Abstract
This work demonstrates that a biodegradable chitosan-based biocomposite packed in mini-reactors successfully removes copper ions from aqueous solutions. The chitosan is obtained by deacetylation of biological chitin, which is extracted from shrimp wastes by lactic acid fermentation. The polysaccharide is embedded in a biodegradable prepolymer matrix before extrusion to produce porous cylindrical pellets of 2 × 80 mm. The highest copper ion removal is 62.5 mg Cu2+ per g of the biodegradable adsorbent. Additionally, the adsorption capacity of the material, below its saturation, allows several cycles of reuse with a hydraulic retention time reduction of 1 h. This chitosan-based material is advantageous when compared with other approaches using non-biodegradable materials or costly commercial adsorbents for removing heavy metal ions in wastewater effluents as well as a filter component in water purification devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of heavy metal ions into the environment from industrial processes, generally through their wastewaters, is a concerning worldwide contamination that affects all life forms on earth. In this regard, the worst contaminants are metal plating facilities, mining operations, and battery manufacturers, although others, such as the paper, fertilizer, tanneries, or pesticide industries, or the manufacturing of stabilizers, thermoplastics, and pigments, also contribute to this global issue (Ngah et al. 2011; Karadas and Kara 2017). The threats to human health from the exposure to these contaminants are well documented. The worst among them are cadmium, chromium, cobalt, copper, nickel, and mercury, which are also common water pollutants (Sprynskyy et al. 2006; Fu and Wang 2011). For example, copper ion poisoning is one of the most feared owing to its high toxicity according to the US environmental protection agency’s maximum contaminant level (MCL) for this metal in drinking water which is 1.3 mg per liter, and the maximum allowable in food is 30 mg/kg (Karadas and Kara 2017). Therefore, the removal of these metals is subjected to intensive research in academic and industrial fields with an eye to low-cost devices with high efficiency and adequate characteristics for a large-scale implementation (Cataldo et al. 2015).
The use of adsorbents is at present the most viable route for removal of these pollutants from wastewaters. However, many commercially available devices present low biodegradability, which becomes a disposal issue after their lifespan. Alternatively, the use of biodegradable adsorbents, besides of their low toxicity, might also allow for the recovery of metals throughout the biodegradation processes. In this regard, several approaches using biopolymers proved to be successful for the removal of dyes and heavy metal ions (Crini 2006), where chitin, especially its deacetylated form chitosan (Ch), is highlighted owing to its high content of primary amino moieties, thereby the highest chelation capacity above all other polysaccharides (Pereira et al. 2017). Importantly, the Ch is highly biodegradable and presents low toxicity as it has been extensively reported elsewhere (Zhang et al. 2016). Nonetheless, the processing of Ch for many applications, like many other polysaccharides, is challenging due to its poor mechanical properties. An alternative is the inclusion of these polymers in adequate biodegradable and low toxic matrices, such as biodegradable polyesters (Haider et al., 2019), thus preserving the desired characteristics in structured forms for enhanced applications (Vakili et al. 2014; Zhang et al. 2016; Muxika et al. 2017).
In this work, we are first to fabricate, to the best of our knowledge, a fully biodegradable Ch-based biocomposite for copper ion adsorption from wastewater, and the results are discussed.
Materials and methods
Materials
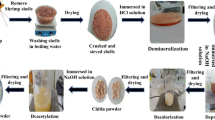
Lactic acid fermentation (Lactobacillus plantarum) of shrimp (Litopenaeus vanameii) wastes to attain the chitin and its further deacetylation produced Ch with Mv of 121.2 kDa and a degree of acetylation of 6.8% (Pacheco et al. 2011) (see supplementary data for FTIR spectra of the synthesized Ch).
Production of Ch-based samples by extrusion
Ch was homogeneously pre-mixed with a thermoplastic prepolymer polyester (PE) in a double weight amount of Ch to PE (CP1), an equal amount of Ch to PE (CP2), and a low Ch amount in a 1:5 (wt/wt) Ch/PE ratio (CP3) prior to adding it into a CSI-laboratory LE-075 extruder with a rotor and a spindle at 85 °C (Willett and Shogren 2002). Non-extrudable Ch flake samples were used as the control for adsorption experiments.
