Abstract
A comparative analysis is performed between the oxidoreductase activity (malate and lactate dehydrogenase: MDH, LDH) in the gills (the lamellae of first gill arch) and brain structures (medulla oblongata, middle brain, forebrain, and diencephalon) of Scorpaena porcus L, 1758 under short-term separated exposure to hypoxia (90 min, 1.7–3.7 mg О2/L and 0.3–1.0 mg О2/L) and hydrogen sulfide (5 min, 37 µМ and 74 µМ Na2S). Under experimental conditions, the increase in hypoxic and hydrogen sulfide loading promotes an increase in the interaction in the MDH ↔ LDH activity system in the tissues under study: the highest value of the correlation coefficient is found in the gills (r = 0.87, p < 0.05) and medulla oblongata (r = 0.96, p < 0.01) This paper further considers the functional relationship between the oxidoreductase activities in the tissues and discusses the metabolic effects of hypoxic and H2S loading on the activity of oxidoreductases and the possible mechanisms of the effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hypoxia and a high content of sulfides is a common problem for coastal and estuarial waters even in aquatic environments not disturbed by humans in the absence of penetrating vertical convection. The Black Sea is an inland sea with extensive natural hydrogen sulfide zone and significant anthropogenic loading. Boundaries of the Black Sea hydrogen sulfide zone exhibit spatial-temporal instability and depend on horizontal and vertical transport, as well as mixing and solar activity. By a depth of 3–7 m, gas seeps are observed, characterized by detrital and microbial mats with an increased concentration of organic matter, where fish can feed on the organisms contained in them (Zaika and Gulin, 2011; Eremeev and Konovalov, 2006). In the wild, black scorpionfish Scorpaena porcus, 1758, or sea ruff, occurs at depths down to 45 m; leads a benthic ambush-predator lifestyle; and is tolerant to hypoxia, anoxia, and, apparently, local concentrations of hydrogen sulfide.
The survival of the animals, which occur in an aquatic environment with a low О2 content, largely depends on the ability to maintain equilibrium between energy exchange and demand for high-energy compounds. The reactions of glycolysis, providing for enzymes of malate dehydrogenase (L-malate: NAD-oxidoreductase; MDH, 1.1.1.37) and lactate dehydrogenase (lactate: NAD-oxidoreductase; LDH, 1.1.1.27), is an essential part of normal metabolism and cell functioning in the course of adaptation to changing environmental conditions.
The cytosolic form of MDH is involved in aerobic glycolysis and participates in malate-aspartate shunt, oxidizing a malate, incoming from mitochondria, to oxaloacetate with the formation of NADH; LDH catalyzes the final stage of anaerobic glycolysis. Both enzymes are involved in energy production and the regulation of oxidation-reduction cell potential; the ratio of their activity is used as an indicator of intensity and directionality of oxidative processes in tissues (Hochachka and Somero, 2002). A change in hydrological characteristics of the environment and oxygen content, in particular, causes shifts in the aerobic and anaerobic metabolism in tissues.
The goal of the present paper is to compare the effect of hypoxia and hydrogen sulfide loading on the activity of oxidoreductases in oxyphilic tissues, specifically, gills and structures of the brain in the Black Sea scorpionfish.
MATERIALS AND METHODS
The investigation included adult individuals of sea ruff Scorpaena porcus (Scorpaenidae) during summer period (34 ind., body length 12–17 cm, and body weight 70–130 g). The fish were captured in July–August with set gillnet and delivered to the laboratory in plastic drums 60 L in volume with aeration. After the transportation, the ruffs were kept in a flow-through aquarium for 1 weak to relieve stress; the study included only actively moving and feeding individuals.
