Abstract
The dependence of the thermodynamic characteristics of adsorption of various classes of organic compounds on the chemical nature of the silica surface modified with 3-bromopropyltrichlorosilane to form a monolayer coating (Br-2 sample), a grafted polymer type layer (Br-5 sample), and additionally silanized layer (Br-5* sample) was studied by gas chromatography. The dispersion interactions on bromosilicas were more active than on the starting support or the NH2 sample with a grafted aminopropyl layer of the polymer type. The role of specific interactions generally decreases in the series Br-2 > Br-5 > Br-5* > NH2. An analysis of the obtained data indicates that the retention mechanism on silicas with bromopropyl groups is determined by the penetration of adsorbed molecules into the grafted layer. In contrast, the aminopropyl polymer layer has a more rigid structure with limited conformational mobility of the grafted aminopropyl groups, the adsorption retention mechanism dominating in this case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chemical modification of the surface of various oxides, including nanoporous silicas, is an indispensable method for creating new materials used in various versions of modern chromatography, sorption, catalysis, and other areas [1, 2]. If it is necessary to synthesize complex grafted compounds on the surface by performing a series of chemical transformations in the surface layer (surface assembly), a limited number of silanes containing a reactive terminal functional group are used as precursors; the most common of these are aminoalkyl- and bromoalkylsilanes [2, 3].
It is believed [2, 3] that almost any compound can be grafted by surface assembly. However, despite the wide synthetic possibilities of the surface assembly method, the occurrence of sequential chemical reactions with 100% selectivity is an exception rather than the rule. Therefore, synthesis of the desired complex end products of chemical modification requires a systematic study of the properties of the surface of not only the original support, but also of the chemically modified silica precursor obtained on its basis for further modification.
The studies of the physicochemical characteristics of the surface of aminosilicas by various methods, including IR spectroscopy and NMR, were reported in numerous publications [2, 4]. It was noted [2, 4] that in the case of sorbents with grafted amino compounds, the so-called arc structure of the grafted layer appears due to the interaction of NH2 groups with the silanols of the silica surface, which affects their protolytic and complexing characteristics, decreases the availability and reactivity of the grafted groups, and changes the surface uniformity and polarity. A systematic study of silicas with chemically grafted aminopropyl polymer-type coating by gas chromatography in [5, 6] showed that, regardless of the characteristics of the staring support, aminosilicas with similar adsorption properties and a fairly uniform surface could be obtained if a dense modifying layer formed with a concentration of grafted groups of more than 4 nm–2. A number of examples of the use of aminosilicas were given to study the relationship between the structure of adsorbed molecules and their retention characteristics and to separate highly polar and reactive compounds, including organic bases.
Bromoalkylsilicas were used to obtain enantioselective and metal complex catalysts and sorbents for ligand exchange chromatography, separation of enantiomers, and isolation and purification of enzymes [2, 7–12]. The use of 3-bromopropyltrichlorosilane led to reliable binding to the walls of the quartz capillary of the ultra-cross-linked polymer based on vinylpyridine proposed in [13] as a stationary phase for capillary electrochromatography.
The use of bromoalkylsilicas makes it possible to perform synthesis under milder conditions compared with chloroalkylsilicas and often with better yields [2, 4]. The positive aspects of the use of 3-bromopropyltrichlorosilane are high density of modifier grafting and the possibility of additional silanization using simple agents, in particular, trimethylchlorosilane, which can favorably affect the properties of materials based on bromosilicas with grafted compounds of complex structure and facilitates the prediction of their adsorption and catalytic activities.
Despite the wide range of practical applications, the surface properties of grafted bromoalkylsilanes were studied insufficiently. A short summary of our works [14, 15] devoted to the study of silicas with bromopropyl coatings was given in [2, p. 397].
This paper presents the results of our study of the surface properties of nanoporous silicas modified with 3-bromopropyltrichlorosilane to form a monolayer coating and a grafted polymer-type layer. It summarizes the relationship between the nature of the grafted functional groups, mainly, bromo- and aminopropyl groups, with the thermodynamic characteristics of adsorption (TCA).