Characterization of samples
Scanning electron microscopies (SEM) were recorded in a JEOL JSM-5900 KV (Japan) microscope. Samples were immersed in 5% (v v−1) glutaraldehyde for 24 h (4 °C) and treated with OsO4 1% (v v−1) for 2 h. Then, they were dehydrated with ethanol and covered with carbon and gold before the analyses. Powder X-ray diffraction (XRD) patterns were acquired in a D-8 Bruker Advance (USA) diffractometer with Bragg-Bretano θ-θ geometry, Kα Cu radiation, and Ni filter (0.5%) of Cu-Kβ. Thermogravimetric analysis (TGA) was recorded on a TGA-Instrument SDT Q600 V20.9 Build 20 unit (USA) with 50 mL/min nitrogen flow from 20 to 650 °C in a 5 °C/min rate. Tensile strength, maximum elongation at break, and Young’s modulus were measured in a SINTECH 1/S, MTS (USA), with a load cell of 100 N at a crosshead speed of 2 mm/min. Samples were cylindrical pellets of 80 mm of length and 2 mm of diameter pretreated at 45% relative humidity and 25 °C for 48 h.
Cu (II) adsorption analysis of samples
A solution of Cu(NO3)2∙2.5 H2O was dissolved in deionized water (70 mg Cu2+/L) and adjusted to pH 6 with NaOH (5% w/v) (Chen and Wang 2011; Wan et al. 2013). Atomic absorption spectrometry (FAAS) was conducted in a Varian FS 220 (USA). Samples were diluted 1:10 with deionized water and acidified with HNO3. Equation (1) displays the Cu2+ adsorbed per gram of adsorbent (mg Cu2+/g ads) (Popuri et al. 2009).
where q is the capacity of adsorption (mg Cu2+/g ads); L is the volume; [Cu2+]0 is the copper concentration in the inlet; [Cu2+]i is the copper concentration in the outlet; and wads is the packed adsorbent in grams.
Adsorption of Cu2+ ions in a continuous system proceeded at room temperature with varied hydraulic retention times (HRT) between 0 and 2 h. The reactor was a tubular system with a 6-mL feed volume in an ascendant way. The amount of CP1, CP2, and CP3 samples was 1.171 ± 0.053 g, 1.973 ± 0.079 g, and 3.201 ± 0.044 g, respectively, with 5 cycles of reuse for each reactor. The pseudo-second-order kinetics ascribed to Eq. (2).
where k (g ads/mg Cu2+ min) is the rate constant of pseudo-second-order adsorption. The plot of \( \frac{t}{q_t} \) versus t gave the pseudo-second-order rate constant k, and \( {q}_ek{q}_e^2=h \) and h are the initial rate of adsorption (mg Cu2+/g ads min) (Ho and Mckay 2002).
Results and discussion
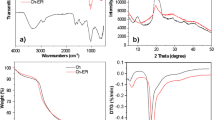
The XRD patterns, shown in Fig. 1, evidenced the production of the biocomposite where the intensity peaks assigned to Ch increased with its content (Pacheco et al. 2011). The TGA data revealed a Td of 216.7 °C with 8.57% mass loss for CP1 sample, whereas CP2 displayed 3.15% loss at 186.2 °C. The less thermally stable was CP3 sample (1.7% mass losses at 164.3 °C). The Ch sample decomposes above 300 °C; therefore, the early decompositions in the biocomposites were assigned to PE. On the other hand, the mechanical determinations for the extruded samples (Fig. 2) displayed a smaller Young’s modulus, thus indicating the consecution of relatively flexible materials (Ren et al. 2017). The results for the Young’s modulus, shown in Fig. 2b, had no significant difference (p > 0.05) between CP1 and CP2; however, this was not the case for CP3 (171.22 ± 22.24 MPa). This behavior was ascribed to the Ch content in agreement to other reported works using polyethylene-based blends with this polysaccharide (Quiroz-Castillo et al. 2014).
Noteworthy, none of the extruded samples displayed a significant difference of maximum elongation. This contradicts the work by Quiroz-Castillo et al. (2014), where the increase in Ch embedded in their polyethylene-based proposal significantly decreased the elongation at break. Similarly to the Young’s modulus, the tensile strength was not significantly different for CP1 and CP2 samples (7.04 ± 0.17 MPa, 6.82 ± 0.2 MPa; p > 0.05), but it was for the lowest Ch content sample CP3 (7.24 ± 0.5). Therefore, the smaller the Young’s modulus, the higher the flexibility of the material, and this work demonstrated that CP1 and CP2 samples might sustain relatively high tensile stress (Mendes et al. 2016).
Adsorption experiments in the continuous system
The results of the adsorption experiments shown in Fig. 3 clearly evidenced that the increase in Ch in the biocomposites led to an increase in the adsorption capacities. However, the fastest adsorption detected at initial times reached a plateau towards the adsorption equilibrium. Another work by Wan et al. (2013) described a similar behavior using a Ch-coated sludge to a maximum HRT of 2 h. Noteworthy, a direct comparison with other reported studies could not be conducted as there are no reports using a fully biodegradable Ch embedded in polyester material to remove metals from wastewater.
