Features are discussed for the microstructure and phase composition of powder prepared by chemical dispersion of aluminum-molybdenum alloy with 10 wt.% molybdenum in caustic soda solution after heat treatment at 1250°C for 90 min. It is concluded that heat treatment leads to significant phase and structural transformations of the powder and is a required stage in treatment before sintering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increase in the level of properties of ceramic materials and creation of new forms of ceramic is impossible without improving starting powder quality. Development of new production solutions and principles for selecting additive facilitates creation of ceramic materials exhibiting improved physicotechnical characteristics and makes it possible to give them a number of unique special properties. In this connection there is considerable interest in material based on powder prepared by chemical dispersion of aluminum alloys in caustic soda solution. This method developed in the Moscow State Industrial University (now Moscow Polytechnic University, or Moscow Polytech) [1,2,3,4,5], makes it possible without significant expenditure to prepare starting raw material with nanoparticles and to alloy it [6, 7].
The aim of the present study is creation of the possibility of maximum use in industry of the unique properties of ceramic based on Al2O3 by developing fundamentally technologically simple and energy saving schemes for preparing starting raw material, i.e., alloyed nano-powders, and also the principles of controlling their fineness and aprticle shape. Preparation of Al2O3 nanopowder has been described by chemical dispersion of aluminum-molybdenum alloy (Al–Mo alloy) in a caustic soda solution and the properties of powder has been studied after heat treatment. The results obtained may be proposed as a basis for clarifying the production parameters of large-scale manufacture of this form of raw material in order to create unique ceramics for special purposes.
Powder Preparation by Chemical Dispersion of Al–Mo Alloy and Study Using X-Ray Phase Analysis (XPA)
Chemical dispersion of Al–Mo alloy was accomplished by treatment with 20% aqueous solution of caustic soda [2]. The residue obtained was washed in distilled water: 2Al + Mo + 4NaOH + 8H2O → 2Na[Al(OH)4] + Na2MoO4 + 6H2↑ and filtered in vacuum unit [7]. The pH of the medium before washing was 12.8, and after washing it was 8.7. It should be noted that the original unwashed power was dried at elevated temperature of 200°C, and in this case there is formation of a strong skin that is difficult to break down. This is apparently connected with occurrence aluminum hydroxide polymerization with excess content of alkali ions OH– with formation of a a polymer chain (HO)2·Al·O·Al·(OH)2.
Polymerization requires thermal activation (200°C) [8], whereas evaporation of adsorbed chemically unbonded water (in the case of drying washed residues after 18 decantations, i.e., with n = 18) is observed at a significantly lower temperature (60°C). In addition, after heat treatment in air (1250°C) the original residue (n = 0) is solid aggregates, which are extremely difficult to refine to a state of fine powder, used for charge preparation. Aggregates were ground manually using a porcelain pestle and mortar, reacting intensely with impact and wear, whereas washed residue (n = 18) is easily pulverized in a mortar with a pestle to a fine powder condition.
A feature of the residue washing process (Fig. 1) is constancy of medium pH after 12 decantations, which is connected with the impossibility of removing the ionic layer from hydroxyl groups OH– from a residue particle surface, exhibiting increased absorption capacity. The number of hydroxyl groups (their concentration) within the composition of an ionic layer is connected with the degree of residue particle fineness: the greater particle fineness, the more concentrated are hydroxyl groups. pH indices of the medium for deposition, i.e., the products of chemical dispersion of Al–Mo alloy are provided below:
n | pH* | n | pH | n | pH |
0 | 12.8 | 6 | 10.5 | 12 | 8.7 |
1 | 12.2 | 7 | 10.1 | 13 | 8.7 |
2 | 12.2 | 8 | 9.4 | 14 | 8.7 |
3 | 12.1 | 9 | 9.1 | 15 | 8.7 |
4 | 11.9 | 10 | 8.9 | 16 | 8.7 |
5 | 11.4 | 11 | 8.7 | 17 | 8.7 |
18 | 8.7 |
* pH with n = 0 concerns the original deposit before washing, pH = 8.7 corresponds to the maximum degree of washing.
