Abstract
Thermodynamic characteristics of adsorption of different classes of organic compounds on nanoporous silicas with chemically grafted methylsiloxane have been determined by gas chromatography. It was shown that an increase in the concentration of grafted groups from 1.5 to 1.7 nm–2 leads to an increase in the Henry’s constants and heats of adsorption and, as a rule, to a decrease in the standard entropies of adsorption. With respect to arenes, the polarity of the samples is practically the same as that of the liquid phase OV-1. The set of gas chromatographic and static data on vapor adsorption, taking into account the values of the contact angles of wetting with water and hexadecane for similar monolayers fixed on silicon wafers, allows us to conclude that the degree of lyophobization of the surface of the methylsiloxane layers is significantly higher than that of the hexadecyl layers, but inferior to that of the polyfluoroalkyl layers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Lyophobization of surfaces of different nature is a processing method widespread in various fields of industrial, scientific, and medical–biological activity, the purpose of which is to give the required properties to materials used in various media. Lyophobization protects materials of various origins, from fabric to metal, from uncontrolled wetting; prevents their contamination and caking; reduces adhesion; and drastically reduces adhesion and condensation, as well as giving them anticorrosion and dustproof properties. The need for superhydrophobic, hydrophobic, and oleophobic materials, along with the development of the latest biomimetic methods, has stimulated the further development of chemical modification of solid surfaces with alkyl, polyfluoroalkyl and polymer coatings, which allows the directed synthesis of materials with given properties of different applications, including as adsorbents, catalysts, and supports [1–5]. For these purposes, the inorganic material most widely used as a matrix for modification is mechanically strong, thermally resistant silicon oxide SiO2, which is well established, in many ways, as an optimal and convenient object of the chemical method of changing surface properties. This is facilitated by the variety of types of SiO2 offered by industry, including ones with high chemical and geometrical homogeneity of the structure, as well as the possibility of varying the degree of hydroxylation that the most important factor in the modification process [1, 2]. Polymethylsiloxanes have been uniquely popular organosilicon polymers for many years, which either create nonpolar, chemically stable, and heat-resistant coatings with low surface energy [4–6] or are very most important components of organic–inorganic hybrid materials for various applications, including nanotechnologies, as protective, self-cleaning, and optical coatings; membranes; and semiconductor and antistatic coatings resistant to UV radiation and with water-repellent properties [7–9]. In recent years, a new type of silicon-containing materials based on polysiloxanes chemically modified by both organic and inorganic compounds with improved properties has been developed [10, 11]. Current trends in the synthesis and use of organosilicon sorption materials are described in detail in review [12].
Among polysiloxanes, the use of polydimethylsiloxanes (PDMSs) is widespread: from drilling mud lubricants [13] and fillers in polymer materials [14] to adsorption-type additives in the electrolyte of lithium-ion batteries [15], in microelectronics [16, 17] and polydimethylsiloxane microfluidic technology and its biological applications [8, 18]. The use is well known of PDMSs as stationary phases in gas–liquid and high-performance capillary gas chromatography, in particular, OV-1 and OV-101, both for the separation of organic compounds of different classes [19] and for studying the thermodynamic characteristics of sorption on these stationary phases [20, 21]. Sorbents for the isolation and concentration of organic compounds by direct supercritical fluid extraction and gas chromatography that are resistant to solvent washing have been developed by grafting OV-101 onto trimethylsilylated glass beads [22]. In [23], a simple and effective way of obtaining superhydrophobic self-cleaning films was demonstrated by modifying the surface of composite particles of CaCO3/SiO2 by strong stirring and self-assembly of PDMSs. The well-known high-tech properties of SiO2/PDMS composites, such as thermal stability and hydrophobicity, turned out to be improvable by additional modification of the systems with metal oxides [24, 25].
The physicochemical and operational properties of chemically modified materials obviously depend on the chemical structure and structure of grafted molecules. Given the dominant role played by surface phenomena in their interaction with the environment, it seems necessary at the initial stages to apply adsorption methods for the study of such objects, which can provide predictions about such critical features as hydrophobicity and oleophobicity, as well as their association with various characteristics of the grafted layer.
In gas chromatography, thermodynamic characteristics of adsorption of a set of organic compounds, including saturated and aromatic hydrocarbons, on nanoporous silicas with chemically grafted methylsiloxane, were determined. The results obtained by adsorption and wetting methods for methylsiloxane coatings and well-studied hydrophobized materials with chemically grafted hexadecyl and perfluorohexyl groups were compared for the purpose of assessing the lyophobicity of the samples.
