Abstract
The review presents the main works of Professor A.A. Borisov. The material is presented in several sections written by his students and colleagues. The subjects discussed include detonations in gaseous reactive systems and systems with inhomogeneous heat release are considered. The expediency of using detonation modes in aircraft and rocket engines is also considered. In addition, the results of mathematical modeling of shock waves in air are presented and questions of chemical kinetics are discussed. Based on the experimental study of vortex flows, an approach to the analysis of the formation of hot spots in real fuel combustion devices is formulated. A separate part is devoted to the kinetics of chemical transformations and their role in studying and predicting combustion processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
August 2022 marks the 90th anniversary of the birth of Professor A.A. Borisov. The years that have passed since his death made it possible to fully realize and appreciate the loss suffered by the science of combustion and explosion. The research carried out under his leadership and with his direct participation allowed him to take a worthy place in the community of scientists involved in chemical physics and the physics of combustion and explosion.
His scientific interests were focused on theoretical and experimental studies of the processes of self-ignition and detonation of gas and heterogeneous systems. Here is what Anatoly Alexandrovich wrote about this in his doctoral dissertation [1]: “The theory of self-ignition and the theory of detonation form the base of the science of combustion of gaseous systems. A special role in understanding these phenomena was played by the idea of chain reactions and thermal self-acceleration of reactions, put forward by Academician N.N. Semenov. This idea not only gave a complete explanation of the complex phenomena of self-ignition, but also served as one of the starting points for the theory of detonation, formulated in general terms by Academician Ya.B. Zel’dovich. By now, it can be considered entirely proven that both processes really have a deep internal connection and that the detonation wave is an ignition wave that propagates in combination with the shock wave generated by the release of energy during the reaction.”
At the end of the 1950s, A.A. Borisov, while still an engineer in the laboratory of Professor S.M. Kogarko, began to study the kinetics of chemical reactions at high temperatures [2]. The ignition of heptane in incident and reflected shock waves was studied using the shock tube technique. Having carried out a theoretical and computational analysis of the propagation of shock waves in a reacting medium and using their own experimental results, the authors of [2] were able to determine the areas of applicability of shock tubes for studying chemical reactions. In [3], the heat release in a real gas flow in a shock tube was studied by optical methods of temperature recording and the effect of violation of the one-dimensionality of the flow on it was shown depending on various gas-dynamic factors and nonequilibrium chemical kinetics. The experience gained in early studies in the study of physical and chemical processes behind shock waves allowed Prof. Borisov to create his own scientific school, bringing together researchers in the field of gas-phase and heterogeneous combustion of fuels, shock waves, and detonation.
This review is compiled based on those sections written by the students and colleagues of Prof. Borisov and recent achievements devoted to the main areas of his scientific activity.
DETONATION PROCESSES IN REACTING GAS MEDIUMS
For detonation processes and the flow of reacting media, the most characteristic is the presence of the so-called critical and nonstationary phenomena, which should considered taking into account both the kinetics of chemical processes occurring in a heated reaction medium and convective and conductive mass transfer, as well as various gas-dynamic disturbances that manifest themselves in the form of shock waves and rarefaction waves. Due to the complexity of these processes, their nonequilibrium and nonstationarity, modern modeling methods allow solving only individual problems with the flow of reacting media with exothermic and endothermic reactions, and then they are largely simplified. Therefore, Anatoly Alexandrovich concentrated on experimental research in studying these phenomena. The new experimental data obtained under his leadership are not only of fundamental scientific but also of applied importance for the technology of explosion safety and the improvement of installations based on detonation modes.
Under the leadership of Anatoly Alexandrovich, installations were developed on which experimental studies were carried out of the detonation limits of fuel-air mixtures (FAM) in smooth and rough tubes [4], as well as in flat slot-type channels [5, 6]. It was shown in [4] that the true limits of detonation in smooth tubes are quite close to the limits of flame propagation, especially for poor mixtures of hydrocarbons with air. It has been found that the limit is always preceded by an area of unstable modes with a pulsating velocity. The weak dependence of the detonation limits in tubes on the initiation energy and on the tube diameter, when the latter exceed certain values, makes it possible, at least for a limited range of changes in these parameters, to speak of the detonation limits as a characteristic of the mixture only. At the same time, in the existing theoretical models of detonation limits, the independence of concentrations at the limit on the tube diameter has not been confirmed; thus, their addition is necessary.
Critical phenomena during the propagation of detonation through channels of complex configuration, namely, the transition of detonation from a tube to an expanding cone, are experimentally investigated. An empirical dependence of the critical transition diameter on the opening angle of the conical tube and the critical exit of detonation from the tube into the free volume [7] was obtained, according to which, when the opening angle of the conical tube is greater than 60°, the critical diameter of the detonation transition from a tube of constant cross section to a conical expanding tube ceases to depend on the angle conical tube solution.