The experiments were conducted in a specially designed chamber at water temperature 21 ± 0.5°C (Soldatov et al., 2020). The fish were kept at oxygen concentration in water ranging from 4.5 to 6.7 mg/L (normoxia), from 1.7 to 3.7 mg/L (test 1), and from 0.3 to 1 mg/L (test 2). The time of exposure to hypoxia was 90 min; oxygen content in the water was monitored using the ELWRO PRL T N5221 oximeter (Poland). Short-term hydrogen sulfide loading was created by 5 min of exposure of two experimental groups of fish to different concentrations of sodium sulfide as the hydrogen sulfide donor: 37 µM Na2S (test 3) and 74 µM Na2S (test 4) while relying on data of other researchers (Porteus et al., 2014). No disturbances of external respiration or behavioral responses were observed in the fish during any of the tests.
Tissue dissection, homogenization, and centrifugation were performed under cooling (0 ± 4°С). Samples of tissues, namely, filaments of the lamellae of the first gill arch, the MO, and the MFD were collected after the experiment immediately following decapitation and stored prior to analysis under –80°С in a freezer chamber (Farma 900 Series, Termo Scientific, United States).
The specific activity of LDH (lactate: NAD-oxidoreductase; LDH, 1.1.1.27) and MDH (L-malate: NAD-oxidoreductase; MDH, 1.1.1.37) was measured in spectrophotometric terms at 340 nm and 25°C based on the NADH oxidation rate in the cytoplasm of the tissues; protein content was determined using the microbiuret method. Pyruvate and oxaloacetate served as substrates for determining the LDH and MDH activity, respectively (Mil’man et al., 1974). Activity of the enzymes in tissues of the control group of fish was taken as 100%. The diagram in Fig. 1 shows a variation in activity of enzymes relative to the control. The results are presented in the form of M ± m; the statistical significance of means was assessed using Student’s t-test and differences were considered significant at p < 0.05. Statistical processing and graphic representation were done by the standard Microsoft Excel software.
Activity of (a, b) MDH, (c, d) LDH, and (e, f) MDH/LDH index under (a, c, e) hypoxia and (b, d, f) hydrogen sulfide loading in tissues of Scorpaena porcus. Here and in Fig. 2: (I) first gill arch, (II) medulla oblongata, (III) middle brain, forebrain, and diencephalon; (C) control, (1) 1.7–3.7 mg О2/L, (2) 0.3–1 mg О2/L, (3) 37 µM Na2S, and (4) 74 µM Na2S. *Significantly differ from control, р < 0.05. **Significantly differ from another test, р < 0.05.
RESULTS
Separate exposure to short-term hypoxia and hydrogen-sulfide loading triggered the adaptive fluctuation of activity of carbohydrate metabolism enzymes (Fig. 1).
Test 1. Moderate hypoxia (1.7–3.7 mg O2/L) resulted in a 50% decline in the activity of MDH and LDH in gills (p < 0.05; Figs. 1a, 1b). The trends were noted toward decline in the activity of both enzymes in the MO and their increase in the MFD, which was significant for the MDH (p < 0.05). The value of the MDH/LDH index remained unchanged relative to the control in gills and brain regions (Fig. 1e). The correlation between the MDH and LDH activity reached a maximum in MO (r = 0.96, p < 0.01) (Fig. 2a).
Test 2. Under acute hypoxia (0.3–1 mg O2/L), as LDH activity decreased by 50%, insignificant increase in MDH activity in gills (p < 0.05) triggered an increase in the index by 40% (p > 0.05). In MO, the activity of the enzymes returned to the control level. In the MFD, the activity of both enzymes substantially declined compared to test 1 (p < 0.05), which held an MDH/LDH index constant. Correlation between the MDH and LDH activity showed a maximum increase in gills (r = 0.87) and the MFD (r = 0.81) and remained high in the MO (r = 0.94, (p < 0.05–0.01) (Fig. 2a).
Test 3. Moderate hydrogen sulfide loading (37 µM Na2S) revealed a trend toward a decrease in activity of MDH in gills and, conversely, the increase in the MO and MFD (Fig. 1b). Compared to the control, LDH activity declined 50% in gills (p < 0.05) and remained unchanged in brain tissues (Fig. 1d). There was an emerging trend toward an increase in the MDH/LDH index in gills and MO (Fig. 1f). The correlation between the MDH and LDH activity peaked in gills (r = 0.75, p < 0.05) (Fig. 2b). Gill tissue lamella darkened.
