Abstract
Species with a high resistance to hypoxia are usually characterized by an increased H2S tolerance of hydrogen sulfide; however, high anaerobic potential cannot be the only explanation for survival in an environment with elevated concentrations of sulfides. The activity of oxidoreductases, as well as parameters of adenylate system were studied in the tissues of hypoxia/anoxia-tolerant clam Anadara kagoshimensis (Tokunaga, 1906) under conditions of experimental H2S loading (HSL). Adult specimens with a shell height of 26–38 mm are used. The control group of clams is kept in an aquarium with an oxygen concentration of 7.0–7.1 mg/L (normoxia). The experimental group is exposed to the effect of HSL created by dissolving sodium sulfide (H2S donor) in water to a final concentration of 6 mg S2–/L; exposure time is 24 h. After the first day of the experiment, the level of O2 in water is 1.8 mg/L and there is no hydrogen sulfide. Some of the clams are exposed to repeated hydrogen sulfide loading (second day of the experiment), and Na2S is introduced to a final concentration of 9 mg S2–/L; by the end of the second day, 1.9 mg S2–/L and trace concentration of O2 (0.03 mg/L) are registered. In the first days of HSL, a high activity of malate dehydrogenase (MDH) against the background of a significant suppression of the activity of lactate dehydrogenase (LDH) and an increase in the values of MDH/LDG index persists; this reflects a strengthening of anaerobic processes in the tissues of anadara with relatively high concentrations of О2 in water (1.8 mg/L). After the second day of HSL, the activity of oxidoreductases in the clam tissues does not change when compared with the first day; however, the value of adenylate energy charge (AEC) persists against the background of a relative decrease in [ATP]. The retention of AEC indicates the ability of the anadara to exist under conditions of hydrogen sulfide contamination and acute forms of hypoxia/anoxia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The formation of hydrogen sulfide (H2S) in the shelf zone is usually not regular and is accompanied in most cases by the appearance of local zones with reduced oxygen content (hypoxia) (Zaika et al., 2011), which is a consequence of the absence of cross vertical convection and the formation of local areas of decay of dead organic matter (Orekhova and Konovalov, 2018). The presence of specific vertical currents (upwellings) contributes to the removal of deep waters contaminated with H2S to the coastal zone (Orekhova and Konovalov, 2018). The phenomena mentioned above are widely distributed within the coasts of the Black Sea.
Benthic organisms living in zones of the combined action of acute hypoxia and hydrogen sulfide contamination should have increased resistance to these factors. They include the bivalve clam Anadara kagoshimensis (Tokunaga, 1906) (hereinafter anadara). In the experiments carried out on this species, a tolerance to hypoxia and anoxia was noted (Isani et al., 1989; Soldatov et al., 2010; Golovina, 2019), as well as the ability to tolerate the presence of H2S (Miyamoto and Iwanaga, 2017; Nakano et al., 2017). The level of O2 consumption by anadara does not decrease even at its extremely low concentration in sea water (<1.2% saturation) (Cortesi et al., 1992).
The coupling of glycolysis reaction with the processes of protein metabolism should be attributed to the metabolic aspects of a marine organism’s tolerance to hypoxia. It was demonstrated that, with a decrease in the level of О2 in tissues, the content of compounds not peculiar to aerobic metabolism (alanine and succinate) increases (Buck, 2000), the production of N\({\text{H}}_{4}^{ + }\) increases (Chew et al., 2005), the activity of alanine and aspartate aminotransferases grows (Soldatov et al., 2009), the processes of transamination of amino acid (glutamate, alanine) are activated (Hochachka and Somero, 2002), and the respiratory chain of mitochondria is rearranged to an uncompensated type of functioning (Savina, 1992).
The nature of resistance of hydrobionts to hydrogen sulfide contamination is not entirely clear. In a number of works, symbiotic relationships of clams with sulfide-oxidizing bacteria are noted (Stewart and Cavanaugh, 2006), and the presence of a special O2-transport protein and several H2S-insensitive hemoglobin varieties is registered (Arp and Childress, 1981, 1983). Special attention is paid to the presence of granular inclusions that contain hematin in erythroid blood elements which allow the clams to neutralize the increased concentrations of sulfides (Vismann, 1993). The involvement of granular inclusions of erythrocytes in H2S neutralization was demonstrated for the anadara (Soldatov et al., 2018). At the same time, despite some studies, metabolic aspects of the adaptation of hydrobionts to increased concentrations of sulfides have not been covered enough so far.
The stability of hydrobionts largely depends on the ability to maintain a balance between the intensity of energy metabolism and the request for macroergic compounds (Hochachka and Somero, 2002). The reactions of glycolysis that are provided by the enzymes malate dehydrogenase (L-malate: NAD oxidoreductase; MDH, 1.1.1.37) and lactate dehydrogenase (lactate: NAD oxidoreductase; LDH, 1.1.1.27) are an integral part of the mechanisms of adaptation, as well as an important system for studies (Somero, 2010; Bishop and Iliffe, 2012). A central place in the energy metabolism of all types of cells belongs to the adenylate system, which includes ATP, ADP, and AMP. To estimate the energy state of the cells, the AEC index, which reflects the intensity of physiological processes, is used.