As is seen, the maximum degree of residue washing is achieved in the 11th decantation.
Powder phase composition was established using XPA, and the phase composition of all powders was determined in a D2 PHASER instrument from Bruker (CuKα radiation, Ni filter, tube regime 10 mA, 30 kV). Interpretation of the spectrum and calculation of the phase composition (Fig. 2) was accomplished by means of a JCPDS-ICCD card index using specialized software (Topas x-ray structural analysis package). Results of XPA and the sizes of coherent scattering regions (CSR) of crystal phases are provided in Table 1. It is seen that the main crystal phase after chemical dispersion is gibbsite γ-Al(OH)2, and 2 wt.% of sodium molybdate is also present. Due to its high solubility in water a considerable part of the product was lost during decantation. The size of the gibbsite CSR comprises 86, and for sodium molybdate it is 33 nm.
In order to reconstruct heating conditions during powder synthesis and compact sintering thermal effects were studied in an oxidizing medium with a heating rate of 5°C/min to 1200°C. A powder compact was placed in a tungsten crucible (instead of corundum) for cleanliness of the experiment and reducing the possibility of applying extraneous thermal effects as a result of reaction of the test powder and crucible material under high-temperature and corrosive atmosphere conditions. Differential thermal analysis of aluminum hydroxide powder with sodium molybdate showed that their decomposition proceeds in several stages: in the range to 300°C a thermal effect corresponds to adsorbed water removal during subsequent heating in the range 300 – 550°C a clearly defined endothermic effect is observed, which is connected with final removal of crystalline water; in the range 750 – 850°C there is sodium molybdate melting. Results of thermogravimetric analysis point to an overall weight loss of 43%.
Powder Analysis by Scanning Electron Microscopy (SEM)
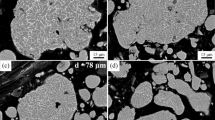
Results of SEM for Al–Mo alloy powder after chemical dispersion show that within the material a considerable amount of agglomerated prismatic particles are present (Fig. 3), which is typical for gibbsite of monoclinic syngony [9]. The predominant size of agglomerates is 50 – 80 μm, and sizes of crystals are 8 – 15 (60 vol.%) and 15 – 40 μm (40 vol.%). Powders, both ultra- and nano-dispersed, have a tendency towards agglomeration and aggregation.
Powder was calcined in a furnace with a heating rate of 200°C/h and isothermal exposure at 1200°C for 1 h; cooling was conducted in the furnace. With this temperature range quite actively sintering powder is obtained with no shrinkage. Results of SEM for powder after heat treatment are shown as an increase in sodium molybdate content after heat treatment is connected with the high intensity of diffusion processes, and therefore the average crystal size within agglomerates of a powder sample is an order of magnitude greater than for powder after chemical dispersion.
Sintering proceeds by a liquid-phase mechanism. During heat treatment in the range 750 – 850°C there is melting of NaMoO4, which wets the powder surface and therefore intensifies the sintering diffusion processes.
Conclusion
These studies are a logical continuation of work for preparing fine boehmite powder, conducted the scientific group of the Moscow Polytech. Powders prepared by a chemical method, i.e., by decomposition of commercial aluminum and aluminum alloys with caustic soda, are classified as finely dispersed powders, containing a significant proportion particles of the nanosize range. Powders exhibit a suitable combination of structure, phase composition, and rheological properties. The fine structure of the powder composition has been studied for the first time, i.e., a product of chemical dispersion of Al–Mo alloy containing 10 wt.% molybdenum with an aqueous alkaline solution. A production process has been developed for preparing nano- and ultrafine aluminum oxide powders containing refractory element, i.e., molybdenum.
