Abstract
The phase composition and morphology of a synthesized material based on aluminum powder modified with various concentrations of V2O5 (3, 5, 10 wt %)—compacted in the form of tablets and sintered at temperatures of 820 and 900°C in He and air—have been studied by X-ray analysis and electron microscopy. It is shown that the phase components are uniformly distributed in the plane of the microsection, and their ratio is determined by the temperature and time of annealing of the pellets. It has been established that, due to the activation of processes on the surface of modified particles of aluminum powder, it becomes possible to control the properties of interphase boundaries in oxidizing and inert media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Interest in alloys of the Al–V system, which have a light metal matrix and a high modulus of elasticity [1], appeared in the first half of the 20th century and is not weakening even at the present time, which is due to the prospects for their commercial use in the manufacture of products for military and aerospace technology [2], in addition to as biomaterials with high mechanical characteristics [3, 4]. The development of advanced industries is impossible without the creation of the modern materials, especially composites, necessary for the production of parts with light weight, flexibility, and high strength and abrasion resistance. Therefore, the creation of metal–matrix composites [5] for the automotive industry, railway transport, and aerospace industries [6] is of great importance. In addition, Al–V alloys are very important master alloys that are widely used for the production of both heat-resistant alloys and some special ones [7, 8]. The addition of vanadium and aluminum makes it possible to significantly increase the mechanical properties of titanium alloys—wear, fatigue, corrosion, and thermal resistance—and improves the cold working process of alloys [8, 9]. However, there is a very large difference in the melting temperatures of the components of the Al–V system (that of Al is 660°C, and that of V is 1910°C), which leads to difficulties in direct synthesis of alloys even when using modern methods of heat treatment. In this regard, in recent years, much attention has been paid to the creation of alternative methods for obtaining Al–V alloys and composite materials, among which a special place is occupied by methods based on aluminothermal reduction of vanadium upon heating mixtures of aluminum and vanadium pentoxide [6, 7, 10]. Despite their obvious efficiency and versatility in the production of aluminum-vanadium alloys, these methods have a common disadvantage, which is the need to use high-energy methods of physical impact—for example, high-energy grinding for homogenization of the components of the Al–V2O5 system [7, 9–11]. The use of these methods is associated with the likelihood of contamination of the material with undesirable impurities, as well as the need to strictly adjust the mixing mode of the reagents due to the possibility of their spontaneous combustion under mechanical action [8, 10].
We have developed a method [12] for modifying aluminum powders with vanadium pentoxide, which allows direct contact of V2O5 with the surface of Al particles without changing their original shape and introducing undesirable impurities that are inevitable when using mixers and attritors. Vanadium pentoxide is added to the aluminum powder as a V2O5·nH2O hydrogel, which has a high covering ability with respect to the surfaces of oxides and metals [13, 14]. The completeness of combustion of the modified powder is achieved by mixing the components of the Al–V2O5 system at the molecular level.
We proposed the developed method in order to activate the oxidation of aluminum powders as components of metallic fuels in energy condensed systems [13, 15–17]. By adjusting the concentration of V2O5 in the gel, it is possible to obtain dispersed systems with different amounts of vanadium pentoxide, as well as other oxides of transition metals [18]. In this regard, it is of interest to use the developed approach not only to control the reactivity of fuels based on powdered aluminum, but also to synthesize aluminum–vanadium alloys and composite materials.
The main goal of this work is to assess the possibilities of using the modification of aluminum particles with vanadium pentoxide hydrogels to obtain composite materials. A concentrated gel containing 5.5–6.0 wt % V2O5 was used as a modifier. Within the framework of the general task of the work, a study was undertaken of the influence of the concentration of V2O5 and heat-treatment conditions for morphology and phase composition of the sintered material.