Test 4. Increased hydrogen sulfide loading (to 74 µM Na2S) led to a slight increase in the MDH and LDH activity in gills compared to test 3. Darkening of the gill tissue progressed. In MO, the activity of MDH persisted at test 3 level; LDH activity remained steady. The activity of both enzymes, however, spiked in the MFD compared to the control and test 3 (p < 0.05); that is, it attained 212% for MDH and 185% for LDH. The trend toward a large value of the MDH/LDH index persisted in gills and the MO compared to the control; the ratio of activity of the enzymes remained unchanged in MFD. Coefficient r between the MDH and LDH activity increased to 0.65 (p ≥ 0.05) in the MFD and 0.94 (p < 0.01) in the MO.
DISCUSSION
Hypoxia has a substantial impact on the abundance and diversity in microfauna, as well as structure and function of marine ecosystems (Vaquer-Sunyer and Duart, 2010). Ambient hypoxia and the corresponding shift of anaerobic metabolism have been known to pose a significant threat to energy balance in fish and limit ability of the animals to produce a sufficient amount of ATP for satisfying their metabolic demands. Despite the critical importance of aerobic respiration to sustain metabolic functions, multiple organisms dwell and thrive under various hypoxic and even oxygen-free ambient conditions.
During biochemical adaptation to hypoxia in an aquatic habitat (Almeida-Val, 1993), metabolic reorganization obeys two generalized patterns: either the anaerobic synthesis of ATP increases (the Pasteur Effect) or the level of ATP decreases (metabolic depression). This kind of change in metabolism is based on the activation of glycolysis with the involvement of glycogen or glucose as substrates and lactate as intermediate product. “Enzymatic adaptation” to hypoxia also implies changes in the affinity of indivi-dual enzymes involved in aerobic and anaerobic metabolism (Lushchak et al., 1998).
All ectotherms and fish, in particular, employ adaptive biochemical strategies to attain metabolic homeostasis under the variation of oxygen dissolved in water (Hochachka and Somero, 1984). In hypoxia, survival of teleost fish primarily depends on the ability to maintain cellular energy balance (steady ATP level), despite a drop in the aerobic energy production (Richards, 2009).
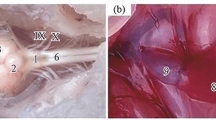
Hypoxia vs. gills. Similar to other benthic fish species, the gill apparatus in the scorpionfish is small in area compared to active, faster swimming fishes (Gray, 1954). We employed tissue of the first gill arch only to study features of the scorpionfish metabolic profile. First gill arches of many teleost fish, including the scorpionfish, are innervated through (IX) glossopharyngeal and (X) vagus nerves. The epithelium of filaments of this gill arch includes NEC, which fulfills the function of the special О2 sensor. As PwО2 declines, the activation of NEC, responding to О2 concentration, launches a cascade of cardiorespiratory reflexes, which ensure the survival and adaptation of the body to conditions of hypoxia. Due to gill epithelium, the gill apparatus features other rather diverse physiological functions. In addition to being considered the main organ of respiratory gas exchange, gills play an important role in ionic and osmotic balance and are the primary locus of elimination of nitrogenous matters (Mommsen, 1984b). Except for water, oxygen, and carbon dioxide exchange, which appears to be determined by simple diffusion, all other abovementioned processes require the application of considerable metabolic activity. Gill tissues are characterized by a substantial “internal” absorption of О2 by gill filaments, which are not immediately related to a respiratory function of gill apparatus (Johansen and Pettersson, 1981). The oxidative ability of gill tissue is additionally represented by the excess of mitochondria in specialized gill cells with particular functions. Due to high oxidative ability of gill tissue, glucose and lactate serve as the most important sources of carbon (Mommsen, 1984a).
The second-highest MDH activity (next to brain tissues) was recorded by our study from gill tissue of the scorpionfish. Under moderate hypoxia, the MDH and LDH activity displayed a downward trend; however, under severe hypoxia, the MDH activity increased to an extent, which produced rather high values of MDH/LDH index, nearly twice exceeding the comparable index of brain tissues.