The aim of this work was to study the activity of oxidoreductases and adenylate status of anadara tissues with hydrogen sulfide loading under experimental conditions.
MATERIALS AND METHODS
This work was carried out on adult individuals of anadara collected in June 2021 in the waters of Laspi Bay (44°25′ N, 33°42′ E). The height of the clam shell from the lock to sash edge was 26–38 mm.
Scheme of experiment. The control group of clams (seven individuals) was kept in an aquarium with an oxygen concentration of 7.0–7.1 mg/L (normoxia). The experimental group (14 individuals) was exposed to the effect of hydrogen sulfide loading. Sodium sulfide (Na2S) (used as a donor of H2S) was dissolved to a final concentration of 6 mg S2–/L in the water where the clams were. As a result of Na2S hydrolysis, hydroxide ion (ОН–) is produced, which gives an alkaline reaction (2Na+ + S2– + HOH → Na+ + HS– + Na+ + OH–). Since the formation of ОН– in water led to its alkalization, the shift in the acid–base balance of the aquatic environment was eliminated by the introduction of 0.1 n HCl, keeping pH values in the range of 8.20–8.27. H2S formed during the hydrolysis of Na2S partially evaporates from the solution and is oxidized during the interaction with O2 to SO2 (with a lack of O2 up to S), which over time is accompanied by a decrease in the content of both gases in the water of the aquarium. Twenty-four hours later, the level of oxygen in water was 1.8 mg/L, and no traces of hydrogen sulfide were found. In some clams (seven individuals), the tissue samples were collected after 24 h exposure. The remaining seven individuals were exposed to repeated hydrogen sulfide loading. The Na2S sample was additionally introduced in the water of the aquarium to a final concentration 9 mg S2–/L. Twenty-four hours later (the second day of the experiment), traces of oxygen (0.03 mg/L, the level of sulfides was 1.9 mg S2–/L) were found in the water of the aquarium. Tissue samples were also taken from the clams of this group for the analysis.
The content of oxygen in the water was controlled using a oximeter DO Meter ST300D RU (Ohaus, United States). The values of pH were measured on a InoLab pH 720 pH meter (Germany). The value of sulfide ion in water was determined potentiometrically using an MSBS sulfide selective sensor (The Netherlands).
Biochemical studies. The preparation of tissues (legs, gills, and hepatopancreas), homogenization, and centrifugation were performed when cooling (0 ± 4°С). The tissue samples were stored at a temperature of –80°С in the freezer (Farma 900 Series, TermoScientific, Unite States). The activity of cytoplasmic MDH and LDH was measured spectrophotometrically at a wavelength of 340 nm according to the rate of oxidation of the reduced form of NADH coenzyme using a 0.2 M Tris-HCl buffer, pH 7.5, as an extraction medium (according to Kolesnikova and Golovina, 2020). Pyruvate was used as a substrate for determining LDH activity and oxaloacetate for MDH. The activity of MDH and LDH was determined at an incubation temperature of the reaction mixture of 25°C in three repetitions for each tissue. The specific activity of enzymes was expressed in µmol NADH/(min·mg supernatant protein). The protein content was determined by a microbiuret method; extinction was measured at the wavelength 330 nm.
The content of adenylic nucleotides was registered by a chemiluminescent method (Holm-Hansen and Booth, 1966), carrying out two repetitions of measurements. The results were expressed in µmol/g wet weight of tissue. The studied tissues were homogenized in 0.1 M Tris-acetate buffer, pH 7.75, in the cold. The adenylate complex was extracted in boiling buffer in a water bath for 5 min. The extracts were frozen until further analysis. The determination of ATP was carried out according to a standard method, by light emission with the addition of luciferin-luciferase on an ATP-Luminometer 1250 device (LKB, Sweden). ADP and AMP were reduced to ATP using pyruvate kinase and adenylate kinase enzymes. AEC was calculated based on the obtained values (Atkinson, 1968). The average weight of leg tissue sample was 47 mg, gills 42 mg, and hepatopancreas 26 mg.
The results of the appropriate repetitions were averaged, and then average values and indices of variation were calculated. The data were presented as M ± m. The normality of distribution was checked using Pearson’s criterion. Two-sided Student’s t criterion was used for comparison. Differences were considered significant at p < 0.05; a linear coefficient of correlation (r) was calculated. The statistical processing and graphic presentation of the received information was performed using the standard Microsoft Excel 2010 package.