EXPERIMENTAL
Sorbents
Chemically modified silicas (CMS) with grafted methylsiloxane were obtained by modifying a silica support—aerosilogel (designation ASG in the text, specific surface area ssp = 100 m2/g, effective pore radius 20 nm)—with silane ClSi(CH3)2[OSi(CH3)2]2Cl, which is a derivative of oligomethylsiloxane, according to the method [5, 6]. Samples of OMS-1 (concentration of grafted groups N = 1.5 nm–2, ssp = 95 m2/g) and OMS-2 (N = 1.7 nm–2, ssp = 94 m2/g) were studied. According to the data on the adsorption of nitrogen and benzene, modification has practically no effect on the effective pore radius, since the thickness of the grafted methylsiloxane layer is less than 1 nm [5]. The results are compared with those obtained earlier [26] for hydrophobic CMS with grafted hexadecyl groups (C16, modifier ClSi(CH3)2(n-C16H33), N = 2.8 nm–2, ssp = 77 m2/g) and perfluorohexyl groups (CF, modifier ClSi(CH3)2(CH2)3(n-C6F13), N = 2.1 nm–2, ssp = 79 m2/g), as well as on the liquid phase of methylsiloxane OV-1 with the composition [ОSi(CH3)2]n (10% on Gas Chrom Q) [27].
Methods
The concentration of grafted groups was determined from the results of elemental analysis for carbon (PerkinElmer 2400 CHN Analyzer, Schwarzkopf Microanalytical Lab., United States) according to the formula given in [1]. The determination error was ≤07%.
Nitrogen adsorption isotherms were measured on an ASAP-2020 sorbtometer (Micromeritics, Norcross, United States) at 77 K, and the adsorption isotherms of water, hexane, and benzene vapors were obtained by the gravimetric static method (on a McBan–Bakr spring balance [28]) at 298 K.
Gas chromatography (GC) studies were performed on a Chrom-5 chromatograph with a flame ionization detector and on an Agilent HP-6890 chromatograph with a thermal conductivity detector (katharometer) in the single-column mode. Helium was used as the carrier gas. The dead time in the case of a flame ionization detector was determined by methane and, in the case of a katharometer, by air. The length of the glass columns was 1–1.2 m, and the inner diameter was 2–2.5 mm. Samples of substances were typically administered in the form of 2–30 times diluted vapors by means of a 0.1-mL syringe.
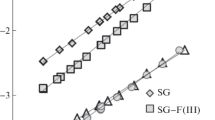
The advantages of using GC include high sensitivity, the ability to study samples in nano- and even picogram quantities, when the adsorbate–adsorbate interaction can be neglected. GC allows to relatively quick determinate of the thermodynamic characteristics of adsorption (TCA) of a wide range of test molecules. A necessary condition for confirming the possibility of using GC for determining thermodynamic values is an absence of dependence of retention volumes Va calculated from the data measured in the GC experiment on the carrier gas velocity [28, 29]. This was confirmed in the work for all samples, and, for example, Va of heptane and benzene on silica with a methylsiloxane layer remained constant (within 1–2%) when the flow rate changed from 11 to 32 mL/min (Fig. 1). These data made it possible to assume that Va is numerically equal to the Henry constant of adsorption equilibrium KH.
Kovats indices I and Henry constant of adsorption equilibrium KH were calculated from the GC parameters using the formulas given in [28, 29]. As standard states of the substance, 1 μmol/mL in the gas phase and 1 μmol/m2 in the adsorbed state were used.
Initial (Henry region) adsorption heats q and the standard adsorption entropies ΔS° in the approximation of their temperature independence were calculated from temperature dependences KH by the equation [28, 29]
The error in determining q and ΔS° ranged from 2 to 7%.
The contribution of CH2 groups to the lnKH \(({\text{ln}}{{K}_{{{\text{H}}({\text{C}}{{{\text{H}}}_{2}})}}})\) values of n-alkanes was calculated using the equation [29]
where n is the number of carbon atoms in the n-alkane molecule and a is the value corresponding to the contribution of two H atoms to lnKH.