Hypoxia vs. the brain. The brain is the most complex organ sensitive to О2, consisting of multiple structural and functional components with remarkably distinct and independently regulated levels of functional and metabolic activity. Aerobic oxidation, which is the main pathway of glucose utilization by brain, determines the extremely high sensitivity of the brain to hypoxia. In addition, part of intermediate products of glucose oxidation is used by the brain to form mediators (acetylcholine and GABA), which (GABA in particular) sustain the brain’s resistance to hypoxia, as well as store acetyl residue in the form of acetyl aspartate (Yazykova, 2004; Marshal, 1995).
Five major regions are outlined in the brain of teleost fishes, namely, the MFD and MO. The latter is involved in the formation of the brainstem and contains basic reflex centers regulating the respiration, cardiac activity, vascular tone, and nuclei of six pairs (V–X) cranial nerves and is a pathway for ascending and descending neural tracts. Fish the MFD include centers of olfaction, vision, and hearing; fulfill functions of integration and regulation of the body functions and motor coordination; and is involved in control over feeding (Kotrschal and Kotrschal, 2020; Smirnov and Kuz’mina, 2020).
At the baseline level (normoxia), the MFD and MO of the scorpionfish were reported to differ slightly in a degree of MDH activity, which appears to reflect a trend of higher intensity of aerobic processes in the evolutionary younger MFD structures. As РwО2 declined (moderate hypoxia), MDH activity increased in the MFD and remained within the limits of measurement error in the MO. Under low РwО2 values (acute hypoxia), MDH activity remained high in the MO, while appreciably declining in the MFD, suggesting a pronounced metabolic stability of MO, which is required to maintain homeostasis. The adaptive stability of the MO at low РwО2 values is maintained by high LDH activity (within limits of control level).
At the baseline (normoxia), MO and MFD had comparable LDH values. The high LDH activity in brain structures discovered by us determines the “anaerobization” of pathways of energy metabolism supporting the production of high-energy compounds during change of РwО2 in bottom layers. Under exposure to acute hypoxia, this degree of LDH activity was maintained steady in MFD and MO. At the same time, moderate hypoxia was accompanied by an increase in LDH activity in MFD and decrease in MO. Data obtained by us on the MDH and LDH activity in the scorpionfish brain extend existing knowledge of the preferred use of glycogen in the brain of some jawless and benthic teleost fish species as the closest source of glucose, as well as utilization in metabolic conversions of lactate or ketones if glucose supply is limited (Soengas and Aldegunde, 2002).
Importantly, under exposure to hypoxia, the MDH/LDH index remained at a relatively steady level within the limits of measurement error in all studied brain regions; the ratio of MDH to LDH in MFD and MO suggested that brain tissues retain oxidative ability under various short-term hypoxia gradations. The persistence of a high intensity of aerobic and anaerobic processes in MFD and MO of the scorpionfish under acute hypoxia may apparently suggest the existence of various metabolic strategies in energy exchange for evolutionary unevenly aged brain structures within the range of РwО2 not critical for the particular species. Additionally, the ability to sustain steady ATP levels in tissues, especially in sensitive tissues such as the brain, in particular, during hypoxia is considered an indicator of tolerance to hypoxia (Hochachka et al., 1996), which agrees with the “retention” of the MDH/LDH index.
H2S as toxin and mediator. Sulfides (generalized term for dissociated ions H2S ↔ НS– ↔ S2–) are always present in the oxygen-free (anoxic) layer of marine sediments; their diffusion to bottom water horizons is governed by the diffusion rate and consumption of oxygen in oxygenated sediments (Vaquer-Sunyer and Duart, 2010). Therefore, the quantity of sulfides increases during hypoxia. Specifically, the mass mortality of hydrobionts during this time might result from sulfide-induced toxicity and hypoxia proper (Vaquer-Sunyer and Duart, 2010). The content of sulfides in the water column might reach strong concentrations and actively affect the physiology of fish dwelling in shallow waters along the sea coasts (Bagarinao and Vetter, 1992). The toxicity of sulfides is largely determined by the inhibition of cytochrome с-oxidase and interference with other important enzymes (Bagarinao and Vetter, 1992).