RESULTS
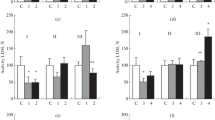
Activity of oxidoreductases. The maximal activity of MDH was registered in the foot of a clam (control group), 0.575 ± 0.067 µmol NADP/(min/mg protein), which exceeded the enzyme activity in the gills and hepatopancreas by 34–35% (p < 0.05) (Fig. 1a). Hydrogen sulfide loading caused a significant increase in the activity of MDH in the foot in the first day of the experiment: 0.974 ± 0.180 µmol NADP/(min/mg protein). The differences reached ~70% (p < 0.05). On the second day, the enzyme activity returned to the control values. No significant changes in the activity of MDH occurred in the gills and hepatopancreas.
Activity of MDH (a), LDH (b) (µmol NADH/(min mg protein)), and MDH/LDH index (c) in the tissues of Anadara kagoshimensis under conditions of hydrogen sulfide loading. (I) Foot, (II) gills, and (III) hepatopancreas; (C) control; (1) first day of the experiment and (2) second day of the experiment. \(*\) Significantly, when compared with the control, р < 0.05; \(*{\kern 1pt} *\) significantly, when compared with another experiment, р < 0.05.
A maximal activity of LDH was noted in the gills of the control group of clams: 0.040–0.047 µmol NADP/(min/mg protein) (Fig. 1b). The activity was minimal in the foot, 0.025 ± 0.003 µmol NADP/(min/ mg protein) (89% lower than in the gills, p < 0.05). All tissues responded to hydrogen sulfide loading by a significant decrease in LDH activity. The minimal activity of the enzyme was registered in the tissues on the second day of the experiment. As compared with the control values, the differences were 1.9–5.7 times (p < 0.05).
Under conditions of hydrogen sulfide loading, a significant increase in MDH/LDH index was noted in the studied tissues (Fig. 1c) (2.5–4.9 times relative to the control, p < 0.01). The change in the MDH/LDH index value is mainly caused by a decrease in the activity of LDH in the tissues. A correlation coefficient (r) between the activity of LDH in the values of MDH/LDH index in the foot (from –0.70 to –0.80) and gills (from –0.70 to –0.80) had a negative value in the control and experiments (p < 0.05).
With hydrogen sulfide loading, the linear correlation coefficient increased significantly in the MDH–LDH system in the hepatopancreas (r = 0.74, р < 0.05, first day; r = 0.84, р < 0.05, second day) and foot (r = 0.94, р < 0.05, second day). The correlation between the activity of MDH and LDH in the tissues of the control individuals was insignificant.
Adenylate system. A maximal pool (AP) was noted in the tissues of the clam foot (13.44 ± 0.12 µmol/g wet weight) (Table 1). It was 43–44% lower in other tissues (p < 0.01). This difference extended to all components of the adenylate complex (ATP, ADP, AMP). The AEC values coincided in all tissues and was at the level 0.53–0.56.
Hydrogen sulfide loading reduced AP in all studied tissues. In the first day of the experiment, its values decreased by 13–14% (p < 0.01). Changes affected the concentration of ATP ([ATP]) to a greater extent. The resource of this compound decreased by 24–29% (p < 0.05). The content of ADP also decreased: by 15% in the hepatopancreas, 23% in the foot, and 32% in the gills (p < 0.05). With respect to AMP, the results were the opposite. The content of this fraction was 16–25% higher (p < 0.05). AEC in the foot tissues of the clam remained at the level of the control values (0.52). A decrease in this value by 14–17% was noted in the gills and hepatopancreas (p < 0.05).
No fundamental changes were observed on the second day of the experiment. Almost all controlled indices were at the level noted in the first day. [ATP], which continued to decrease, was an exception. As compared with the first day, the decrease in [ATP] in the second day was 5–6% for foot and gill tissues and was more of a trend; for hepatopancreas, the drop in the values of this index reached 16% (p < 0.05).
DISCUSSION
It follows from the results that the hydrogen sulfide load was accompanied by a significant decrease in oxygen concentration in the water of the aquarium. Moreover, a two-time introduction of Na2S samples during 2 days of the experiment led to certain changes in the water environment: after the first day, a moderate hypoxia (1.8 mg О2/L) developed in the water of the aquariums and hydrogen sulfide was not detected; on the second day, a stable anoxia was formed with the preservation of hydrogen sulfide contamination (1.9 mg S2–/L).