EXPERIMENTAL
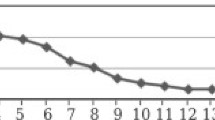
The modifier was synthesized according to the following procedure. Ten grams of vanadium pentoxide of analytical grade are taken and melted in a platinum crucible at a temperature of 750°C. The resulting homogeneous melt is poured into a porcelain mug with a capacity of 1000 mL with distilled water (900 mL) with vigorous stirring. The resulting colloidal solution with a vanadium concentration of about 6.5 g/L is evaporated to form a gel with a vanadium-pentoxide content of 5.5–6 wt %, which is used further as a modifier. The mixing of the initial aluminum powder with the modifier at a given concentration of vanadium oxide was carried out in a porcelain mortar, the resulting mass was dried at a temperature of 80°C for 1 h, then heated at 350°C for 0.5 h for complete dehydration of the material, which is limited by dehydration. V2O5 · nH2O [19]. Typically, to obtain a modified powder containing 5.0 wt % V2O5, 9.5 g of ASD-4 aluminum powder in a porcelain mortar was mixed with 8.4 g of gel of nominal composition V2O5·nH2O with a vanadium pentoxide content of 5.96 wt %. The resulting mixture was dried at 80°C for 1 h with stirring and finally heated at 350°C for 0.5 h. The effective combustion point of the obtained sample in air is 776°C, which is 265°C is lower than the value of this parameter for unmodified ASD-4 powder (Fig. 1). The powders were then pressed into tablets with a diameter of 10 mm (0.7 g of powder) at a pressing pressure of 3.92 MPa (40 kg/cm2). All tablets obtained a mirrorlike flat surface. According to the scheme described above, compacted samples with a content of 3, 5, and 10 wt % V2O5.
X-ray phase analysis was performed on a Shimadzu diffractometer (СuKα radiation) using the YCPDSDICDD Powder Diffraction File (PDF2). The surface morphology of the synthesized material was studied using a JEOL JSM-6390LA scanning microscope equipped with an energy dispersive X-ray analyzer (EDX). The density and volume of the samples were determined on an AccuPyc II 340 helium pycnometer.
RESULTS AND DISCUSSION
Table 1 shows the results of X-ray full-profile analysis by the Rietveld method [20] of compacted powders (tablets) after annealing for 1 h in He at a temperature of 820°C. The analysis was performed on the real (external) surfaces of the tablets after annealing and the internal sections from a depth of 0.5–0.7 mm, which were subjected to grinding and polishing.
It follows from Table 1 that, after sintering in He from the end (outer) surface of the tablet, a decrease in the content of metallic aluminum is observed with an increase in the concentration of vanadium pentoxide. At the same time, the formation of aluminum oxides of both modifications (γ- and α-Al2O3), the total concentration of which increases with increasing concentration of V2O5. Along with this, there are intermetallic compounds of Al3V, Al10V composition corresponding to the region of the Al–V diagram that is rich in aluminum [21]. As the V2O5 content increases, the concentration of intermetallic compounds decreases, but the amount of the V2O3 phase increases from 0.8 to 4 wt % with the addition of 10 wt % modifier.
One of the important characteristics of composite materials is their specific gravity (density), since the mass of the structure, in combination with the strength characteristics of the material, often determine the possibility and prospects of its use.
Table 2 shows the values of the volume and density of some of the obtained samples under various synthesis conditions: a content of V2O5 of 3–5–10 wt %, a surrounding environment of O2 and He, a sintering duration of 1–2 h, and a temperature of 820°C.
It follows from Table 2 that, with an increase in the sintering time of the pellets in He, their density increases, which is associated with a change in the ratio of the phase components in the material (see Table 1 and the following text). When synthesized in air (O2), the density changes to a lesser extent (Al–3% V2O5 sample). From the reference data [22], only the density of V2O5 (3.362 g/cm3) is less than the density of Al2O3 (3.96 g/cm3). The rest of the phases in the composite have densities higher than that of Al (2.699 g/cm3) and its oxide. The maximum increase in the density of the obtained materials does not exceed 7.5%.
The results of X-ray phase analysis after grinding and polishing showed that, under similar annealing conditions in an inert atmosphere, as in the first case, the concentration of metallic aluminum decreases. However, in this case, only γ-Al2O3 is fixed and, after annealing, the content of the intermetallic compound decreases in correspondence with the concentration of V2O5. At 10% pentoxide, intermetallic phases are absent, but oxide VO1.15 (2.3%) is fixed.