EXPERIMENTAL
Objects of Investigation
The support was silica SiO2 (mesoporous adsorbent) with an effective pore radius of 20 nm and a specific surface area of ssp = 97 m2/g [16]. The chemically modified silicas (CMSs) were synthesized at BioKhimMak ST using the trifunctional modifier 3-bromopropyltrichlorosilane. This usually ensures the presence of additional hydroxyls at the anchor silicon atom belonging to the modifier in the grafted coating.
The Br-2 sample with a monolayer coating (the concentration of the grafted groups was N ≈ 2 nm–2) was synthesized under anhydrous conditions (at the first stage) according to the following scheme:

The Br-5 sample (the concentration of the grafted groups was N ≈ 4.9 nm–2) was prepared in the presence of water:

In this case, the modifier molecules interact with both the silica surface and with each other, forming a grafted layer of the polymer type.
The Br-5* sample is Br-5 additionally treated with trimethylchlorosilane (TMS).
To analyze the effect of the functional group on the TCA, we used the known [5, 6] and new data for the aminosilica NH2 sample with a grafted polymer type layer (N ≈ 4.7 nm–2) prepared by modifying SiO2 with 3-aminopropyltriethoxysilane.
Methods
The specific surface areas ssp of the samples were calculated by the BET method from the nitrogen adsorption isotherms (the area of the molecule is 0.162 nm2) measured on an ASAP-2020 Micromeritics sorptometer at 77 K. In addition, \(s_{{{\text{sp}}}}^{*}\) of CMSs were calculated by the equation similar to that given in [2]:
where \(s_{{{\text{sp}}}}^{0}\) is the specific surface area of the starting support, and m is the mass fraction of the grafted layer in the CMS.
The gas chromatographic studies were performed on a Chrom-5 chromatograph with a flame ionization detector and glass columns (length 0.6–0.8 m, inner diameter 2.5 mm). Helium was used as a carrier gas (gas flow rate 15–35 mL/min). Before the measurements, the samples were heated in a helium flow at a temperature of 453 K for 30 h.
The test molecules were n-alkanes, adamantane, trans-decalin, aromatic hydrocarbons, and oxygen- and nitrogen-containing compounds (“kh.ch.” (chemically pure)). The samples were introduced three to six times in the form of vapor–air mixtures in amounts of 0.1–0.2 mL. The dead time was determined according to methane [17].
The Henry constants of adsorption equilibrium KH and Kovats retention indices I were calculated from the gas chromatographic (GC) parameters using the formulas given in [1, 17].
The thermodynamic characteristics of adsorption were determined using the following standard states of the substance: 1 μmol/cm3 in the gas phase and 1 μmol/m2 in the adsorbed state [1, 17].
The initial differential molar heats of adsorption q and standard adsorption entropies ΔS° (in the Henry region) were determined from the temperature dependence of KH in the range from 372 to 423 K at a step of 4–5 K by the equation [1, 17]
The error in the experimental thermodynamic characteristics of adsorption did not exceed 5% for all the test compounds.
The contribution of the CH2 groups to ln KH (\(\ln {{K}_{{\text{H}}}}({\text{C}}{{{\text{H}}}_{2}})\)) and q (\({{q}_{{{\text{C}}{{{\text{H}}}_{2}}}}}\)) for n-alkanes was calculated using the equations [1, 18]
where n is the number of carbon atoms in the n-alkane molecule, and а and a' are the quantities corresponding to the contribution of two H atoms to lnKH and q, respectively.
The contributions of the specific interaction were evaluated by the equation [1, 19]
where ΔG° is the Gibbs energy of adsorption, and ΔGd is the energy of the dispersion forces of the compound under study equal to the ΔGd of the real or hypothetical n-alkane with the same polarizability.