The main changes in the activity of energy metabolism enzymes under the influence of H2S was noted after the first days of the experiment, which was reflected in a significant decrease in the activity of LDH while maintaining the activity of MDH at the level of control values in most tissues. Previously, a similar reaction was noted in the anadara under conditions of anoxia (Soldatov et al., 2009), which means a complete cessation of the functioning of the mitochondrial respiratory chain. In our study, a similar set of metabolic processes developed under the action of H2S loading and in the presence of relatively high oxygen concentrations (1.8 mg/L), although the anadara is able to maintain the oxidative metabolism with a critical degree of hypoxia (<0.5 mg О2/L) (Cortesi et al., 1992). It is generally accepted that the state of oxygen starvation develops in hydrobionts at the content of oxygen <2 mg/L (Rosenberg et al., 2001). It is obvious that the development of the state of limited oxygen availability that borders with anoxia (1.8 mg O2/L) in the anadara should be considered a consequence of the toxic effects of sulfides. It is known that the main toxic effect of hydrogen sulfide is manifested due to binding to cytochrome c oxidase, which limits its interaction with oxygen (Cao et al., 2011) and actually blocks cellular respiration, stopping the functioning of the mitochondrial respiratory chain (histotoxic hypoxia). In addition, the situation can also worsen in the interaction of H2S with the elements of the mechanism of sulfide detoxification in bivalves and other marine invertebrates. These complex compounds (hematins) of the gill tissue (Power and Arp, 1989; Kraus, 1995) bind H2S with two types of hemoglobin found in the hemolymph of the anadara (Doeller et al., 1988), which leads to the development of progressive hypoxemia. In our opinion, hydrogen sulfide loading determines the compatibility/conjugation of its direct toxic effects with a hypoxia factor in the aquatic environment, as well as with the development of oxygen starvation of different genesis at the level of an individual hydrobiont. Taking into account the above facts, 48-h hydrogen sulfide loading had apparently no additional effect on changes in energy metabolism processes manifested after 24 h of exposure.
It can be assumed that the factor of hypoxia/anoxia developing under hydrogen sulfide loading under the influence of sulfides plays a decisive role in the formation of metabolic reaction of the anadara and the transition of energy metabolism of the clam to the anaerobic pathway. Bivalves are recognized as one of the groups of organisms most resistant to hypoxia and anoxia, for which a high enzymatic activity of glycolytic enzymes (first and foremost, phosphofructokinase, pyruvate kinase, and MDH) is typical (Hand and Somero, 1983). At the same time, clams have an extremely low activity of LDH, since it is the MDH (performing a dual function) that plays the leading role in the reactions of glycolytic redox balance with anaerobic metabolism (Hand and Somero, 1983). It is believed (Yusseppone et al., 2018) that, during anoxia, the induction of MDH activity and the accumulation of succinate are significant traits of the activation of anaerobic mitochondrial pathways to maintain the survival of clams.
The suppression of the activity of LDH under conditions of acute hypoxia and anoxia in clams has a special adaptive significance. A similar reaction was noted in a number of works (Larade and Storey, 2002a; Washizu et al., 2002), including for the anadara (Soldatov et al., 2009; Golovina, 2019). It should be noted that a decrease in the activity of LDH with a deficiency of О2 is as a kind of reflection of the fundamental reorganization of tissue metabolism, which excludes the accumulation of toxic products in the form of lactate in the clam tissues. The prevention of the appearance of lactate is carried out at the stage of the formation of pyruvate, when under the action of alanine aminotransferase and with the involvement of glutamate, alanine and α-ketoglutarate are synthesized. In turn, α-ketoglutarate is converted by Krebs cycle enzymes into succinate, which makes it possible to obtain the additional resource of macroergs (GTP).
In addition to glutamate formed from α-ketoglutarate, its reserve can arise during the transformation of D-aspartate. It was noted that the shells of the representatives of Anadara genus contain a significant reserve of D-aspartate (Larade and Storey, 2002b; Watanabe, 2005). The reaction of its transformation into glutamate is catalyzed by aspartate aminotransferase. The oxaloacetate is reduced to malate, which enters mitochondria through a special carrier and is converted to succinate, which also makes the resynthesis of ATP possible. Such a sequence of events was considered for the first time in the work of Owen and Hochachka (1974). The accumulation of succinate and alanine in clam tissues as the final products indicate in favor of such a sequence of transformations under conditions of hypoxia (De Zwaan et al., 1991; Larade and Storey, 2002a). In addition, a protector role of succinate in relation to mitochondrial membranes is noted in a number of works (Bacchiocchi and Principato, 2000), counteracting the excess production of reactive oxygen species under conditions of hypoxia (Grivennikova and Vinogradov, 2013; Cadenas, 2018). Simultaneously, a strengthening of the processes of transamination of amino acids (glutamate, alanine) (Hochachka and Somero, 2002) and an increase in the activity of alanine and aspartate aminotransferases (registered in the anadara tissues with anoxia) (Soldatov et al., 2009) find their place within the proposed scheme. It should be noted that glycogen is one of the key substrates determining the set of processes considered above (along with amino acids). The depletion of its reserves significantly increases the likelihood of the death of clams under conditions of hydrogen sulfide contamination (Miyamoto and Iwanaga, 2017).