It follows from the results obtained that annealing of the samples in a He atmosphere is accompanied by the appearance of intermetallic and oxide phases. The reason for their appearance is the thermite reaction
during which a large amount of heat is released (915.6 kJ/mol) [23]. The interaction of oxides V2O5 and Al2O3, as a result of which the oxide phase AlVO4 [23], as well as the formation during the heating of the furnace and the heat of reaction (1) of atomic V and its interaction with aluminum, as a result of which intermetallic Al–V compounds of a certain composition are formed.
During heating, V2O5 loses part of its oxygen, forming oxide phases, in which vanadium is in lower oxidation states (V2O3 and VO1.15). The number of these phases, with an increase in the content of V2O5 in the original powders, increases. At the same time, there is a decrease in the content of intermetallic compounds. Apparently, the process of decomposition of vanadium oxides is not completed during an hour’s exposure.
The results of a 2-h holding in He at a temperature of 820°C showed a decrease in the amount of metallic aluminum and an increase in the concentration of aluminum oxide and intermetallic compound. So, in a sample containing 5% V2O5, after holding in He for 2 h, the amount of Al is 75.4%, the total content of γ- and α-Al2O3 9s 22.5% and that of Al3V is 2.1%, and oxide phases based on V are absent.
Thus, based on the results of X-ray phase analysis, after annealing at 820°, the main phases are γ- and α‑Al2O3 modifications of aluminum oxide, metallic aluminum, intermetallic phases of the Al–V system, and vanadium oxides with a lower valence (V2O3, VO1.15). The ratio of these phases is determined by the temperature and time of pellet annealing.
Additional information on the processes occurring during the high-temperature annealing of tablets with different modifier contents was obtained by analyzing the microstructure of sintered materials. Figure 2 shows images of the surface of thin sections obtained by scanning electron microscopy after an hour of sintering of tablets in He medium.
It follows from Fig. 2 that, with an increase in the concentration of V2O5, not only does the shape of the particles change, but also the contrast in the images of structural elements associated with an increase in the concentration of aluminum oxides formed during the thermite interaction (Eq. 1). This is due to the charging of the surface with a decrease in the electron drain from the dielectric surface of the oxide phases. Morphological analysis, with a simultaneous elemental analysis of the surface of thin sections, showed that the lightest areas are represented by Al3V.
As an example, Fig. 3 shows images of the surface of thin sections obtained by the SEM method after sintering samples in an inert atmosphere at a temperature of 900° within 1 h. It can be seen that the phase components are fairly uniformly distributed in the plane of the microsection. From analysis of the microstructure of the composite by the EDX method, it follows that there are three regions (white, gray, and black), which correspond to three phases in the structure of the sample, which is consistent with the results [14] obtained for samples subjected to joint grinding of powders of aluminum and V2O5. Dark areas correspond to α-Al2O3, white ones to the Al3V intermetallic compound, and gray ones to an aluminum matrix with fine oxide inclusions.
A similar picture is observed in the case of annealing the samples in an air atmosphere. Figure 4 shows an image of the surface of thin sections after sintering in air for 1 h at a temperature of 900° with samples containing 3 wt % V2O5. Comparison with Fig. 3 shows the practical identity of the microstructure of materials synthesized in oxidizing and inert media.
This result can be explained by the fact that the intensification of the oxidation of the starting aluminum is observed at temperatures of about 600°C [22], when the protective properties of the oxide film are lost as a result of the formation of γ-Al2O3 and loss of continuity at the time of aluminum melting. Vanadium pentoxide, being an oxygen supplier, consolidates particles on the surface of samples after melting of aluminum and thermal interaction. During annealing inside the pellet, the amount of the Al2O3 oxide phase depends on the amount of modifier (V2O5) added. As a result of the processes occurring directly in the pressed sample, a composite material is formed with interphase boundaries that are formed directly in the course of interaction. Such conditions are favorable for purposes of eliminating the effect of the real surface of the powders during conventional sintering methods in systems in which thermal interaction of the components does not occur.
REFERENCES
Vol, A.E., Stroenie i svoistva dvoinykh metallicheskikh sistem (Structure and Properties of Binary Metal Systems), Moscow: Fizmatgiz, 1959.
Omran, A.M., J. Al-Azhar Univ. Eng. Sect. (JAUES), 2007, vol. 2, no. 6, p. 36.
Stolecki, B., Borodziuk-Kulpa, A., and Zahorowski, W., J. Mater. Sci.,1987, vol. 22, no. 8, p. 2933.