Potential mechanisms assisting fish in surviving sulfide toxicity include interactions with blood proteins, anaerobic metabolism, a certain insensitivity of cytochrome c-oxidase to sulfides, and methemoglobinemia (Torrans and Clemens, 1982; Bagarinao and Vetter, 1989, 1990, 1992, 1993). Species highly resistant to hypoxia are presumed to be distinguished by high H2S-tolerance (Grieshaber and Völkel, 1998); at the same time, long-term survival in the sulfide-containing environment cannot be explained by a high anaerobic potential (Völkel et al., 2001).
In addition to toxic action, H2S has been known to fulfill the function of physiologically important signaling molecule (Olson, 2012). As a mediator, endogenic H2S is primarily synthesized from the amino acid of L‑cysteine and/or homocysteine. The synthesis of Н2S occurs in many (if not all) body tissues by two cytosolic enzymes, namely, cystathionine β-synthase (CBS) and cystathionine γ-lyase. H2S can also be generated in mitochondria under the action of cysteine aminotransferase and 3-mercaptopyruvate transferase (also present in cytosol). The main enzyme of H2S synthesis (CBS) has been additionally shown to integrate interaction between H2S and other gaseous signaling molecules (CO, NO), while contributing to the regulation of energy metabolism (Giuffréa et al., 2014).
It should be acknowledged, however, that physiological and biochemical responses to sulfide action in fish are still not fully understood. Additionally, modern ideas defining hypoxia consider only the oxygen concentration in the aquatic environment without including a possible synergetic effect of hypoxia and sulfide toxicity (Vaquer-Sunyer and Duart, 2010).
Our study involved provisionally “physiological” concentrations of the H2S–Na2S donor, short-term exposure to which was not accompanied in fish by disorders and the depression of external respiration (Porteus et al., 2014), which suggested the absence of a pronounced toxic effect of the abovementioned doses.
H2S vs. gills. Sulfides, easily penetrating to the gill epithelium, are known to hamper the binding of О2 to blood hemoglobin and promote the development of tissue hypoxia similar to the one induced by cyanide or decrease in О2 supply (Affonso et al., 2004). According to the described mechanism, the scorpionfish might have also acquired a certain degree of hypoxemia (decreased blood PO2) from the interaction of H2S and blood hemoglobin. In fish, sulfide-oxidizing activity of blood is assumed (Bagarinao and Vetter, 1989) to promote the minimization of the number of potentially toxic compounds which enter vital organs with blood flow. Sulfide-oxidizing activity has been also reported from the fish gill tissue proper, spleen, liver, and kidney (Bagarinao and Vetter, 1989).
Processes observed under the hydrogen sulfide loading of gill tissue in the scorpionfish were characterized by some signs of metabolic depression. This was indicated by a decline in MDH activity in the first gill arch, which indirectly suggested an interaction between H2S and gill tissue.
H2S vs. brain. The scorpionfish exposure to low a concentration of Na2S did not cause statistically significant changes in the activity of oxidoreductases in brain tissues, whereas these changes were more pronounced with the twofold increase in Na2S concentration in the medium. This phenomenon of a nearly absent response of oxidoreductase (within the limits of measurement error) to low Na2S concentration on the entire body level could be realized due to sulfide-binding activity of gill tissue and blood, which ensures the scorpionfish protection against the entry of physiologically significant quantity of sulfides to the body. At the same time, high concentration of Na2S synchronously triggered in the MFD an increase in MDH and LDH activity, which implies a close coordination in the functioning of aerobic and anaerobic metabolic pathways (Van Waarde, 1983). Proceeding under the assumption of hypoxemia/tissue hypoxia at a high Na2S dose, a significant boost in MDH activity in the most О2-sensitive part of brain (MFD) could have been primarily associated with the dual role of MDH in aerobic and anaerobic metabolism (Hochachka and Somero, 1984). Due to its high activity compared to other enzymes of energy metabolism, MDH becomes involved in anaerobic processes and glycolysis. Under exposure to H2S, a simultaneous increase in the LDH and MDH activity in oxyphilic brain structures points to the presence of the hypoxemia/tissue hypoxia factor occurring at high Na2S concentration, on the one hand, and, on the other, indicates a direct “response” of oxidoreductases on the presence of Н2S. It should be noted that tentatively less О2-sensitive MO on the level of oxidoreductases showed very little response even to high Na2S concentration, which suggests a distinct metabolic regime in this brain region of the scorpionfish. The processes, occurring under hydrogen sulfide loading, may include sulfide-oxidizing activity under relatively low/nontoxic H2S concentrations in the MO, which features a pronounced resistance to РО2 variations and/or H2S proper.