Results of XPA have shown that a predominant phase of the powder product is gibbsite, and the alloying element is present in the form of sodium molybdate (~2 wt.%). Loss of alloying element proceeds during decantation after chemical dispersion.
A synthesis regime has been selected for powder making it possible to provide retention of the optimum powder activity, to reduce the risk of crack formation and simultaneously to obtain during sintering specimens with low volumetric shrinkage.
Results of powder SEM show that powders before and after firing differ from each other. The original powder of agglomerates with shape similar to spherical has a radial-beam structure. Particles after heat treatment are inclined towards coarsening and have a shape similar to equiaxed. Preliminary sintering of powder will proceed by a liquid-phase mechanism, which makes it possible to reduce the firing temperature and prospectively to improve the physicotechnical properties of corundum ceramic.
References
Yu. G. Trifonov, A. D. Shlyapin, V. D. Alekhin, et al., “Method of chemical dispersion as a procedure for preparing nano-dispersed aluminum oxide powder for preparing structural nano-ceramics with unique properties,” Nanoinzhineriya, No. 3, 9 – 13 (2013).
A. D. Shlyapin, A. Yu. Omarov, and Yu. G. Trifonov, “New ceramic material structure and phase composition,” Refract. Indust. Ceram., 53(6), 387 – 390 (2013).
Tu. G. Trifonov, A. Yu. Omarov, N. A. Kasatova, and A. D. Shlyapin, “Study of powder prepared by chemical dispersion of aliuminum-lithium alloys,” Ogneupory Tehkn. Keram., No. 3, 28 – 32 (2013).
A. Yu. Omarov, O. V. Shikumova, and A. Kh. Khairi, “Kinetics of hydrogen separation during reaction of aluminum with alkaline solution,” Izv. Moskov. Gos. Indust. Univ., No. 1, 54 – 57 (2011).
A. D. Shlyapin, A. Yu. Omarov, A. Kh. Khairi, and Yu. G. Trifonov, “Study of aluminum hydroxide powder prepared by chemical dispersion of aluminum and its alloy,” Refract. Indust. Ceram., 53(5), 317 – 321 (2013).
A. Yu. Omarov and Yu. G. Trifonov, “Structure and phase composition of new ceramic material prepared from products of alloy AMg12 chemical dispersion,” Pis’ma Materialakh, No. 4, 272 – 274 (2013).
A. Yu. Omarov and Yu. G. Trifonov, “Structure and phase composition of ceramic materials prepared from aluminum hydroxide powder by chemical dispersion of aluminum-magnesium alloys,” Refract. Indust. Ceram., 54(5), 362 – 365 (2014).
A. Yu. Omarov, F. Z. Badaev, and Yu. G. Trifonov, “Production scheme for sintering nanodispersed powders prepared by chemical dispersion,” Refract. Indust. Ceram., 53(5), 322 – 325 (2013).
Yu. G. Trifonov, A. Yu. Omarov, A. D. Shlyapin, and A. A. Vasin, “Structure of aluminum oxide powder prepared by chemical dispersion of Al–Ti alloy and ceramic sintered from it,” Innovatsii Invest., No. 4 (2013).
Work was completed with financial support of the Russian Federation Ministry of Education and Science within the scope of a state assignment No. 11.5987.2017/VU for carrying out work “Organization of the performance of scientific research” (Number for publication: 11.5987.2017/6.7) using equipment of the Center for Collective Utilization “High-tech in engineering”, Moscow Polytech.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Novye Ogneupory, No. 6, pp. 46 – 49, June, 2018.
Rights and permissions
About this article
Cite this article
Tarasovskii, V.P., Shlyapin, A.D., Omarov, A.Y. et al. Structure and Phase Composition of Powder Prepared from Chemically Dispersed Aluminum-Molybdenum Alloy. Refract Ind Ceram 59, 318–321 (2018). https://doi.org/10.1007/s11148-018-0228-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11148-018-0228-y