Species with a high resistance to hypoxia are usually characterized by increased H2S tolerance (Grieshaber and Völkel, 1998). At the same time, a high anaerobic potential cannot be the only explanation for long-term survival in the environment with elevated sulfide concentrations (Völkel et al., 2001). Many hypoxia-tolerant species have the ability to reduce their metabolic rate, which is provided due to reversible phosphorylation and the activation and inactivation of the key regulatory enzymes of glycolysis, such as glycogen phosphorylase, pyruvate kinase, and phosphoenolpyruvate carboxykinase (PEPCK) (Yusseppone et al., 2018). Inhibitory agents include fructose 2,6-bisphosphate (allosteric phosphofructokinase activator) and alanine (inhibits the activity of pyruvate kinase). An increase in the content of these factors contributes to the suppression of the glycolytic activity of tissues in general (Oeschger and Storey, 1990).
The presence of alternative oxidase (AOX) is a notable feature of bivalve mitochondria (van Hellemond et al., 2003). Insensitive to sulfide inhibition AOX oxidizes ubiquinol and reduces O2 to water and, consequently, supports electron transport, when the cytochrome oxidase of the mitochondrial respiratory chain is inhibited in the presence of sulfides (Yusseppone et al., 2018). AOX is able to protect “anaerobic” mitochondria from respiratory contamination with hydrogen sulfide. In the electron transport system (ETS), AOX deflects electrons away from classical phosphorylation sites in complexes III and IV (cytochrome oxidase) and reduces oxygen without pumping of protons across the internal mitochondrial membrane when the regular metabolic pathways are “stopped.” It was noted that the AOX reaction is much more pronounced in gills when compared with mantle tissue (Yusseppone et al., 2018), since gills serve as an organ through which H2S enters the organism of animals and where its effect on cytochrome c oxidase will be maximal. Moreover, the gill tissue, which has no appreciable stores of glycogen, reduces the need of its cells for energy (frequency of cilia beats), simultaneously coordinating the shutdown of ETS activity and the production of ATP to maintain the potential of the mitochondrial membrane at a low oxygen level. The additional protection of the integrity of mitochondria during the periods of oxygen depletion is also provided by an increase in the expression of HSP90 heat shock protein (Yusseppone et al., 2018).
The analysis of the state of the adenylate system of the anadara under the action of H2S demonstrated the absence of critical changes in the energetic status of the clams. Initially, the AEC of the control group of clams was 0.53–0.56 (depending on the studied tissue), which, in comparison with higher vertebrates, may reflect a moderate level of intensity of physiological processes (Atkinson, 1968; Luk’yanova, 2004) and a high degree of adaptation to a constantly reduced content of О2 in the bottom layers of the water environment at the range of depths of the anadara habitat from 3 to 60 m (Sahin et al., 2019; Revkov, 2016). It should be noted that this peculiarity is typical for the anadara, which consumes 5–6 times less oxygen under the conditions of normoxia than Mytilus galloprovincialis Lam (Soldatov et al., 2009). Bivalves have a low cytochrome oxidase activity when compared with other animal species (Hand and Somero, 1983), which can also to a large extent determine the value of indices of the energy status of the clam tissues.
Under conditions of hydrogen sulfide loading and accompanying acute hypoxia, the AEC of anadara decreased slightly in the gills and hepatopancreas (in tissues involved in the binding/detoxification of sulfides); however, AEC did not undergo significant changes in the foot tissues, which in general reflects the high degree of the tolerance of this species to the indicated environmental conditions. Similar to that observed in anadara, a decrease in the resource of ATP under the influence of hydrogen sulfide was noted under conditions of hypoxia in Mytilus edulis L. (Wijsman, 1976) and Lima hians (Gmelin, 1791) (Gäde, 1983), which can indicate a similarity of biochemical manifestations with an increase in the concentration of sulfides and oxygen starvation.
CONCLUSIONS
With hydrogen sulfide loading, a high activity of MDH against the background of a significant suppression of LDH activity and an increase in the values of the MDH/LDH index remained in the tissues of anadara, which reflects the intensification of anaerobic processes in the tissue structures of this species with relatively high concentrations of oxygen in water (1.8 mg/L), considered a consequence of the toxic effect of H2S on the respiratory chain of mitochondria. On the second day of the experiment, no significant changes in the activity of oxidoreductases were observed. At the same time, the level of adenylates in tissues decreased slightly, but the level of AEC did not undergo critical changes, which indicates the ability of the clam to exist under conditions of hydrogen sulfide loading and acute forms of hypoxia.
The results of our study allow us to support the proposition that the environmental effect of H2S is similar to the effect of anoxia by its consequences for the energetic metabolism of hydrobionts (Oeschger and Storey, 1990). It is obvious that the hydrogen sulfide loading per se is as a source of the development of hypoxia/anoxia of different genesis (environmental/hypoxic hypoxia, hemic due to the inactivation of hemoglobin, tissue, or histotoxic), that is, a mixed type of hypoxia in invertebrates. The main effect of hydrogen sulfide loading is manifested by the transition of the organism of anadara to anaerobic glycolysis with the involvement of MDH. For anadara, the effect of H2S has no additional or specific effects at the level of glycolytic enzymes and energetic status of the tissues as compared with hypoxia/anoxia. At the same time, a more intense inhibition of individual enzymes is possible under the action of hydrogen sulfide in any hydrobiont, particularly when accompanied by a sharp decrease in the level of fructose-2,6-bisphosphate (an allosteric activator of phosphofructokinase) and the inhibition of pyruvate kinase by alanine, which contributes to a further decrease in the activity of phosphofructokinase and glycolytic activity in general (Oeschger and Storey, 1990).