RESULTS AND DISCUSSION
According to the data on nitrogen adsorption, modification with 3-bromopropyltrichlorosilane has almost no effect on the BET-calculated ssp values (m2/g) of the samples: 106 for Br-2 and 95 for Br-5. The \(s_{{{\text{sp}}}}^{*}\) values calculated by Eq. (1) are 10% lower. In the case of aminopropyl silica NH2, ssp = 71, and \(s_{{{\text{sp}}}}^{*}\) = 89. Some difficulty lies in the choice of the value of the area of the molecule for chemically modified surfaces [20, 21]. However, the differences in the values of ssp (used in the calculations) and \(s_{{{\text{sp}}}}^{*}\) did not affect the interpretation of the results of our work. Modification is accompanied by the normal decrease in the C constant of the BET equation: 100 on the starting SiO2 support and 59, 55, and 44 on the Br-2, Br-5, and NH2 samples, respectively.
The TCA of linear alkanes, whose interaction with the surface is determined mainly by the dispersion forces, are presented in Figs. 1–3. It was shown that the formation of a bromopropyl layer on the surface leads to a significant increase in the Henry constants KH and heats of adsorption q compared to those of the starting substrate. The KH of n-alkanes do not change on passing from the sample with the Br-2 monolayer coating to the sample with the bromosilica Br-5 coating with a grafted layer of polymer type, whose thickness is approximately equal to two monolayers. Additional silanization with trimethylchlorosilane leads to a small decrease (by ≈20%) in KH of n-alkanes on the Br-5* sample, but these values are still much higher than on the starting support and especially on the TMS-modified low-energy silica [22] (Fig. 1). The KH of all the test compounds on the sample containing only trimethylsilyl groups on the surface is almost an order of magnitude lower than on bromosilicas [22]. Therefore, strong dispersion interactions with the grafted bromopropyl radicals compensate for the decrease in the number of silanol groups accessible for adsorption after additional silanization. The heats of adsorption of n-alkanes (Fig. 2), as well as the contributions to the heat of adsorption of CH2 groups, on the samples with a grafted bromopropyl layer of the polymer type are close (\({{q}_{{{\text{C}}{{{\text{H}}}_{2}}}}}\) = 6 kJ/mol) and, like KH, higher than on the starting substrate (\({{q}_{{{\text{C}}{{{\text{H}}}_{2}}}}}\) = 4.3 kJ/mol [16]). A comparison of the standard entropies of adsorption shows that ΔS° decreases after modification with 3-bromopropyltrichlorosilane, the decrease being more pronounced for Br-5* (Fig. 3).
The results suggest that the permeability of the grafted layer for the adsorbed molecules plays a certain role in the mechanism of retention on bromine-containing CMSs. This ensures an increase in the heats of adsorption after modification due to the additional lateral interactions with the grafted groups and a decrease in the entropy of adsorption due to the decrease in the mobility of molecules “dissolved” in the modifier layer.
The formation of an aminopropyl layer of the polymer type on the surface has no effect on the TCA of molecules capable only of dispersion interactions: KH, q, and ΔS° of n-alkanes do not change on passing from SiO2 to aminosilica (Figs. 1–3). At a first glance, the same KH and q values after modification with amino-silane can be explained if we take into account that the additional hydroxyls on the silicon atom, which formed during the modification of silica with trifunctional silane, can take a very active part in the dispersion interaction. Similar results were observed on the grafted layers obtained using trifunctional octyl and perfluorohexyl silanes [22, 23]. It should be borne in mind, however, that the grafted amino compounds can form an arc structure of the grafted layer due to the interaction of NH2 groups with the residual and/or newly formed silanols of the surface, which reduces the participation of both types of groups in the adsorption.
The peculiarities of adsorption equilibria on bromopropyl silicas can be explained if we take into account that the polarizability of the bromine atom is almost two times higher than that of the amino and especially OH group (for example, 6 × 10–3, 4 × 10–3, 4.4 × 10–3, and 3.3 × 10–3 nm3 for CH3Br, CH3NH2, CH3CH3, and СН3ОН, respectively [24]).