RESULTS AND DISCUSSION
Dispersive Interactions
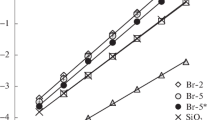
The interaction of saturated hydrocarbons with materials of any nature is predominantly determined by universal dispersion forces, which is characterized by TCA of these molecules. On OMS-2, heats of adsorption q of alkanes with the number of carbon atoms in the molecule from 5 to 11 are slightly higher than on the initial support (Fig. 2) A decrease in the density of the grafted layer on OMS-1 leads to a decrease in q. These results serve as a fairly reliable confirmation of the hypothesis made in [5, 6] that the grafted methylsiloxane layer has a about cyclic structure. The fact is that such modifiers as, for example, a silane of the composition ClSi(CH3)2[OSi(CH3)2]2Cl, have the ability to bind by two silicon atoms to the surface, which leads to the formation of arched structures consisting of three links Si(CH3)2O and effectively shielding the carrier surface. At the same time, it is possible to organize arched structures consisting of six Si(CH3)2O units formed by two radicals interacting with the surface, as well as with each other, with the formation of a siloxane bond. The existence of a larger number of large arches on OMS-2 as compared to OMS-1 may be the reason for the observed change in the adsorption energy. Although the composition of the stationary liquid phase of OV-1 is the same as that of grafted methylsiloxanes, the heat of dissolution in OV-1 is lower (Fig. 2). This effect is explained by the influence of the surface of the silica support on the energy characteristics.
Obviously, the values of the most important TCA, Henry constants KH, are determined by both energy and entropy characteristics. It follows from Fig. 3 that the values of ΔS° on the OMS-2 are less than on the original carrier. This fact dominates in the decrease in KH by OMS-2 after ASG modification (Fig. 4). At the same time, the decrease in KH values for OMS-1 in comparison with ASG is due to the energy factor. A comparison of the properties of grafted methylsiloxanes and other hydrophobized surfaces shows that the KH values are intermediate in this group of CMS, i.e., change in the series С16 > OMS-2 > OMS-1 > CF (Fig. 4). The contributions of CH2 groups to lnKH of n-alkanes change in the same direction as Henry’s constants (Table 1). Analysis of the data given in work [6] shows that the adsorption values of hexane measured under static conditions at 298 K change in a similar way (Table 1).
In this work, we studied the thermodynamic characteristics of the adsorption of adamantane (AD), trans-decalin (D), and decane (C10)—compounds with the same numbers of carbon atoms in the molecule and similar molar masses. Like TCA for linear alkanes, the heats of adsorption AD and D are the highest, and the entropies of adsorption are the smallest on OMS-2 (Table 2). Unlike alkanes, KH AD on OMS-2 can be higher than on ASG (for example, at 403 K, 0.40 and 0.35 for OMS-2 and ASG, respectively). As follows from Fig. 5, on samples with a methylsiloxane layer, the order of the compounds yield is as follows: C10 comes out first from the column, then D, and then AD, and the KH AD reaches values characteristic of undecane. Such ratios differ significantly from those observed for varieties of silica, where the order of release of compounds is: AD < D < C10, and, which is even more the case for adsorbents with a flat surface, for example, Carbopack or WSe2, on which a very strong decrease in interaction energy D and, especially, a compact AD molecule compared to C10 [30].
More detailed information on the effect of the nature of the sorbent on the order of yield of scaffold (AD) and linear (C10) molecules follows from retention selectivity = \(K_{{\text{H}}}^{{{\text{rel}}}}\)(AD)/KH(C10) determined over a wide temperature range. It is these relative characteristics, like Kovats indices I, that are used when comparing the properties of solid and liquid phases for GC [30, 31]. The I values on grafted methylsiloxanes practically do not differ from those obtained upon dissolution in the liquid phase, in methylsiloxane OV-1, one of the most popular liquid phases for GC (Table 3), although they somewhat decrease upon going from OMS-2 to OMS-1, but remain higher than on the original carrier and, moreover, on Сarbopaсk (Fig. 6). Therefore, on silicas with methylsiloxane layers, there is most likely not only adsorption on the outer surface of grafted groups, but also an absorption retention mechanism, i.e., penetration of adsorbed molecules into large arches.