REFERENCES
Arp, A.J. and Childress, J.J., Blood function in the hydrothermal vent vestimentiferan tube worm, Science, 1981, vol. 213, pp. 342–344. https://doi.org/10.1126/science.213.4505.342
Arp, A.J. and Childress, J.J., Sulfide binding by the blood of the hydrothermal vent tube worm Riftia pachyptila, Science, 1983, vol. 219, pp. 295–297. https://doi.org/10.1126/science.219.4582.295
Atkinson, D.E., Energy charge of the adenylate pools as a regulatory parameter. Interaction with feedback modifiers, Biochemistry, 1968, vol. 7, no. 11, pp. 4030–4034. https://doi.org/10.1021/bi00851a033
Bacchiocchi, S. and Principato, G., Mitochondrial contribution to metabolic changes in the digestive gland of Mytilus galloprovincialis during anaerobiosis, J. Exp. Zool., 2000, vol. 286, pp. 107–113. https://doi.org/10.1002/(sici)1097-010x(20000201)286:2<107::aid-jez1>3.0.co;2-8
Bishop, R.E. and Iliffe, T.M., Ecological physiology of the anchialine shrimp Barbouria cubensis: a comparison of epigean and hypogean populations, Mar. Biodiversity, 2012, vol. 42, no. 3, pp. 303–310. https://doi.org/10.1007/s12526-012-0113-8
Buck, L.T., Succinate and alanine as anaerobic end-products in the diving turtle (Chrysemys picta bellii), Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2000, vol. 126, no. 3, pp. 409–413. https://doi.org/10.1016/s0305-0491(00)00215-7
Cadenas, S., Mitochondrial uncoupling, ROS generation and cardioprotection, Biochim. Biophys. Acta, Bioenerg., 2018, vol. 1859, no. 9, pp. 940–950. https://doi.org/10.1016/j.bbabio.2018.05.019
Cao, Y., Wang, H.G., Cao, Y.Y., et al., Inhibition effects of protein-conjugated amorphous zinc sulfide nanoparticles on tumor cells growth, J. Nanopart. Res., 2011, vol. 13, pp. 2759–2767. https://doi.org/10.1007/s11051-010-0163-4
Chew, S.F., Gan, J., and Ip, Y.K., Nitrogen metabolism and excretion in the swamp eel, Monopterus albus, during 6 or 40 days of estivation in mud, Physiol. Biochem. Zool., 2005, vol. 78, no. 4, pp. 620. https://doi.org/10.1086/430233
Cortesi, P., Cattani, O., and Vitali, G., Physiological and biochemical responses of the bivalve Scapharca inaequivalvis to hypoxia and cadmium exposure: erythrocytes versus other tissues, Proceedings of an International Conference Marine Coastal Eutrophication, Bologna, 1992, p. 1041. https://doi.org/10.1016/B978-0-444-89990-3.50090-0
De Zwaan, A., Cortesi, P., Thillart, G., et al., Differential sensitivities to hypoxia by two anoxia-tolerant marine molluscs: A biochemical analysis, Mar. Biol., 1991, vol. 111, no. 3, pp. 343–351.
Doeller, J.E., Kraus, D.W., Colacino, J.M., and Wittenberg, J.B., Gill hemoglobin may deliver sulfide to bacterial symbionts of Solemya velum (Bivalvia, Mollusca), Biol. Bull., 1988, vol. 175, no. 3, pp. 388–396.
Gäde, G., Energy production during anoxia and recovery in the adductor muscle of the file shell, Lima hians, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1983, vol. 76, no. 1, pp. 73–77. https://doi.org/10.1016/0305-0491(83)90173-6
Golovina, I.V., Resistance to negative effects and the ratio of energy metabolism enzyme activity in tissues of the Black Sea molluscs Mytilus galloprovincialis Lamarck, 1819 and Anadara kagoshimensis (Tokunaga, 1906), Morsk. Biol. Zh., 2019, vol. 4, no. 3, pp. 37–47. https://doi.org/10.21072/mbj.2019.04.3.04
Grieshaber, M.K. and Völkel, S., Animal adaptations for tolerance and exploitation of poisonous sulfide, Annu. Rev. Physiol., 1998, vol. 60, pp. 33–53. https://doi.org/10.1146/annurev.physiol.60.1.33
Grivennikova, V.G. and Vinogradov, A.D., Mitochondrial production of reactive oxygen species, Biochemistry (Moscow), 2013, vol. 78, no. 13, pp. 1490–1511. https://doi.org/10.1134/S0006297913130087
Hand, S.C. and Somero, G.N., Energy metabolism pathways of hydrothermal vent animals: adaptations to a food-rich and sulfide-rich deep-sea environment, Biol. Bull., 1983, vol. 165, pp. 167–181.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation: Mechanism and Process in Physiological Evolution, New York: Oxford Univ. Press, 2002.