Adsorption kinetics
The pseudo-second-order kinetic Eq. (2) was best to describe the adsorption processes with high correlation coefficients (0.922–0.999), as shown in Table 1. Similarly, Ho and McKay (2002) described a pseudo-second-order kinetic equation based on the concentration of a copper solution from the adsorption capacity of peat. The rate-limiting factor was ascribed to chemisorption involving valence forces through electron sharing between the amino groups and metal ions (Pradhan et al. 2005). In the present study, the constant rate k, which was experimentally determined by plotting the slopes and the interceptions of \( \frac{t}{q_t} \)against time, indicated the lowest absorption capacity (7.0) for the sample with the lowest Ch; whereas a 9-fold increase was observed for the highest Ch content sample (CP1), which corresponds to a 99% adsorption efficiency (Table 2). Noteworthy, the adsorption capacity for non-extruded Ch flakes was 60 mg Cu2+/g ads, close to the CP1 sample, although the assay with Ch showed a higher initial rate than that for CP1. A high adsorption equilibrium resulted in fast adsorption rates and short equilibrium times (Wan et al. 2013), and therefore, these experimental evidences substantiates a high affinity of the Cu2+ ions to the adsorbent following the pseudo-second-order kinetic (Ho and McKay 2002).
In another work by Wan et al. (2013), batch experiments using Ch-coated sludge described a Langmuir isotherm model for the maximum adsorption of 18.83 mg Cu/g in 24 h; nonetheless, in the present work, the CP1 sample displayed 3.5-fold higher adsorption than this earlier report. It is fair to mention other approaches using non-biodegradable adsorbents such as the Ch-coated polyvinyl chloride beads in Popuri et al. (2009) with up to 87.9 mg Cu/g removal and the Ch-coated polyethylene terephthalate by Niu and co-workers (Niu et al. 2017) with a maximum adsorption of 23.70 g Cu/g, as well as the study by Niu et al. (2017) with ca. 160 mg/g by using a Ch-coated polyethyleneimine adsorbent. However, these composites contain non-biodegradable polymer pollutants, and some of them are regarded as environmentally dangerous plastics.
SEM, EDS, and FTIR analyses for the copper ion adsorption
The cycles of use and Cu2+ adsorption capacities of the samples were monitored by SEM before and after treatment, as shown in Fig. 4. The results evidenced the presence of Ch flakes on the sample surfaces. The slight changes after Cu removal might be ascribed to the swelling effect of water on the polysaccharide. On the other hand, the EDS analysis, shown in Fig. 5, displayed the presence of the metal on the areas where the Ch was present, and thus substantiating the action of the polysaccharide to retain the metal ions. Additionally, the experimental evidence indicated that the saturation point of the materials was not reached as the copper signals are between 1 and 3%. Additionally, the FTIR (Fig. 6) spectra corroborated that CP1 was the best sample for Cu2+ adsorption owing to the evident decrease in the characteristic N-H stretching bands of the polysaccharide at 1650–1550 cm−1 and that for O-H at 3200 cm−1 owing to the coordination with the metal. Another feature worth to note is the robustness of the materials (Table 2) with less than 1wt% weight losses after each cycle for the samples.
Conclusion
The present work concludes that the Ch-based biodegradable biocomposite successfully removes Cu2+ in contaminated model water allowing cycles of re-use and the biocomposites present high robustness. The best material is produced with double weight amount of Ch to the polyester prepolymer to attain up to 99.16 ± 0.1% of metal removal. This work also demonstrates that Ch-based biocomposites are advantageous over commercial absorbents and other Ch-based approaches, which are costly and present poor biodegradability and toxicity concerns regarding some of the components thereof. Further work is in progress towards an engineered application of the material for heavy metal ion recoveries in aqueous effluents.