Based on our data, it is possible to explain, in a first approximation, the strong noncovalent binding of proteins by the surface of affinity sorbents obtained using bromosilica precursors [12]. The main reason for this, in our opinion, is the high dispersion potential of the surface with grafted bromopropyl groups, which increases the hydrophobic interactions.
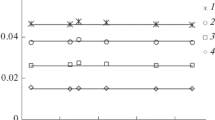
To discuss the possible retention mechanism in systems involving modified silicas and saturated hydrocarbons, we studied the adsorption equilibria in systems involving molecules with the same number of carbon atoms, but with different spatial structures: adamantane (AD), trans-decalin (D), and decane (C10). The Kovats indices and heats and entropies of adsorption for AD and D are given in Table 1. As in the case of n-alkanes, q of the cyclic compounds decreases in the following order: Br-5* > Br-5 > SiO2 ≈ NH2, while ΔS° increases in the opposite direction: Br-5* < Br-5 < SiO2 ≈ NH2.
As is known, the individual links of the cyclic AD and D molecules are remote from the gas– solid interface. Therefore, on a flat surface, for example, on graphitized thermal soot GTS [16], AD and D are held much more weakly than C10, whose molecule in a conformation most favorable for interaction can contact with the surface by all of its links. Adamantane leaves the GTS column almost together with heptane: I(AD) = 690, and trans-decalin leaves it before nonane: I(D) = 870. C10 is also adsorbed on SiO2 more strongly than the cyclic compounds, but the differences in the Kovats indices of AD and D are barely noticeable (Table 1). This is illustrated in Fig. 4, where the results are presented in an easily perceptible and compact form as the temperature dependences of the ratios of the Henry constants of cyclic hydrocarbons and decane. This is probably due to the peculiarities of the molecular topography (roughness, curvature) of the surface of amorphous silicas formed by randomly arranged silicon–oxygen tetrahedra. On bromosilicas, the order of exit is different: the cyclic hydrocarbons exit later than decane. A similar picture is observed during the dissolution in liquid phases, for example, on methylsiloxane OV-1 I(AD) = 1103, and I(D) = 1056 [16]. The determining factor in retention on liquid phases is a decrease in the pressure of saturated vapors of AD and D (~9 and 16.8 kPa at 403 K, respectively) compared to decane (28.3 kPa at 403 K), and the shape and size of the molecule have only a secondary effect. On bromosilicas, the absorption mechanism of retention is probably preferable. It can be assumed that the increase in the relative parameters of cyclic compounds (Table 1, Fig. 4) on passing from Br-5 to Br-5* is associated with a decrease in the effect of the adsorption field of the silica surface as a result of additional silanization, and the grafted layer probably becomes less structured.
A distinction of aminosilica relative to the previously studied CMSs [16] is the fact that cyclic compounds are retained on this sample more weakly than decane over the whole range of temperatures, which resembles the tendencies of adsorption of AD and D on the solid surface of the starting support (Table 1, Fig. 4). Probably, as a result of the interaction of aminopropyl radicals with the residual silanol groups of the surface, forming the arc structures, the mobility of the grafted groups decreases, and the modifying layer becomes more “rigid.”
Note that the linear dependences of ln KH for n‑alkanes on the number of carbon atoms in the molecule in the series of seven homologs with a correlation coefficient R > 0.999 (Fig. 1) and hence the additivity of the methylene link make it possible to rightfully argue that n-alkanes, including decane, interact with the silica surface or with the grafted layers by all of their chain links.
Thus, the analysis of the results indicates that the penetration of adsorbed molecules into the grafted layer plays an important role in the mechanism of retention on silicas with bromopropyl groups. In contrast, the aminopropyl polymer layer has a more rigid structure with limited conformational mobility of the grafted aminopropyl groups; in this case, the adsorption mechanism of retention appears to be dominant.