Holm-Hansen, O. and Booth, C.R., The measurement of adenosine triphosphate in the Ocean and its ecological significance, Limnol. Oceanogr., 1966, vol. 11, no. 4, pp. 510–519. https://doi.org/10.4319/lo.1966.11.4.0510
Isani, G., Cattani, O., and Tacconi, S., Energy metabolism during anaerobiosis and recovery in the posterior adductor muscle of Scapharca inaequivalvis (Bruguière), Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1989, vol. 93, no. 1, pp. 193–200.
Kolesnikova, E.E. and Golovina, I.V., Oxidoreductase activities in oxyphilic tissues of the black sea ruff Scorpaena porcus under short-term hydrogen sulfide loading, J. Evol. Biochem. Physiol., 2020, vol. 56, no. 5, pp. 459–470. https://doi.org/10.1134/S0022093020050099
Kraus, D.W., Heme proteins in sulfide-oxidizing bacteria/mollusc symbioses, Am. Zool., 1995, vol. 35, no. 2, pp. 112–120. https://doi.org/10.1093/icb/35.2.112
Larade, K. and Storey, K.B., A profile of the metabolic responses to anoxia in marine invertebrates, Cell and Molecular Responses to Stress, Vol. 3: Sensing, Signaling and Cell Adaptation, Amsterdam: Elsevier Sci. B, 2002a.
Larade, K. and Storey, K.B., Reversible suppression of protein synthesis in concert with polysome disaggregation during anoxia exposure in Littorina littorea, Mol. Cell. Biochem., 2002b, vol. 232, nos. 1–2, pp. 121–127.https://doi.org/10.1023/a:1014811017753
Luk’yanova, O.N., ATP-ases as nonspecific molecular biomarkers of the state of aquatic organisms under anthropogenic pollution, Tezisy Dokladov II Mezhdunarodnoi nauchnoi konferentsii “Biotekhnologiya – okhrane okruzhayushchei sredy” (Proc. II Int. Sci. Conf. “Biotechnology in Environmental Protection”), Moscow: Mosk. Gos. Univ., 2004.
Miyamoto, Y. and Iwanaga, C., Effects of sulphide on anoxia-driven mortality and anaerobic metabolism in the ark shell Anadara kagoshimensi, J. Mar. Biology. Assoc. U. K., 2017, vol. 97, no. 2, pp. 329. https://doi.org/10.1017/S0025315416000412
Nakano, T., Yamada, K., and Okamura, K., Duration rather than frequency of hypoxia causes mass mortality in ark shells (Anadara kagoshimensis), Mar. Pollut. Bull., 2017, vol. 125, nos. 1–2, pp. 86–91. https://doi.org/10.1016/j.marpolbul.2017.07.073
Oeschger, R. and Storey, K.B., Regulation of glycolytic enzymes in the marine invertebrate Halicryptus spinulosus (Priapulida) during environmental anoxia and exposure to hydrogen sulfide, Mar. Biol., 1990, vol. 106, pp. 261–266.
Orekhova, N.A. and Konovalov, S.K., Oxygen and sulfides in bottom sediments of the coastal Sevastopol Region of Crimea, Oceanology, 2018, vol. 58, no. 5, pp. 679–688.https://doi.org/10.1134/S0001437018050107
Owen, T.G. and Hochachka, P.W., Purification and properties of dolphin muscle aspartate and alanine transaminases and their possible roles in the energy metabolism of diving mammals, Biochem. J., 1974, vol. 143, no. 3, pp. 541–553. https://doi.org/10.1042/bj1430541
Powell, M.A. and Arp, A.J., Hydrogen sulfide oxidation by abundant nonhemoglobin heme compounds in marine invertebrates from sulfide-rich habitats, J. Exp. Zool., 1989, vol. 249, no. 2, pp. 121–132. https://doi.org/10.1002/jez.1402490202
Revkov, N.K., Colonization’s features of the Black sea basin by recent invader Anadara kagoshimensis (Bivalvia: Arcidae), Morsk. Biol. Zh., 2016, vol. 1, no. 2, pp. 3–17. https://doi.org/10.21072/mbj.2016.01.2.01
Rosenberg, R., Nilsson, H.C., and Diaz, R.J., Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient, Estuarine, Coastal Shelf Sci., 2001, vol. 53, no. 3, pp. 343–350. https://doi.org/10.1006/ecss.2001.0810
Sahin, C., Erbay, M., Kalayci, F., et al., Life-history traits of the Black Scorpionfish (Scorpaena porcus) in southeastern Black Sea, Turk. J. Fish. Aquat. Sci., 2019, vol. 19, no. 7, pp. 571–584. https://doi.org/10.4194/1303-2712-v19_7_04
Savina, M.V., Mekhanizmy adaptatsii tkanevogo dykhaniya v evolyutsii pozvonochnykh (Adaptation Mechanisms of Tissue Respiration in Vertebrate Evolution), St. Petersburg: Nauka, 1992.
