Abstract
The results of studying the processes of sorption of copper(II) ions from aqueous solutions by unmodified and modified chitosan granules are presented. As a result of the surface immobilization of nickel 2-ethylimidazolate in the presence of a surfactant, the maximum sorption capacity of the sorbent based on chitosan increases to 19.5 mol/kg. In this case, there is a reduction in the time to reach adsorption equilibrium in the “sorbent–CuSO4 solution” system up to 60 min and an increase in the degree of extraction of copper(II) ions up to 99.9%. IR spectra, microphotographs, and the elemental composition of initial and modified chitosan granules were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Due to global industrialization, heavy metals rapidly spread with sewage [1, 2]. Wastewater from fine organic synthesis, electroplating, paper production, and mining enterprises are the main pollutants of surface water bodies with heavy metals. A significant contribution to the discharge of heavy metals with water flows is made by the petroleum refining industry, as a result of the refinement of petroleum feedstock using supported catalysts based on d-metals. Unlike organic pollutants, heavy metals are not biodegradable and tend to accumulate in living organisms, exhibiting a cytotoxic effect on living organisms—in particular, by binding to protein amino acids through –NH2 and –SH groups, inactivating a number of enzymes and other biologically active substances [3]. Therefore, the effective removal of heavy metals remains an important task. Copper, cadmium, lead, and chromium are toxic heavy metals deserving special attention in cleaning waters [1, 4].
The most common and, in a number of parameters, expedient process of water purification from heavy-metal ions is adsorption from solution on solid porous adsorbents [5], which are zeolites, activated carbon, carbon nanotubes, and metal oxides; however, these materials have low sorption potential and selectivity [6].
Currently, sorbents that are easily separated from solutions, the components of which are biodegradable and/or chemically inert, are promising sorbents for the extraction of heavy-metal ions [7–9]. Both pure chitosan and many of its known modified forms satisfy these requirements. The efficiency of using chitosan in the extraction of heavy metals is due to the participation of cationic groups of chitosan in the sorption process.
The sorption capacity of chitosan can be increased by crosslinking chitosan at the stage of gel preparation [10, 11], by including zeolites [12] and starch [13] into the gel, and by obtaining sorbents based on chitosan and imidazolate framework structures (zeolitic imidazolate frameworks (ZIFs)). Sorbents based on granules of cross-linked chitosan and ZIF are relatively new biodegradable and nontoxic sorbents with a high potential for sorption of Cr(VI), Cu(II), and U(IV) [14–16].
Imidazole framework structures have a number of properties, such as being nanoscale and having a high specific surface area and porosity, and some representatives of this wide class of materials have the potential to extract Cr(III), Hg(II), and Cd(II) [17, 18]. It should be noted that porous ZIFs, which are used in adsorption processes, are obtained in the presence of deprotonating agents and soft templates (for example, surfactant micelles). However, when obtaining sorbents based on chitosan and ZIF, such auxiliary substances are not used, which leads to a slight improvement in adsorption characteristics compared to cross-linked chitosan.
The purpose of this work is to create an effective sorbent based on chitosan by its targeted modification with nickel 2-ethylimidazolate both in the absence and in the presence of a surfactant, as well as to establish the patterns of sorption of copper(II) ions from aqueous solutions.
EXPERIMENTAL
The following materials and reagents were used in the work without additional purification: nickel chloride (NiСl2⋅6H2O, chemically pure), 2-ethylimidazole (Sigma Aldrich, >98.0%), ammonia water 20% (NH4OH, analytical grade), chitosan (SD = 88%, M = 200 kDa), epichlorohydrin, sodium hydroxide (NaOH, chemically pure), dodecyldimethylamine-N-oxide, and copper sulfate (CuSO4⋅5H2Oh, chemically pure).
Preparation of Chitosan Granules (Sample 1)
Chitosan in an amount of 3 g was dissolved in 97 mL of 1% acetic acid and stirred for 20 min. After homogenization, 550 µL of epichlorohydrin was gradually added as a cross-linking agent to the mixture and stirred for 5 min. The gel prepared in this way was placed dropwise with a syringe into a container with 1 M NaOH solution. The granules were kept in the solution for 20 min and then washed with distilled water until neutral pH values were reached. The resulting granules were used for surface modification.
Assembly of Nickel 2-Ethylimidazolate on the Surface of the Beads (Sample 2)
The obtained and washed chitosan granules were immersed in 100 mL of distilled water containing NiCl2 in an amount of 1.07 g and kept for 5 min at constant stirring (200 rpm). Next, 25 mL of distilled water containing 100 µL of 20% NH4OH and 2-ethylimidazole in an amount of 1.75 g. The resulting reaction mixture was kept for an hour at room temperature (25°C) and constant stirring, 300 rpm. The modified chitosan granules were separated from the solution and washed with distilled water until pH was neutral.