Specific Interactions
Shielding and substitution of silanol groups, which relatively actively adsorb molecules with π-bonds, leads to the usual decrease in q, but a relatively small change in the entropy characteristics of arenes as a result of modification (Table 2). Despite the small difference in the number of grafted Si(CH3)2O units on the surface of the samples with methylsiloxane coating, the KH of hydrocarbons of different chemical structures is more than twice as high on OMS-2 than on OMS-1. For both linear alkanes and arenes, the KH values on methylsiloxanes are intermediate in the group of hydrophobized surfaces, i.e. change in the series С16 > OMS-2 > OMS-1 > CF (Fig. 4). On the whole, GC data are in good agreement with those obtained for benzene adsorption under static conditions (Table 1). We can also note a decrease in constant C of the BET equation for nitrogen from 107 at ASG to 21 at OMS-2.
It is known [29, 32] that a comparison of the temperature dependences of relative Henry constants \(K_{{\text{H}}}^{{{\text{rel}}}}\) for molecules with relatively similar sizes and polarizabilities, including pairs such as benzene–hexane, makes it possible to characterize the role of π-bonds in specific intermolecular interactions. It has been shown that, in a wide temperature range, a change in the concentration of methylsiloxanes grafted to the surface has little effect on \(K_{{\text{H}}}^{{{\text{rel}}}}\) (Fig. 7). It is interesting that on grafted methylsiloxanes they differ insignificantly from those observed upon dissolution in methylsiloxane OV-1 (Fig. 7). Taking into account that OV-1 belongs to nonpolar liquid phases, it is possible that arenes in the case of the studied CMSs interact mainly with Si(CH3)2O units, and the role of residual silanols in the adsorption process is insignificant. Table 3 shows the Kovats I retention indices for a number of studied samples, indicating that, according to the traditional GC parameter for assessing the polarity of materials, the role of specific interactions of arenes noticeably decreases as a result of modification, but depends little on the concentration of grafted groups. In this case, within a range of 2% for OMS-1 and OMS-2, the values of I arenes are approximately the same as for OV-1. Consequently, silicas with methylsiloxane can be used as sorbents in analytical GC of hydrocarbons. A different picture is observed in the case of the sorption of acetonitrile, diethyl ether, and ethyl acetate; i.e., molecules capable of stronger specific interactions. The I values on the methylsiloxane layers are much higher than on OV-1 (Table 3). In this case, the interaction of acetonitrile and ethers with unreacted with the modifier and apparently available residual silanol groups on the CMS surface is undoubtedly manifested. However, the values of I on methylsiloxane layers for such molecules differ by no more than 4%. In addition, the degree of decrease in KH on going from OMS-2 to OMS-1 depends little on the nature of the molecules, which is illustrated in Fig. 8, which shows the temperature dependences of KH for acetonitrile and toluene. Therefore, it can be supposed that the concentration of residual silanol groups on OMS-2 is close to OMS-1.
Water is a compound that is extremely sensitive to the presence of hydrophilic centers on the surface—in our case, silanol groups [1]. Work [6] shows that modification with methylsiloxanes reduces water adsorption by 45 times at p/p0 → 1 compared to the original carrier. At the same time, prolonged exposure in water vapors for 4 months increases water adsorption on OMS-2 from 4 μmol/m2 by only 3 μmol/m2, remaining below the limit value of the capacity of the conditional idealized monolayer.
According to Fig. 9, where the initial isotherms of water adsorption are given, coatings with Si(CH3)2O units regardless of grafting density well shield the hydrophilic groups of the carrier. The same course of water adsorption isotherms on methylsiloxane layers confirms the assumption of close concentrations of residual silanol groups on OMS-2 and OMS-1. In terms of water-protecting properties, methylsiloxanes are not inferior to the С16 sample, although perfluorohexyl layers are more effective in this regard.
It should be emphasized that, in contrast to the detailed study of perfluorohexyl-coated silicas by adsorption and IR spectroscopic methods [33, 34], the reduction in the concentration of methylsiloxane groups and, therefore, the flow rate of the modifier contributes to the reduction of the adsorption activity of the material and does not affect its high hydrophobicity.