Correlational relationship between the MDH and LDH under hypoxia and hydrogen-sulfide loading. An analysis of correlational relationship (r) between the MDH and LDH activity in oxyphilic tissues has shown an increase in the former as PwO2 declined, which suggests an enhancement of the interaction between these two enzymes against О2 deficiency. Importantly, tissues of the first gill arch and MO, which are the first to provide for cardiorespiratory reactions under hypoxia, display the largest r values compared to MFD, which may imply a functional interrelationship of these two structures within the regulatory cardiac and respiratory functions loop. The obtained r values point to a more balanced interaction of oxidoreductases in the MO compared to MFD, which suggests a higher resistance of MO to hypoxia and agrees with the absence of dramatic changes in the MDH and LDH activity in MO tissue under different PwO2 levels.
Compared to hypoxia, the pronounced “shift” of correlation relation (r) between the MDH and LDH activity of the first gill arch under hydrogen sulfide loading appear to be mediated by the specific involvement of gill tissue in the inactivation of H2S excess and accompanying exposure to H2S hypoxemia. The maximum hydrogen sulfide loading revealed a significant correlational relationship of MO oxidoreductases, which sustain the production level of high-energy compounds to provide for the generation of cardiorespiratory “commands” aimed at the organism’s survival, which intensify against the background of hypoxemia/tissue hypoxia.
CONCLUSIONS
These findings suggest a similarity in the alteration of energy metabolism under hypoxia and hydrogen sulfide loading between О2-sensitive brain tissues and gills, which agrees with a previously proposed idea about the identity of metabolic consequences of exposure to hypoxia and H2S and responses to the activation of oxidoreductases of energy exchange (Oeschger and Storey, 1990; Olson et al., 2006). Responses of oxyphilic tissues to hydrogen sulfide loading in the scorpionfish were largely manifested in the alteration of energy metabolism oxidoreductase activity (MDH and LDH), occurring as a result of inactivation of the H2S excess. The oxyphilic structures that are more functionally active/О2-sensitive or evolutionary younger and require more spending from high-energy compounds might be more susceptible to action of H2S vs. hypoxia, based on competitive/reciprocal relations between О2 and H2S. The response of the first gill arch oxidoreductases to hypoxia and action of H2S have certain similarity, which appears to be due to anatomical disposition of gills towards environmental factors. The first gill arch and MO, composing the regulatory cardiac and respiratory functions loop, feature the tightest/most pronounced coupling (r) of MDH and LDH activity under exposure to hypoxia and hydrogen sulfide.
REFERENCES
Affonso, E.G., Polez, V.L.P., Correa, C.F., et al., Physiological responses to sulfide toxicity by the air-breathing catfish, Hoplosternum littorale (Siluriformes, Callichthyidae), Comp. Biochem. Physiol., 2004, vol. 139, no. 4, p. 251. https://doi.org/10.1016/j.cca.2004.11.007
Almeida-Val, V.M., Val, A.V., and Hochachka, P.W., Hypoxia tolerance in Amazon fishes: status of an under-explored biological “goldmine,” in Surviving Hypoxia: Mechanisms of Control and Adaptation, Boca Raton: CRC, 1993, p. 435.