The procedure for obtaining a sorbent based on chitosan by assembling nickel 2-ethylimidazolate on the surface of chitosan granules in the presence of dodecyldimethylamine-N-oxide (sample 3) is similar to the method for obtaining sample 2 but differs in that, before adding an aqueous solution of NiCl2, chitosan granules were immersed in 100 mL of distilled water containing 100 μL of dodecyldimethylamine-N-oxide and kept for 5 min with constant stirring (200 rpm).
The obtained granules, both unmodified and modified, were used in the sorption of copper(II) ions without preliminary drying.
The morphology of the obtained samples was analyzed by scanning electron microscopy using a Tescan VEGA 3 SBH scanning electron microscope at an accelerating voltage of 5 kV. Data on the elemental composition were obtained using an energy dispersive X-ray analyzer (Oxford Instruments X-Act) at a resolving voltage of 20 kV.
Infrared spectra were obtained in the range of 4000–500 cm–1 using a Shimadzu IRAffinity-1S FT-IR spectrometer using the frustrated total internal reflection method.
Sorption of copper(II) ions was carried out under static conditions from aqueous solutions of copper(II) sulfate with stirring and temperature control at 298 K. The sorption kinetics of copper(II) ions was studied by the limited solution volume method. Obtaining kinetic curves of sorption was carried out as follows: weighed portions of granules equal to 0.1 g in terms of dry chitosan were placed in test tubes with a volume of 15 cm3, followed by 10 mL of an aqueous solution of copper sulfate with a concentration of copper(II) ions equal to 1.5 × 10–3 and 1.75 × 10–3 mol/L. After the contact time, the solutions were separated from the sorbents by filtration, and the concentration of copper(II) ions was determined using a 210 VGP atomic-absorption spectrophotometer.
Sorption capacity (qτ, mg/g) was calculated by formula (1):
Degree of extraction (α, %) of copper(II) ions from the solution was estimated by formula (2):
where C0 is the initial concentration of copper(II) ions, mg/L; Cτ is the concentration of copper(II) ions after the specified contact time; m is the mass of the sorbent, g; and V is the volume of the solution, mL.
Processing of the kinetic curves of sorption of copper(II) ions was carried out within the framework of models of the first (3) and second (4) order:
where k1 and k2 are the rate constants of the sorption process according to the model of the first (min–1) and second (mg min g–1) order, respectively.
The Boyd–Adamson and Weber–Morris diffusion models were used to identify the limiting stage of the sorption process. Within the framework of the Boyd–Adamson model, when external diffusion is limited, the kinetic curve should be linear in coordinates –ln(1 – F) = f(τ). If the limiting stage of the process is sorption in the sorbent phase (internal diffusion), the linearity of the kinetic curves must be observed in coordinates F = f(τ1/2). F is the degree of equilibrium in the system calculated by Eq. (5):
According to Weber–Morris, internal diffusion is described by Eq. (6):
where kid is the rate constant of internal diffusion and c is a parameter related to the thickness of the boundary layer.
To obtain the sorption isotherms of copper(II) ions, sorbents containing 0.1 g of dry chitosan were placed in 10 mL of an aqueous solution of copper sulfate. The initial concentrations of Cu(II) varied within 5 × 10–3–0.5 mol/L. Sorption of copper was carried out at a temperature of 298 K. The contact time corresponded to the time to reach the adsorption equilibrium determined in the kinetic experiment.
To obtain the constants of the process of sorption of copper(II) ions, the adsorption isotherms were processed in the linear coordinates of the Langmuir (7), Freundlich (8), and Dubinin–Radushkevich (9) models:
RESULTS AND DISCUSSION
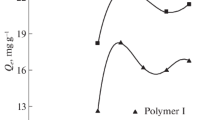
The work proved the effectiveness of chitosan modification by constructing ZIFs both in the presence and in the absence of a surfactant on the surface of the granules. By changing the values of the sorption capacity with time (Fig. 1), it was found that the equilibrium in the “sorbent–CuSO4 solution” system is achieved with a contact time of 90 min with the solution for sample 1 and for samples 2 and 3 at a contact time of 50 min with the solution. It should be noted that, when heavy metals are removed by known imidazolate framework compounds, but with other structural units, sorption equilibrium is achieved at a contact time of 180–420 min [1]; when sorbents based on chitosan are used for the same purposes, sorption equilibrium occurs in the range from 180 to 1440 min [8].
The degree of extraction of copper(II) ions for the sample 3 obtained in the presence of a surfactant is 99.9%.
The sorption kinetics of copper(II) ions on the obtained samples was approximated in first and second order kinetic models. All kinetic parameters are summarized in Table 1.