There are many approaches to gas chromatographic evaluation of the surface “polarity” of materials, i.e., the contributions of electrostatic and donor-acceptor interactions and hydrogen bonding to sorption, and hence of their selectivity with respect to various classes of organic compounds. Table 2 presents the contributions of the specific interaction ΔGsp to the standard Gibbs energy of adsorption on the starting support, bromo- and aminosilicas, for some of the test compounds. It also gives (for comparison) data on the other two conventional nonpolar adsorbents—silicas modified with octyl trichlorosilane [22], with a grafting density of octyl groups close to that of functional CMSs, namely, the C8 sample (N ≈ 4.4 nm–2) and the sample with additional silanization C8*.
The –ΔGsp values generally decrease when SiO2 is modified, which is associated with the replacement and screening of the silanol groups of the silica surface. In the group of bromosilicas, the lowest –ΔGsp values are usually observed on Br-5*. The polarity of the Br-5* surface is higher than that of amino silica, which corresponds to a change in the dipole moments μ of the molecules of the similar alkane derivatives: μ (1-Br-propane) = 1.9 D, μ (1-aminopropane) = 1.3 D [25]. Unlike amino groups (nucleophiles), bromopropyl groups possess rather strong electrophilic properties. This factor can affect the –ΔGsp values of molecules with pronounced donor ability. For example, on passing from amino silica to Br-5*, –ΔGsp of diethyl ether, dioxane, and tetrahydrofuran increase more than twofold, while for alcohols, they differ only 1.2-fold. The Kovats indices I, which are the most popular characteristics of the stationary phases for GC (Table 3), change similarly: on Br-5*, they are higher than on NH2 by 120–300 units for ethers and by only ≈30 units for alcohols.
The decrease in –ΔGsp compared to that of C8 octyl silica, i.e., the sample with nonpolar grafted groups, is an unexpected characteristic of amino silica. The only reason for this phenomenon may be the active interaction of NH2 groups with silanols of the starting support and/or additional SiOH groups, which reduces the degree of participation of the groups of both types in adsorption and leads to the creation of a denser coating. As in the case of octyl silica, the additional silanization of bromosilica is usually accompanied by a decrease in –ΔGsp on Br-5 > Br-5*.
Additional silanization has long been considered a technique that improves the quality of sorbents, in particular, the chemical homogeneity of their surface. Octyl silica C8* with additional silanization has the most nonpolar surface with respect to many compounds under study. However, only a few works presented quantitative adsorption data that confirm the efficiency of this process [2, 22, 26–28]. Therefore, let us consider in detail the Henry constants of a number of compounds measured in a wide temperature range on the Br-5 and Br-5* samples.
A comparison of the temperature dependences of the relative Henry constants \(\ln K_{{\text{H}}}^{{{\text{rel}}}}\) for molecules with relatively close sizes and polarizabilities, including traditional pairs such as benzene–hexane or diethyl ether–pentane [1, 20, 28], made it possible to determine the role of π-bonds or hydrogen bonds in intermolecular interactions (Fig. 5). It was shown that additional silanization significantly reduces \(\ln K_{{\text{H}}}^{{{\text{rel}}}}\) for the diethyl ether–pentane pair on Br-5* compared to that on Br-5 due to the replacement and screening of the silanol groups of the starting support and the additional SiOH groups that formed as a result of the hydrolysis of the unchanged Cl atoms during modification with 3-bromopropyltrichlorosilane. Additional silanization has almost no effect on \(\ln K_{{\text{H}}}^{{{\text{rel}}}}\) for the benzene–hexane pair. Interestingly, the contributions of the methylene links to the adsorption ln KH(CH2), generally used to evaluate the energy of dispersion forces, are close on these samples, but higher than \(\ln K_{{\text{H}}}^{{{\text{rel}}}}\) for the benzene–hexane pair.
Dependences of the contributions of the methylene links to adsorption lnKH(CH2) and \(\ln K_{{\text{H}}}^{{{\text{rel}}}}\) on the reciprocal temperature on Br-5 and Br-5*; Et2O: \(K_{{\text{H}}}^{{{\text{rel}}}}\) = KH(diethyl ether)/KH(pentane); С6Н6: \(K_{{\text{H}}}^{{{\text{rel}}}}\) = KH(benzene)/KH(hexane).