For the theory of detonation and technology of explosion safety, the study of the phenomenon of detonation in a free charge without a shell is important. The study of this type of detonation is useful for understanding the structure of gaseous detonation, its stability, and the nature of limiting phenomena. The quantitative characteristics of such a detonation are necessary when assessing the possibility of propagation of the detonation process in clouds of gas mixtures of complex configuration, which can form during the accidental release of fuel into the atmosphere. Under the guidance of Prof. Borisov, an apparatus was developed for studying detonation in a free cylindrical gas charge [8], in which the latter was formed in a solid shell by blowing it with a combustible gas mixture from an accelerating detonation tube installed vertically, and then a solid shell under the action of gravity. When studying the propagation of detonation over free cylindrical charges of stoichiometric acetylene-oxygen mixtures diluted with nitrogen, detonation was obtained in an acetylene-air mixture and it was shown that the ratio of the critical detonation diameter of a free charge to the critical diameter of the detonation exit from the tube into the volume was not constant, but decreased with an increase in the value of the latter [9].
In the further works [10–12], studies of detonation in free charges of various hydrocarbons were carried out. It was shown that when the diameter of the free charge approaches the critical one for the mixture under study, the detonation velocity decreases and a pulsating character of motion appears with periodic initiation of the mixture at the periphery of the charge. The characteristic size of a multifront detonation cell increases when the process propagates in a free charge compared to propagation in a charge with rigid walls. For the critical diameter of detonation propagation in a free charge, dcr.fr, the empirical dependence is obtained:
dcr.fr/dcr = A + B/dcr – C/\(d_{{{\text{cr}}}}^{2},\)
where dcr is the critical diameter of the detonation exit from the tube into the volume; a A, B, and C are numerical coefficients equal to 1.55 ± 0.05, 40 mm, and 7 mm2, respectively. It was also shown that the outflow of the detonating mixture into the atmosphere creates a region outside the installation, through which detonation can propagate from the hole with a length not exceeding four hole diameters [11, 12].
In [12], an experimental study of the propagation of detonation in a combustible mixture layer above a rigid surface was carried out on an installation representing a flat channel, the side surface of which was removed before detonation initiation in the combustible mixture filling this channel. As a result, a gas charge with a free surface was formed, simulating a combustible mixture layer above a rigid surface. Stoichiometric acetylene-air and hydrogen-air mixtures were studied. It is shown that the critical height of the combustible mixture layer for detonation propagation is half the critical diameter of detonation propagation in a free charge.
The experimental studies conducted on the diffraction of detonation waves made it possible to develop a semiempirical formula for estimating the critical detonation initiation energy of charges of any geometric shape based on the measured critical diameters for the transition of detonation from a tube to an unlimited volume. The formula agrees quite well with the available experimental data [13, 14].
DETONATION IN SYSTEMS WITH NONMONOTONIC HEAT RELEASE
In direct relationship to the studies of low-velocity detonation (LVD), traditionally carried out in the laboratory, Prof. Borisov was interested in the question of the effect of the reactions occurring behind the LVD front on the stability of the latter. This problem is a special case of detonation in systems with nonmonotonic heat release. Stationary detonation models predict [15] that stable propagation of both normal and LVD is possible in such systems. However, our analytical study [16] of the effect of exothermic reactions beyond the Chapman–Jouguet (CJ) point on detonation stability showed that conditions are possible under which the evolution of a secondary compression wave due to afterburning reactions behind the CJ point will lead to the formation of a secondary shock wave that overtakes the LVD front and violates its stability.
A striking example of systems with nonmonotonic heat release are two-phase mixtures of suspensions of aluminum particles in a gas explosive, in which B. Veyssiere observed two-front detonation [17]. In [18], a theoretical stationary model of two-front detonation was proposed, which is in good agreement with the experiment. Later, a nonstationary model of detonation in such hybrid mixtures was developed, which made it possible to visualize the process of the formation of secondary detonation (see Fig. 1) for the case of aluminum particles with a diameter of 13 μm. In the case of Al particles of about 1 μm, the secondary wave quickly overtakes the leading front and eventually leads to the propagation of a normal detonation corresponding to the total heat release [19].
Another example of systems with nonmonotonic heat release is gaseous explosive mixtures of nitromethane with tetranitromethane [20], in which a two-level cellular structure is observed. A numerical example of such a structure is shown in Fig. 2 [21]. Interestingly, in such gas mixtures, the propagation of LVD, which corresponds to the first stage of the reaction, is also possible (Fig. 3). However, the evolution of the secondary compression wave, which is due to the second stage of the reaction, eventually leads to the transition of LVD to normal detonation corresponding to total heat release. If there were sufficiently strong losses in the tube wall, which were not taken into account here, it would be possible to stabilize the LVD. The fundamental work of Prof. Borisov and et al. [22], devoted to the experimental and theoretical study of the detonation of an aluminum suspension in air and oxygen, marked the beginning of a cycle of similar studies in France and Canada (see, for example, [23–25]).
