric carcase of secondary SiC.
Conclusion
By varying the content of different fractions of boron carbide, carbon, and silicon carbide additions ceramic materials have been prepared by reaction bonding based on boron carbide whose density is in the range 2.55 – 2.7 g/cm3. The optimum microstructure and mechanical properties (σben = 330 – 340 MPa, HV = 27 – 28 GPa) are exhibited by compositions containing 70% F 240 fraction.
It has been established that during siliciding of boron carbide there is formation of a volumetric material structure consisting of primary boron carbide and silicon carbide, secondary silicon carbide, complex compounds in the Si–B–C system, and also free silicon admixture. Secondary silicon carbide forms on grains of both primary silicon carbide and primary boron carbide, thereby creating a strong interconnected structure.
It has been shown that presence within ceramic composition of silicon carbide, solid solution of silicon carbide in boron carbide, and metallic silicon, point to formation of a microstructure by a secondary silicon carbide formation mechanism with direct reaction of carbon with silicon and boron carbide with silicon.
References
M. K. Aghajanian, B. N. Morgan, J. R. Singh, et al., “A new family of reaction bonded ceramics for armor applications,” in: Ceramic Armor Materials by Design (J. W. McCauley and A. Crouson), Ceram. Trans., 134, 527 – 539 (2002).
G. G. Gnesin, Silicon Carbide Materials [in Russian], Metallurgiya, Moscow (1977).
T. Ya. Kosolapova, T. V. Andreeva, and T. S. Bartnistkaya, Intermetallic Refractory Compounds [in Russian], Metallurgiya, Moscow (1985).
USA patent 6.862.970, Method for creating armor from B4C (2005).
S. N. Perevislov, A. V. Maev, D. A. Trubin, et al., “Ceramic protection materials based on silicon carbide and boron carbide,” Proc. XII Sci.-Pract. Conf. “New trends in the field of construction and use of ballistic materials and protective means,” 1 – 19 October 2012, Moscow (2012).
A. I. Ovsienko, Ya. G. Dyatleva, I. N. Manina, et al., “Comparison of structure and properties of boron carbide produced in OOO VIRIAL,” Proc. XXII Internat. Sci.-Tech. Conf. (2103).
E. F. Kharchenko, V. A, Aniskovich, V. V. Lenskii, I. S. Gavrikov, and V. A. Bykov, RU Patent 2440956, Method for preparing ceramic armor material based on silicon carbide and boron carbide, Claimant and patent holder OAO NPP ARMOKOMTsENTR, No. 2010133558 / 03, Claim 08.10.10, Publ. 01.27.12.
I. Yu. Kelina, N. A. Golubeva, V. V. Lenskii, et al., “Shock-resistant ceramic material based on SiC and B4C,” in: Questions of Armor Technology, Sci.-tech,. Ser. 15, Composite Nonmetallic materials in Engineering [in Russian], NTTs Informtekhnika, No. 1(164) – 2(165), 59 – 69 (2012).
H. Shmuel, W. Amir, P. D. Moshe, and F. Nahum, “Microstructural evolution during the infiltration of boron carbide with molten silicon,” J. Europ. Ceram. Soc., 30, 1007–1014 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 10, pp. 42 – 46, October 2014.
Rights and permissions
About this article
Cite this article
Golubeva, N.A., Plyasunkova, L.A., Kelina, I.Y. et al. Study of Reaction-Bonded Boron Carbide Properties. Refract Ind Ceram 55, 414–418 (2015). https://doi.org/10.1007/s11148-015-9736-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-015-9736-1