It can be seen that the linearization of the experimental data for all samples is observed in second-order kinetic model. The values of the equilibrium sorption capacity calculated in second-order kinetic model qe agree with the experimental data. That is, the stage that determines the rate of sorption of copper(II) ions can be the exchange of ions between the adsorbent and adsorbate and further complexation with functional groups of chitosan.
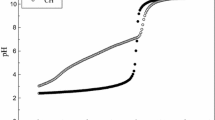
The process of diffusion of copper(II) ions from the bulk phase of the solution into the bulk phase of the sorbent was studied within the framework of the Boyd–Adamson and Weber–Morris models. Based on the presented dependences (Fig. 2), neither the stage of external nor the stage of internal diffusion are uniquely limiting throughout the entire adsorption process, which is probably due to the non-constant distribution of pores over the radii in the volume.
It is also shown that the dependences plotted in the coordinates of the Weber–Morris equation are not linear and do not leave the origin (Fig. 3). That is, the adsorption of copper(II) ions for both cross-linked chitosan granules and modified granules is not unambiguously limited by external or internal diffusion, which is consistent with the conclusions obtained from the Boyd–Adamson theory.
It follows from the type of dependences that two successive stages of mass transfer of copper ions can be distinguished in the course of sorption for all samples. The first linear section of the graph characterizes the diffusion of Cu(II) from the bulk of the solution through the outer diffusion layer to the surface of the sorbent (external diffusion mass transfer). Here, the stage of diffusion through the boundary near the surface limits. At the break point, the influence of the external diffusion factor decreases and the intradiffusion factor increases. The second linear section refers to the diffusion of Cu(II) ions into the sorbent (intradiffusion mass transfer). Here, the sorption process is limited by the transfer of copper(II) ions in the sorbent phase. Thus, the adsorption of copper(II) ions for both cross-linked chitosan granules and modified granules occurs in a mixed diffusion mode and is not diffusion controlled.
For cross-linked chitosan granules (sample 1) and modified granules (samples 2 and 3) adsorption isotherms of copper(II) ions were obtained at a temperature of 298 K in the concentration range of 5 × 10–3–0.5 mol/L (Fig. 4).
The maximum values of the adsorption capacity obtained during the experiment for samples 1, 2, and 3 are 4.91, 8.35, and 19.44 mol/kg, respectively. The linearization of the experimental isotherms of adsorption of copper(II) ions was carried out in linear coordinates of the Langmuir, Freundlich, and Dubinin–Radushkevich models. All adsorption parameters are summarized in Table 3.
It is shown that the linearity of the experimental data on the adsorption of copper(II) ions is observed in the coordinates of the Langmuir isotherm for all samples. Linearization in Dubinin–Radushkevich coordinates is most applicable for samples 2 and 3, which indicates the presence of adsorption in the volume of an energetically homogeneous porous adsorbent. In the course of processing the kinetic experiment, it was shown that the adsorption is of an ion-complexation nature, which is also confirmed by the obtained values of characteristic energy of the adsorbent E, with the values for all samples being in the range of 8–16 kJ/mol.
From the linear coordinates of the Freundlich isotherm, which describes the processes of adsorption on energetically inhomogeneous surfaces, the values of characteristic constants KF and n were found. For all samples, the value of n varies from 1 to 10, which indicates a favorable course of adsorption.
From the linear coordinates of the Langmuir isotherm, the values of the maximum sorption capacity (mol/kg) were obtained; sample 3 has the highest adsorption capacity. From the values of KL, it was also found that the adsorption process proceeds more intensely in the presence of sample 3, which is associated with an increase in the number of adsorption centers.
The morphology of cross-linked chitosan granules (sample 1), chitosan with an immobilized ZIF on the surface of the granules in the absence (sample 2), and in the presence of dodecyldimethylamine-N-oxide (sample 3) is shown in Fig. 5.
It can be seen that the initial chitosan granules are characterized by a predominantly smooth surface. When the surface of the initial granules is modified, nickel 2-ethylimidazolate particles are organized predominantly with lamellar morphology, which is associated with the possibility of framework growth only in the direction perpendicular to the surface. The addition of surfactants at the stage of crystal growth causes the appearance of particles with a bulk structure on the surface of the granules due to the template effect, as a result of which the specific surface area of the sorbent increases significantly.
The elemental composition of chitosan-based adsorbents is shown in Fig. 4.
Infrared spectra of (1) chitosan and (2) modified granules are shown in Fig. 6.