Bagarinao, T. and Vetter, R.D., Sulfide tolerance and detoxification in shallow-water marine fishes, Mar. Biol. (Berlin), 1989, vol. 103, p. 291.
Bagarinao, T. and Vetter, R.D., Oxidative detoxification of sulfide by mitochondria of the California killifish Fundulus parvipinnis and the speckled sanddab Citharichthys stigmaeus, J. Comp. Physiol., 1990, vol. 160, p. 519.
Bagarinao, T. and Vetter, R.D., Sulfide-hemoglobin interactions in the sulfide-tolerance salt marsh resident, the California killifish Fundulus parvipinnis, J. Comp. Physiol., 1992, vol. 162, p. 614.
Bagarinao, T. and Vetter, R.D., Sulphide tolerance in the California killifish, Fundulus parvipinnis, a salt marsh resident, J. Fish. Biol., 1993, vol. 42, p. 729. https://doi.org/10.1111/j.1095-8649.1993.tb00381.x
Eremeev, V.N. and Konovalov, S.K., On the formation of the budget and the regularities of the distribution of oxygen and hydrogen sulfide in the waters of the Black Sea, Morsk. Ekol. Zh., 2006, vol. 5, no. 3, p. 5.
Giuffréa, A., Colaço, H.G., Mastronicola, D., et al., Cystathionine β-synthase and the interplay between the bioenergetically relevant gasotransmitters NO, CO and H2S, Biochim. Biophys. Acta, Bioenerg., 2014, vol. 1837, art. e71.
Gray, I.E., Comparative study of the gill area of marine fishes, Biol. Bull., 1954, vol. 107, p. 219.
Grieshaber, M.K. and Völkel, S., Animal adaptations for tolerance and exploitation of poisonous sulfide, Annu. Rev. Physiol., 1998, vol. 60, p. 33.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation, New Jersey: Princeton Univ. Press, 1984.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation: Mechanism and Process in Physiological Evolution, Oxford: Oxford University Press, 2002.
Hochachka, P.W., Buck, L.T., Doll, C.J., and Land, S.C., Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, p. 9493. https://doi.org/10.1073/pnas.93.18.9493
Johansen, K. and Pettersson, K., Gill O2 consumption in a teleost fish, Gadus morhua, Respir. Physiol., 1981, vol. 44, p. 277. https://doi.org/10.1016/0034-5687(81)90023-2
Kotrschal, A. and Kotrschal, K., Fish brains: anatomy, functionality, and evolutionary relationships, in Animal Welfare, vol. 20: The Welfare of Fish, p. 129. Cham: Springer, 2020. https://doi.org/10.1007/978-3-030-41675-1_6
Lushchak, V.I., Bahnjukova, T.V., and Storey, K.B., Effect of hypoxia on the activity and binding of glycolytic and associated enzymes in sea scorpion tissues, Braz. J. Med. Biol. Res., 1998, vol. 31, no. 8, p. 1059. https://doi.org/10.1590/s0100-879x1998000800005
Marshall, W.J., Clinical Biochemistry, Amsterdam: Elsevier, 1995.
Mil’man, L.S., Yurovetskii, Yu.G., and Ermolaeva, L.P., Determination of the activity of the most important enzymes of carbohydrate metabolism, in Metody biologii razvitiya (Methods of Developmental Biology), Moscow: Nauka, 1974, p. 346.
Mommsen, T.P., Metabolism of the fish gill, Fish Physiol., 1984a, vol. 10, p. 203. https://doi.org/10.1016/S1546-5098(08)60186-7
Mommsen, T.P., Biochemical characterization of the rainbow trout gill, J. Comp. Physiol., 1984b, vol. 154, no. 2, p. 191.
Oeschger, R. and Storey, K.B., Regulation of glycolytic enzymes in the marine invertebrate Halicryptus spinulosus (Priapulida) during environmental anoxia and exposure to hydrogen sulfide, Mar. Biol. (Berlin), 1990, vol. 106, p. 261.