References
Cataldo S, Muratore N, Orecchio S, Pettignano A (2015) Enhancement of adsorption ability of calcium alginate gel beads towards Pd (II) ion. A kinetic and equilibrium study on hybrid Laponite and Montmorillonite–alginate gel beads. Appl Clay Sci 118:162–170. https://doi.org/10.1016/j.clay.2015.09.014
Chen Y, Wang J (2011) Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem Eng J 168:286–292. https://doi.org/10.1016/j.cej.2011.01.006
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085. https://doi.org/10.1016/j.biortech.2005.05.001
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Haider TP, Völker C, Kramm J, Landfester K, Wurm FR (2019) Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew Chem Int Ed 58:50–62. https://doi.org/10.1002/anie.201805766
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper (II) on to peat. Adsorpt Sci Technol 20:797–815. https://doi.org/10.1260/026361702321104282
Karadas C, Kara D (2017) Dispersive liquid–liquid microextraction based on solidification of floating organic drop for preconcentration and determination of trace amounts of copper by flame atomic absorption spectrometry. Food Chem 220:242–248. https://doi.org/10.1016/j.foodchem.2016.09.005
Mendes JF, Pascholain RT, Carmona VB, Sena A, Marques ACP, Marconcini JM, Mattoso LHC, Medeiros ES, Oliveira JE (2016) Biodegradable polymer blends based on corn starch and thermoplastic chitosan by extrusion. Carbohydr Polym 137:452–458. https://doi.org/10.1016/j.carbpol.2015.10.093
Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: processing, properties and applications. Int J Biol Macromol 105:1358–1368. https://doi.org/10.1016/j.ijbiomac.2017.07.087
Ngah WW, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456. https://doi.org/10.1016/j.carbpol.2010.11.004
Niu Y, Ying D, Li K, Wang Y, Ji J (2017) Adsorption of heavy-metal ions from aqueous solution onto chitosan-modified polyethylene terephthalate (PET). Res Chem Intermed 43:4213–4225. https://doi.org/10.1007/s11164-017-2866-y
Pacheco N, Garnica-Gonzalez M, Gimeno M, Bárzana ET, Stéphane DL, Shirai K (2011) Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 12:3285–3290. https://doi.org/10.1021/bm200750t
Pereira FS, Lanfredi S, Perez-Gonzalez ER, da Silva Agostini DL, Marques-Gomes H, dos Santos-Medeiros R (2017) Thermal and morphological study of chitosan metal complexes. J Therm Anal Calorim 129:291–301. https://doi.org/10.1007/s10973-017-6146-2
Popuri SR, Vijaya Y, Boddu VM, Abburi K (2009) Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour Technol 100:194–199. https://doi.org/10.1016/j.biortech.2008.05.041
Pradhan S, Shukla SS, Dorris KL (2005) Removal of nickel from aqueous solutions using crab shells. J Hazard Mater 125:201–204. https://doi.org/10.1016/j.jhazmat.2005.05.029
Quiroz-Castillo JM, Rodríguez-Félix DE, Grijalva-Monteverde H, del Castillo-Castro T, Plascencia-Jatomea M, Rodríguez-Félix F, Herrera-Franco PJ (2014) Preparation of extruded polyethylene/chitosan blends compatibilized with polyethylene-graft-maleic anhydride. Carbohydr Polym 101:1094–1100. https://doi.org/10.1016/j.carbpol.2013.10.052
Ren L, Yan X, Zhou J, Tong J, Su X (2017) Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int J Biol Macromol 105:1636–1643. https://doi.org/10.1016/j.ijbiomac.2017.02.008
Sprynskyy M, Buszewski B, Terzyk AP, Namieśnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304:21–28. https://doi.org/10.1016/j.jcis.2006.07.068
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Gholami Z, Amouzgar P (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr Polym 113:115–130. https://doi.org/10.1016/j.carbpol.2014.07.007
Wan MW, Wang CC, Chen CM (2013) The adsorption study of copper removal by chitosan-coated sludge derived from water treatment plant. Int J Environ Sci Dev 4(5):545. https://doi.org/10.7763/IJESD.2013.V4.411
Willett JL, Shogren RL (2002) Processing and properties of extruded starch/polymer foams. Polymer 43:5935–5947. https://doi.org/10.1016/S0032-3861(02)00497-4
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175–191. https://doi.org/10.1016/j.molliq.2015.12.013
Acknowledgments
Mr. Heriberto Alonso Gómez and Mr. Ricardo Rosas are greatly acknowledged for their assistance in Atomic Absorption Spectrometry and XRD analyses, respectively, at Universidad Autonoma Metropolitana-Iztapalapa. Dr. José D. Sepulveda-Sánchez is also greatly acknowledged for his assistance in EDS and SEM studies. Ms. Claudia Barrera is greatly acknowledged for the diligent proofreading of this paper.
Funding
The authors would like to thank the Secretary of Education, Science Technology, and Innovation of Mexico City (SECTEI) of the Government of Mexico City, for funding Project No. SECTEI/196/2019 and CONACYT for scholarship grants (OVG and MEM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Velasco-Garduño, O., Martínez, M.E., Gimeno, M. et al. Copper removal from wastewater by a chitosan-based biodegradable composite. Environ Sci Pollut Res 27, 28527–28535 (2020). https://doi.org/10.1007/s11356-019-07560-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07560-2