Rahmati, B., Sarhan, Ahmed A.D., Basirun, W.J., and Abas, W.A.B.W., J. Alloys Compd., 2016, vol. 676, p. 369. https://doi.org/10.1016/j.jallcom.2016.03.188
Woo, K.D. and Lee, H.B., Met. Mater. Int., 2010, vol. 16, no. 2, p. 213.
Omran, A.M., E3 J. Sci. Res., 2014, vol. 2, no. 2, p. 026.
de Souza, D.A. and Nunes, C.A., Int. J. Refract. Met. Hard Mater., 2000, vol. 18, no. 1, p. 55.
Wan, H., Xu, B., Li, L., Yang, B., Li, D., and Dai, Y., Metals, 2019, vol. 9, p. 558. https://doi.org/10.3390/met9050558
Liu, Y., Wang, D., Deng, C., Huo, L., Wang, L., and Fang, R., J. Alloys Compd., 2015, vol. 628, p. 208. https://doi.org/10.1016/j.jallcom.2014.12.144
Keller, J.G. and Douglasst, D.L., Oxid. Met., 1991, vol. 36, nos. 5/6, p. 439.
Yang Liu, Dongpo Wang, Caiyan Deng, Lixing Huo, Lijun Wang, and Rui Fang, J. Alloys Compd., 2015, vol. 628, p. 208. https://doi.org/10.1016/j.jallcom.2014.12.144
Shevchenko, V.G., Eselevich, D.A., Konyukova, A.V., and Krasil’nikov, V.N., RF Patent 2509790, Byull. Izobret., 2014, no. 8.
Shevchenko, V.G., Eselevich, D.A., Konyukova, A.V., and Krasil’nikov, V.N., Russ. J. Phys. Chem., B, 2014, vol. 8, no. 5, p. 634.
Eselevich, D.A., Cand. Sci. (Chem.) Dissertation, Yekaterinburg: Institute of Solid State Chemistry of the Ural Branch Russ. Acad. Sci., 2016.
Shevchenko, V.G., Krasil’nikov, V.N., Eselevich, D.A., and Konyukova, A.V., Prot. Met. Phys. Chem. Surf., 2017, vol. 53, no. 5, p. 835.
Shevchenko, V.G., Eselevich, D.A., Ancharov, A.I., and Tolochko, B.P., Univers. J. Phys. Appl., 2017, vol. 11, no. 4, p. 115.
Shevchenko, V.G., Eselevich, D.A., Vinokurov, Z.S., and Konyukova, A.V., Combust., Explos., Shock Waves, 2019, vol. 55, no. 3, p. 289.
Shevchenko, V.G., Krasil’nikov, V.N., Yeselevich, D.A., and Konyukova, A.V., Prot. Met. Phys. Chem. Surf., 2019, vol. 55, no. 1, p. 21.
Wang, Y., Shang, H., Chou, T., and Cao, G., J. Phys. Chem. B, 2005, vol. 109, p. 11361. https://doi.org/10.1021/jp051275+
A Rietveld Extended Program to Perform the Combined Analysis: Diffraction, Fluorescence and Reflectivity Data Using X-Ray, Neutron, TOF or Electrons. http://maud.radiographema.eu. Accessed February 10, 2020.
Okamoto, H., J. Phase Equilib. Diffus., 2012, vol. 33, no. 6, p. 491.
Rabinovich, V.A. and Khavin, Z.Ya., Kratkii khimicheskii spravochnik (Abridged Chemical Handbook), Leningrad: Khimiya, 1977.
Shevchenko, V.G., Eselevich, D.A., Popov, N.A., et al., Combust., Explos., Shock Waves, 2018, vol. 54, no. 1, p. 58.
Funding
This work was carried out in accordance with state order to the Institute of Solid State Chemistry, Ural Branch, Russian Academy of Sciences, no. AAAA-A19-119031890028-0, structural unit no. 2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shevchenko, V.G., Krasilnikov, V.N., Eselevich, D.A. et al. Physicochemical Studies of Material Obtained by Pressing and Sintering Al Powder Modified with V2O5. Prot Met Phys Chem Surf 58, 84–89 (2022). https://doi.org/10.1134/S207020512201018X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512201018X