After immobilization of a ZIF on the surface of chitosan granules, the absorption intensity is about 2921 and 2877 cm–1 (C–H symmetrical and asymmetric stretching respectively) and 1423 and 1375 cm–1 (CH2 oscillations and CH3 symmetric deformation vibrations) increases due to the presence of an ethyl substituent in the imidazole ring. Also, after immobilization with ZIF, the absorption intensity at 1325 cm–1 (vibration range of the tertiary amine) decreases, which is due to the formation of active ZIF growth centers through the binding of nickel to –NH2 groups at the first stages of chitosan surface modification. It should be noted that after ZIF immobilization on chitosan granules, a weak band at 1646 cm–1 is observed in the final spectrum, which indicates the presence of C=C vibrations of the imidazole ring. The bands at 1066 and 1028 cm–1 correspond to C–O tension. The reasons for the decrease in intensity are associated with the formation of active ZIF growth centers with the participation of –OH groups [19]. The main sign of ZIF formation is the appearance of bands in the region of 500–800 cm–1 (especially 530 cm–1), indicating the presence of a Ni–N bond between the metal center and the imidazolate ligand [20]. The intensity of the signals of the corresponding groups of atoms, which determine the features of the structure and properties of chitosan, does not change after modification.
CONCLUSIONS
The processes of sorption of Cu(II) ions from aqueous solutions in the presence of unmodified and modified chitosan granules are characterized. It has been shown that modification of the surface of chitosan granules with nickel 2-ethylimidazolate in the presence of a surfactant and a deprotonating agent leads to an increase in the sorption capacity to 19.5 mol/kg and a reduction in the time to reach adsorption equilibrium to 60 min. It has been established that the adsorption of metal ions in the presence of a modified chitosan-based sorbent proceeds in a mixed-diffusion mode and retains its ion-complexation nature. Thus, the developed sorbents based on chitosan can be proposed as an alternative to industrial cation exchangers for additional purification of aqueous solutions from heavy-metal ions.
REFERENCES
Chen, Y., Bai, X., and Ye, Z., Nanomaterials, 2020, vol. 10, no. 8, p. 1481.
Azimi, A., Azari, A., Rezakazemi, M., et al., ChemBioEng Rev., 2017, vol. 4, no. 1, p. 37.
Xu, S., Lv, Y., Zeng, X., and Cao, D., Chem. Eng. J., 2017, vol. 323, p. 502.
Rasheed, T., Chemosphere, 2020, vol. 259, p. 127369.
Karadaş, C. and Kara, D., Food Chem., 2017, vol. 220, p. 242.
Velasco-Garduño, O., Martínez, M.E., Gimeno, M., et al., Environ. Sci. Pollut. Res., 2020, vol. 27, p. 28527.
Rehman, M., Liu, L., Wang, Q., et al., Environ. Sci. Pollut. Res., 2019, vol. 26, p. 18003.
Li, X., Wang, B., Cao, Y., Zhao, S., et al., ACS Sustainable Chem. Eng., 2019, vol. 7, p. 4548.
Manos, G. and Dunne, L., Nanomaterials, 2018, vol. 8, no. 10, p. 818.
Huang, Y., Zeng, X., Guo, L., et al., Sep. Purif. Technol., 2018, vol. 194, p. 462.
Li, M., Ren, G., Wang, F., et al., Inorg. Chem. Front., 2019, vol. 6, no. 5, p. 1129.
Chakraborty, R., Asthana, A., Singh, A.K., et al., Int. J. Environ. Anal. Chem., 2020, vol. 100, p. 1.
Arora, R., Mater. Today: Proc., 2019, vol. 18, p. 4745.
Wang, K., Tao, X., Xu, J., et al., Chem. Lett., 2016, vol. 45, no. 12, p. 1365.
Liu, L., Yang, W., Gu, D., et al., Front. Chem., 2019, vol. 7, p. 607.
Sugashini, S. and Gopalakrishnan, S., Res. J. Chem. Sci., 2012, vol. 2, no. 6, p. 55.
Huang, R., Yang, B., and Liu, Q., J. Appl. Polym. Sci., 2013, vol. 129, no. 2, p. 908.
Boamah, P.O., Huang, Y., Hua, M., et al., Carbohydr. Polym., 2015, vol. 122, p. 255.
Fernandes Queiroz, M., Melo, K., Sabry, D., et al., Mar. Drugs, 2015, vol. 13, p. 141.
Zhang, Y., Jia, Y., Li, M., et al., Sci. Rep., 2018, vol. 8, no. 1, p. 1.
Funding
This work was funded by the Ministry of Science and Higher Education of the Russian Federation (project no. FZZW–2020–0010).The study was carried out using the resources of the Center for Shared Use of Scientific Equipment of the ISUCT (with the support of the Ministry of Science and Higher Education of Russia, grant no. 075-15-2021-671).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fufaeva, V.A., Nikiforova, T.E. Extraction of Copper Ions by Chitosan-Based Sorbents Modified with Nickel 2-Ethylimidazolate. Prot Met Phys Chem Surf 58, 262–268 (2022). https://doi.org/10.1134/S2070205122020058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205122020058