It is known that the main method for studying the lyophobicity of coatings is to determine the contact angles of wetting on flat surfaces. The corresponding results obtained in [35, 36] on chemically modified silicon wafers are presented in Table 4. The grafted methylsiloxane layers are extremely hydrophobic. In terms of degree of oleophobization of the surface, the methylsiloxane layers significantly exceed the hexadecyl ones, but are inferior to the polyfluoroalkyl ones. However, the low wetting hysteresis on methylsiloxanes, no more than 1°, in contrast to perfluorohexyl coatings, where the wetting hysteresis reaches 20°, suggests the possibility of preparing very effective, uniform protective layers using the investigated modifiers [4]. It is also necessary to note the high thermal stability of the OMS layers in air: in the case of OMS, there is no weight loss up to 623–653 K, while C16 and CF begin to decompose at 443–463 K [5]. In general, taking into account the high cost of fluorine-containing reagents and more stringent requirements for the synthesis conditions, the use of methylsiloxane modifiers for the creation of highly effective coatings, in particular, water- and dirt-protective coatings, remains relevant.
CONCLUSIONS
The results of a joint consideration of GC data and adsorption-static studies of silicas with grafted methylsiloxane, alkyl and polyfluoroalkyl groups are consistent with the literature data on the determination of contact angles. It has been shown that the GC method, which allows one to obtain a more detailed understanding of the presence of active adsorption centers on a modified silica surface, as well as the effect of the structure of the grafted group on the regularities of adsorption processes, can be used as a simple and accessible method for assessing the lyophobicity of the surface of porous bodies.
In general, given the high thermal stability and the ease of obtaining and controlling the concentration of grafted groups, methylsiloxanes are advisable for recommendation for the preparation of sorbents for various purposes and highly effective protective coatings.
REFERENCES
Khimiya privitykh poverkhnostnykh soedinenii (Chemistry of Grafted Surface Compounds), Lisichkin, G.V., Ed., Moscow: Fizmatlit, 2003.
Zaitsev, V.N., Kompleksoobrazuyushchie kremnezemy: sintez, stroenie privitogo sloya i khimiya poverkhnosti (Complexing Silicas: Synthesis, Structure of Grafted Layer and Chemistry of Surface), Kharkiv: Folio, 1997.
Latthe, S.S., Gurav, A.B., Maruti, C.S., and Vhatkar, R.S., J. Surf. Eng. Mater. Adv. Technol., 2012, no. 2, p. 76.
Fadeev, A.Y., in Encyclopedia of Surface and Colloid Science, Somasundaran, P., Ed., New York: Taylor & Francis, 2006, p. 2854.
Kazakevich, Y.V. and Fadeev, A.Y., Langmuir, 2002, vol. 18, p. 2665.
Shoniya, N.K., Roshchina, T.M., Nikol’skaya, A.B., et al., Russ. J. Phys. Chem. A, 2010, vol. 84, no. 11, p. 1945.
Kuroda, K., Shimojima, A., Kawahara, K., et al., Chem. Mater., 2014, vol. 26, no. 1, p. 211.
Sia, K. and Whitesides, G.M., Electrophoresis, 2003, vol. 24, no. 21, p. 3563.
Yoshikawa, M., Wakabayashi, R., Tamaia, M., and Kuroda, K., New J. Chem., 2014, vol. 38, p. 5362.
Lakiza, N.V., Neudachina, L.K., Yatluk, Yu.G., et al., Anal. Kontrol, 2005, vol. 9, no. 4, p. 391.
Neudachina, L.K., Golub, A.Ya., Yatluk, Yu.G., et al., Inorg. Mater., 2011, vol. 47, no. 4, p. 435.
Pozhidaev, Yu.N., Izv. VUZov, Prikl. Khim. Biotekhnol., 2014, no. 4 (9), p. 7.
Petrov, N.A. and Davydova, I.N., Elektron. Nauchn. Zh. Neftegazov. Delo, 2013, no. 5, p. 54.
Katz, H.S. and Mileski, J.V., Handbook of Fillers for Plastics, Springer, 1987.
Kuksenko, S.P., Tarasenko, Yu.A., and Kartel’, N.T., Materialy 13-ogo Vserossiiskogo simpoziuma s uchastiem inostrannykh uchenykh “Aktual’nye problemy adsorbtsii, poristosti i adsorbtsionnoi selektivnosti” (Proc. 13th All-Russian Symposium with International Participation “Topical Problems on Adsorption, Porosity, and Adsorptive Selectivity”), Moscow-Klyazma, April 2–24, 2009, p. 147.
Sizov, S., Anisimov, D.S., Agina, E.V., et al., Langmuir, 2014, vol. 30, p. 1532.
Pan, T., McDonald, S.J., Kai, E.M., et al., J. Micromech. Microeng., 2005, vol. 15, p. 1021.