The KH values for 2-methylpropan-2-ol (Fig. 6), like those for diethyl ether, decrease noticeably after additional silanization because the dominant interaction for these molecules is a strong interaction of the type of hydrogen bonding with the residual silanol groups of the surface (whose number decreases after additional silanization of TMS). The case is different for the adsorption of acetonitrile (Fig. 6). Additional silanization leads only to an insignificant decrease (by ≈5%) of KH of acetonitrile, which resembles the data for alkanes, for example, pentane (Fig. 6), and to a change in the order of exit of diethyl ether and acetonitrile on Br-5* compared to Br-5. According to Table 3, on Br-5* acetonitrile is adsorbed more strongly than diethyl ether. This can be explained if we take into account that acetonitrile is capable of stronger electrostatic interactions with polar bromopropyl groups (the dipole moment is μ(CH3CN) = 3.9D) than diethyl ether (μ = 1.1D). Consequently, the adsorption of acetonitrile on bromosilicas occurs predominantly on the bromopropyl links, but not on SiOH groups. Similar results are obtained when comparing the I values of nitromethane (μ = 3.5D), which, unlike, e.g., those of alcohols, increase after the treatment of bromosilica with TMS (Table 3).
Thus, modification with bromopropylsilane, increasing the density of the grafted layer, and additional silanization in most cases lead to a decrease in the contribution of specific interactions in the series SiO2 > Br-2 > Br-5 > Br-5*. However, this tendency is violated for molecules with a large dipole moment, as shown in the case of acetonitrile and nitromethane. The –ΔGsp and I values increase on Br-5* compared with those on Br-5 (Tables 2 and 3).
Regarding the use of silicas with a grafted bromopropyl coating in analytical chromatography, an important property of bromosilicas Br-5 and Br-5* with a polymer-type modifier film are symmetric chromatographic peaks of almost all the polar compounds under study. Therefore, in practice, it is quite acceptable to use a less expensive (without additional silanization) adsorbent with bromopropyl grafted groups. Br-5 has fairly high selectivity in separation of substances with similar physicochemical properties [14]. The thermal stability of bromosilicas (not less than 473 K), nonvolatility, and storage stability of at least 5 years suggest that 3-bromopropyltrichlorosilane can be used to prepare packed and capillary columns for GC with relatively high polarity.
Thus, a summary of the results indicates that there are significant differences in the properties of the surface of bromo- and aminosilicas. This is of interest from the viewpoint of predicting the adsorption and catalytic activity of materials obtained from these CMSs. The advantages of silicas with a grafted polymer-type layer over those with a monolayer coating generally include significantly lower accessibility of residual silanol groups and higher yields in the syntheses of grafted compounds of complex structure due to the increased concentration of reactive centers on the precursor surface, which facilitates the solution of the problems of synthesis of sorbents and catalysts with desired properties.
REFERENCES
A. V. Kiselev, Intermolecular Interactions in Adsorption and Chromatography (Vysshaya Shkola, Moscow, 1986) [in Russian].
Chemistry of Graft Surface Compounds, Ed. by G. V. Lisichkin (Fizmatlit, Moscow, 2003) [in Russian].