Olson, K.R., Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling, J. Comp. Physiol., 2012, vol. 182, p. 881. https://doi.org/10.1007/s00360-012-0654-y
Olson, K.R., Dombkowski, R.A., Russell, M.J., et al., Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation, J. Exp. Biol., 2006, vol. 209, p. 4011. https://doi.org/10.1242/jeb.02480
Porteus, C.S., Abdallah, S.J., Pollack, J., et al., The role of hydrogen sulphide in the control of breathing in hypoxic zebrafish (Danio rerio), J. Physiol., 2014, vol. 592, no. 14, p. 3075. https://doi.org/10.1113/jphysiol.2014.271098
Richards, J.G., Metabolic and molecular responses of fish to hypoxia, Fish Physiol., 2009, vol. 27, p. 443. https://doi.org/10.1016/S1546-5098(08)00010-1
Smirnova, E.S. and Kuz’mina, V.V., Effect of cholinolytics on the rate of feeding reaction of carp Cyprinus carpio L., Inland Water Biol., 2020, vol. 13, no. 2, p. 308. https://doi.org/10.1134/S1995082920020121
Soengas, J.L. and Aldegunde, M., Energy metabolism of fish brain, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, vol. 131, no. 3, p. 271. https://doi.org/10.1016/s1096-4959(02)00022-2
Soldatov, A.A., Golovina, I.V., Kolesnikova, E.E., et al., Activity of energy metabolism enzymes and atp content in the brain and gills of the Black Sea scorpionfish Scorpaena porcus under short-term hypoxia, J. Evol. Biochem. Physiol., 2020, vol. 56, no. 3, p. 224. https://doi.org/10.31857/S0044452920010143
Torrans, E.L. and Clemens, H.P., Physiological and biochemical effects of acute exposure of fish to hydrogen sulfide, Comp. Biochem. Physiol., 1982, vol. 71, no. 2, p. 183. https://doi.org/10.1016/0306-4492(82)90034-x
Vaquer-Sunyer, R. and Duart, C.M., Sulfide exposure accelerates hypoxia-driven mortality, Limnol., Oceanogr., 2010, vol. 55, no. 3, p. 1075. https://doi.org/10.4319/lo.2010.55.3.1075
Völkel, S., Berenbrink, M., Heisler, N., et al., Effect of sulfide on K+ flux pathways in red blood cells of crusian carp and rainbow trout, Fish Physiol. Biochem., 2001, vol. 24, p. 213.
Van Waarde, A., Aerobic and anaerobic ammonia production by fish, Comp. Biochem. Physiol., 1983, vol. 74, no. 4, p. 675. https://doi.org/10.1016/0305-0491(83)90127-x
Yazykova, M.Yu., Biokhimiya tkanei (Tissue Biochemistry), Saratov: Sarat. Univ., 2004.
Zaika, V.E. and Gulin, M.B., The greatest depths of fish habitat in the Black Sea and the peculiarities of their feeding at the border of the hydrogen sulfide zone, Morsk. Ekol. Zh., 2011, vol. 10, no. 2, p. 39.
Funding
This work was carried out as a part of the topic “Functional, Metabolic, and Toxicological Aspects of Life of Hydrobionts and their Populations in Biotopes with Different Physical and Chemical Regimes,” state reg. no. 121041400077-1, and supported by the Russian Foundation for Basic Research, project no. 20-04-00037.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by E. Kuznetsova
Abbreviations: GABA, γ-aminobutyric acid; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; NADH, nicotinamide adenine dinucleotide + hydrogen; NEC, neuroepithelial cells; MO, medulla oblongata; MFD, middle brain, forebrain, and diencephalon; and РwO2, oxygen tension in aquatic medium.
Rights and permissions
About this article
Cite this article
Golovina, I.V., Kolesnikova, E.E. Comparative Aspects of Hypoxia and Hydrogen Sulfide Effects on the Activity of Oxidoreductases in the Gills and Brain of the Sea Ruff Scorpaena porcus. Inland Water Biol 14, 758–765 (2021). https://doi.org/10.1134/S1995082921060055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082921060055