Hill, S., Qian, W., Chen, W., and Fu, J., Biomicrofluidics, 2016, vol. 10, p. 054114.
Hyver, K.J. and Sandra, P., High Resolution Gas Chromatography, Hewlett-Packard Co., 1989.
Kudasheva, N.V. and Yashkin, S.N., Sorbtsionnye Khromatogr. Protsessy, 2009, vol. 9, no. 5, p. 726.
Zhabina, A.A., Krasnykh, E.L., and Levanova, S.V., Russ. J. Phys. Chem. A, 2014, vol. 88, no. 9, p. 1590.
Kuznetsov, M.P., Glazkov, I.N., Revel’skii, I.A., and Luzyanin, B.P., J. Anal. Chem., 2006, vol. 61, no. 12, p. 1144.
Yang, J., Pi, P., Wen, X., Zheng, D., et al., Appl. Surf. Sci., 2009, vol. 255, no. 6, p. 3507.
Sulim, I.Ya. and Borisenko, N.V., Khim., Fiz. Tekhnol. Poverkhn., 2007, no. 13, p. 159.
Bogatyrev, V.M., Galaburda, M.V., and Borisenko, N.V., Poverkhnost, 2012, no. 4, no. 19, p. 239.
Gurevich, K.B., Roshchina, T.M., Shonia, N.K., et al., J. Colloid Interface Sci., 2002, vol. 254, no. 1, p. 39.
Roshchina, T.M. and Shepeleva, M.S., Izv. Akad. Nauk, Ser. Khim., 2005, no. 1, p. 140.
Eksperimental’nye metody v adsorbtsii i molekulyarnoi khromatografii (Experimental Methods for Adsorption and Molecular Chromatography), Nikitin, Yu.S. and Petrova, R.S., Eds., Moscow: Moscow State Univ., 1990.
Kiselev, A.V., Mezhmolekulyarnye vzaimodeistviya v adsorbtsii i khromatografii (Intermolecular Interactions under Adsorption and Chromatography), Moscow: Vysshaya Shkola, 1986.
Roshchina, T.M. and Shoniya, N.K., Sbornik materialov Vserossiiskogo internet-simpoziuma s mezhdunarodnym uchastiem “Fiziko-khimicheskie problemy adsorbtsii, struktury i khimii poverkhnosti nanoporistykh materialov” (Proc. All-Russian Internet-Symposium with International Participation “Physical and Chemical Problems on Adsorption, Structure, and Chemistry of Nano-Porous Materials’ Surfaces”), Moscow: Frumkin Institute of Physical Chemistry and Electrochemistry Russ. Acad. Sci., 2019, p. 109.
Handbuch der Gaschromatographie, Leibnitz, E. and Struppe, H.G., Eds., Leipzig: Akademische Verlagsgesellschaft Geest & Portig, 1966, vol. 2.
Roshchina, T.M., Shoniya, N.K., Nikol’skaya, A.B., et al., Prot. Met. Phys. Chem. Surf., 2009, vol. 45, no. 2, p. 152.
Roshchina, T.M., Shoniya, N.K., Tayakina, O.Ya., et al., Russ. J. Phys. Chem. A, 2012, vol. 86, no. 3, p. 437.
Roshchina, T.M., Shoniya, N.K., Tayakina, O.Ya., et al., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 8, p. 1367.
Nikol’skaya, A.B., Lagutova, M.S., Shoniya, N.K., et al., Tezisy dokladov 3-ei Mezhdunarodnoi konferentsii po kolloidnoi khimii i fiziko-khimicheskoi mekhanike (Proc. 3rd Int. Conference on Colloid Chemistry and Physicochemical Mechanics), Moscow, 2008, p. 03.
Roshchina, T.M., Shoniya, N.K., Zubareva, N.A., and Fadeev, A.Yu., Russ. J. Phys. Chem. A, 2003, vol. 77, no. 9, p. 1482.
Funding
The work was carried out in accordance with the Plan of the Scientific Council of the Russian Academy of Sciences for Physical Chemistry, topic registration nos. 17-03-460-18 and 19-03-460-15.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Roshchina, T.M., Shonia, N.K. Thermodynamics of Adsorption on Nanoporous Silicas with Grafted Methylsiloxane and Wettability. Prot Met Phys Chem Surf 57, 22–29 (2021). https://doi.org/10.1134/S2070205121010172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205121010172