Soldatov, A.A., Andreenko, T.I., Sysoeva, I.V., and Sysoev, A.A., Tissue specificity of metabolism in the bivalve mollusc Anadara inaequivalvis Br. under conditions of experimental anoxia, J. Evol. Biochem. Physiol., 2009, vol. 45, no. 3, pp. 349–355. https://doi.org/10.1134/S002209300903003X
Soldatov, A.A., Andreenko, T.I., Golovina, I.V., and Stolbov, A.Ya., Peculiarities of organization of tissue metabolism in mollusks with different tolerance to external hypoxia, J. Evol. Biochem. Physiol., 2010, vol. 46, no. 4, pp. 341–349. https://doi.org/10.1134/S0022093010040022
Soldatov, A.A., Kukhareva, T.A., Andreeva, A.Y., and Efremova, E.S., Erythroid elements of hemolymph in Anadara kagoshimensis (Tokunaga, 1906) under conditions of the combined action of hypoxia and hydrogen sulfide contamination, Rus. J. Mar. Biol., 2018, vol. 44, no. 6, pp. 452–457. https://doi.org/10.1134/S1063074018060111
Somero, G.N., The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’, J. Exp. Biol., 2010, vol. 213, no. 6, pp. 912–920. https://doi.org/10.1242/jeb.037473
Stewart, F.J. and Cavanaugh, C.M., Bacterial endosymbioses in Solemya (Mollusca: Bivalvia) —Model systems for studies of symbiont–host adaptation, Antonie Leeuwenhoek, 2006, vol. 90, pp. 343–360. https://doi.org/10.1007/s10482-006-9086-6
van Hellemond, J.J., van der Klei, A., van Weelden, S.W., and Tielens, A.G., Biochemical and evolutionary aspects of anaerobically fuctioning mitochondria, Philos. Trans. R. Soc., B, 2003, vol. 358, no. 1429. https://doi.org/10.1098/rstb.2002.1182
Vismann, B., Hematin and sulfide removal in hemolymph of the hemoglobin-containing bivalve Scapharca inaequivalvis, Mar. Ecol. Prog. Ser., 1993, vol. 98, pp. 115–122.
Völkel, S., Berenbrink, M., Heisler, N., and Nikinmaa, M., Effect of sulfide on K+ flux pathways in red blood cells of crusian carp and rainbow trout, Fish Physiol. Biochem., 2001, vol. 24, pp. 213–223. https://doi.org/10.1023/A:1014050001585
Washizu, T., Nakamura, M., Izawa, N., et al., The activity ratio of the cytosolic MDH/LDH and the isoenzyme pattern of LDH in the peripheral leukocytes of dogs, cats and rabbits, Vet. Res. Commun., 2002, vol. 26, no. 5, pp. 341–346. https://doi.org/10.1023/a:1016278409138
Watanabe, T., Effects of hypoxic and osmotic stress on the free D-aspartate level in the muscle of blood shell Scapharca broughtonii, Amino Acids, 2005, vol. 28, no. 3, pp. 291–296. https://doi.org/10.1007/s00726-005-0188-7
Wijsman, T.C.M., Adenosine phosphates and energy charge in different tissues of Mytilus edulis under aerobic and anaerobic conditions, J. Comp. Physiol., 1976, vol. 107, no. 1, pp. 129–140.
Yusseppone, M.S., Rocchetta, I., Sabatini, S.E., et al., Inducing the alternative oxidase forms part of the molecular strategy of anoxic survival in freshwater bivalves, Front. Physiol., 2018, vol. 9, art. ID 100. https://doi.org/10.3389/fphys.2018.00100
Zaika, V.E., Konovalov, S.K., and Sergeeva, N.G., The events of local and seasonal hypoxia at the bottom of the Sevastopol bays and their influence on macro-benthos, Morsk. Biol. Zh., 2011, vol. 10, no. 3, p. 15.
Funding
This work was supported by State Task no. 121041400077-1 and the Russian Foundation for Basic Research, project no. 20-04-00037.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Barkhash
Abbreviations: AEC, adenylate energy charge; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; AP, adenylate pool; ATP, triphosphate, ADP, diphosphate, and AMP, adenosine 5-monophosphate; HSL, H2S loading.
Rights and permissions
About this article
Cite this article
Soldatov, A.A., Golovina, I.V., Kolesnikova, E.E. et al. Effect of Hydrogen Sulfide Loading on the Activity of Energy Metabolism Enzymes and the Adenylate System in Tissues of the Anadara kagoshimensis Clam. Inland Water Biol 15, 632–640 (2022). https://doi.org/10.1134/S1995082922050194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082922050194