G. V. Lisichkin, in Modern Science, Encyclopedia (Nauka, Flinta, Moscow, 1999), Vol. 1, p. 206 [in Russian].
V. A. Tertykh and L. A. Belyakova, Chemical Reactions Involving Silica Surface (Naukova Dumka, Kiev, 1991) [in Russian].
T. M. Roshchina, V. Y. Davydov, N. M. Khrustaleva, et al., Adsorpt. Sci. Technol. 15, 147 (1997).
V. Ya. Davydov, T. M. Roshchina, N. M. Khrustaleva, and A. A. Mandrugin, Zh. Fiz. Khim. 67, 2428 (1993).
V. A. Davankov, J. Navratil, and H. Walton, Ligand Exchange Chromatography (CRC, Boca Raton, 1988).
G. V. Kudryavtsev, S. Z. Bernadyuk, and G. V. Lisichkin, Russ. Chem. Rev. 58, 406 (1989).
V. V. Krotov, S. M. Staroverov, P. N. Nesterenko, and G. V. Lisichkin, Zh. Obshch. Khim. 56, 2460 (1986).
V. V. Krotov, S. M. Staroverov, P. N. Nesterenko, and G. V. Lisichkin, Zh. Obshch. Khim. 57, 1187 (1987).
P. G. Mingalev, Extended Abstract of Cand. Sci. (Chem.) Dissertation (Mosc. State Univ., Moscow, 1993).
P. G. Mingalev and A. Y. Fadeev, J. Chromatogr. A 719, 291 (1996).
K. V. Maerle, Extended Abstract of Cand. Sci. (Chem.) Dissertation (Nesmeyanov Inst. Element-Org. Compd. RAS, Moscow, 2009).
T. M. Roshchina, V. Ya. Davydov, M. S. Timoshik, et al., Vestn. Mosk. Univ., Ser. Khim. 39, 236 (1998).
T. M. Roshchina, O. V. Kokhanov, E. V. Vlasenko, and A. Yu. Fadeev, in Proceedings of the 9th International Conference on Theoretical Problems of Adsorption and Adsorption Chromatography, The Current State and Prospects for the Development of the Theory of Adsorption, on the 100th Anniversary of the Birth of Academician M.M. Dubinin (Niopik, Moscow, 2001), p. 250.
T. M. Roshchina, K. B. Gurevich, A. Y. Fadeev, et al., J. Chromatogr., A 844, 225 (1999).
Experimental Methods in Adsorption and Molecular Chromatography, Ed. by Yu. S. Nikitin and R. S. Petrova (Mosk. Gos. Univ., Moscow, 1990) [in Russian].
E. Papirer, H. Balard, and C. Vergelati, Adsorption on Silica Surfaces, Vol. 90 of Surfactant Science Series (Marcel Dekker, New York, 2000), p. 205.
A. Voelkel, B. Strzemiecka, K. Adamska, and K. Milczewska, J. Chromatogr., A 1216, 1551 (2009).
S. Gregg and K. Sing, Adsorption, Surface Area and Porosity (Academic, New York, 1982).
A. P. Karnaukhov, Adsorption. The Texture of Dispersed and Porous Materials (Nauka, Novosibirsk, 1999) [in Russian].
T. M. Roshchina, N. K. Shoniya, O. Ya. Tayakina, and A. Yu. Fadeev, Russ. J. Phys. Chem. A 85, 298 (2011).
T. M. Roshchina, N. K. Shoniya, M. S. Lagutova, and A. Yu. Fadeev, Russ. J. Phys. Chem. A 82, 390 (2008).
T. M. Miller, in CRC Handbook of Chemistry and Physics, Ed. by D. R. Lide, 83rd ed. (CRC, Boca Raton, FL, 2002).
O. A. Osipov, V. I. Minkin, and A. D. Garnovskii, Dipole Moments: Handbook (Vyssh. Shkola, Moscow, 1971) [in Russian].
A. V. Kiselev, G. V. Lisichkin, Yu. S. Nikitin, et al., Zh. Fiz. Khim. 57, 1829 (1983).
N. K. Shoniya, S. M. Staroverov, Yu. S. Nikitin, and G. V. Lisichkin, Zh. Fiz. Khim. 58, 702 (1984).
A. A. Serdan, Yu. S. Nikitin, S. M. Staroverov, and G. V. Lisichkin, Zh. Fiz. Khim. 60, 147 (1986).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Roshchina, T.M., Shoniya, N.K. Adsorption Equilibria in the Nanopores of Silicas with Grafted Bromopropyl Groups. Russ. J. Phys. Chem. 93, 1931–1938 (2019). https://doi.org/10.1134/S0036024419100224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419100224