Basic case of two-stage heat release: A → B, B → C considered in [21].
Pressure profiles during weak detonation initiation in a gaseous explosive in a two-stage reaction [21].
A.A. Borisov’s interests were not limited to the study of detonation only in gas and two-phase systems. In 1981, he and his colleagues proposed an informal two-phase viscoplastic model for the initiation of high-density solid explosives [26], which was subsequently included in many gas-dynamic codes for numerical simulation of the detonation of solid explosives. The main advantage of this model is that, in accordance with the experimental data, it takes into account the fact that the microstructure of explosives in different ways affects their sensitivity to ignition and to the combustion process, which is illustrated in detail in the review [27]. In addition, in the review [28], based on the viscoplastic model of hot spot formation [26], an explanation was given for the whole complex of experimental data on the reversal of the shock-wave sensitivity of solid explosives, according to which, at high pressures, a fine-grained explosive was more sensitive than a coarse-dispersed one, and at at low pressures of the shock wave, the situation was reversed.
USE OF THE DETONATION MODES IN AIRCRAFT AND ROCKET ENGINES
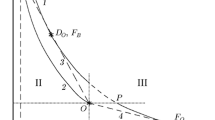
In [29], a comparative analysis of the thermodynamic cycles of power plants operating on combustion (cycles with combustion at a constant pressure P = const and at constant volume V = const) and detonation (Zel’dovich cycle) of the fuel. Ethylene was considered as a fuel simulating kerosene with a ratio of hydrogen and carbon atoms close to 2. Figure 4 shows the calculated dependences of the coefficient of thermodynamic efficiency (CTE) of cycles, \(\eta ,\) on the degree \(\pi \,\,( \geqslant {\kern 1pt} 1)\) of the precompression of the air-fuel mixture. The maximum thermodynamic efficiency of converting the chemical energy of the fuel into useful work is achieved in the Zel’dovich cycle; moreover, the efficiency of the cycle with combustion at a constant volume V = const is a few percent lower, and the efficiency of the combustion cycle at constant pressure P = const is significantly lower, especially for small values of \(\pi .\) Preliminary compression of the mixture increases the efficiency of all the considered cycles, but the Zel’dovich cycle remains the most energy-efficient even at very high values of \(\pi .\)
The advantages of the Zel’dovich cycle can be demonstrated by the following example. Let us imagine a power machine in which a cycle with fuel combustion is implemented at P = const with \(\pi ~\) = 50 (dot 1 in Fig. 4). The transition to the Zel’dovich cycle, other things being equal (transition from point 1 to 2) would increase the efficiency from 54 to 62%; i.e., the work done would increase by 15%. Transition to the Zel’dovich cycle while maintaining the work done (transition from point 1 to 3) would allow reducing the compression ratio from 50 to 20, i.e., by a factor of 2.5. The conclusions made in [29] have already found experimental confirmation in tests of models of liquid rocket engines [30], afterburners [31], and ramjets [32].
MATHEMATICAL MODELING OF AIR SHOCK WAVE PARAMETERS
Mathematical modeling of the parameters of air shock waves generated during explosive processes is an important scientific and practical task. The practical significance of these works is obvious: it is necessary to accurately assess the consequences of air explosions (hereinafter, an explosion means both detonation and various deflagration modes, including turbulent and diffusive ones). From a scientific point of view, this problem is a complex problem, including, in the general case, a mathematical description of a turbulent heterogeneous flow of a compressible multicomponent chemically reacting radiating medium.
For the correct modeling of pressure waves generated in air, three main factors must be correctly taken into account:
– the amount of released energy;
– the rate of energy release;
– the spatial distribution of fuel in the course of the energy release.
Depending on the combination of these factors, the formation of waves in a wide range of parameters is possible. In the simplest case (explosions of condensed explosives, expansion of a compactly compressed gas), to describe wave parameters, it is sufficient to know only the amount of released energy [33, 34]. If it is possible to implement various modes of energy release in the system, for example, detonation or deflagration, it is necessary to take into account the second of the listed factors, the rate of energy release (detonation propagation velocity or, accordingly, deflagration) [35]: during FAM detonation, shock waves with a higher pressure at the front are generated than during deflagration; however, during deflagration, the duration of the compression phase is longer.
Finally, the third factor is significant in two aspects of its influence on the parameters of air mixtures: firstly, the initial dimensions of the areas occupied by FAM determine the nature of product unloading and, accordingly, the parameters of pressure waves; and secondly, the motion of unreacted fuel (especially in the presence of a condensed phase) and its gradual mixing with air can lead to an increase in shock waves during afterburning.
In the works of Prof. Borisov and colleagues, mathematical modeling of the parameters of pressure waves occupies a large place. It was carried out mainly for the air–gas, air–condensed suspension, and air–dust systems, including solid oxidizer dusts.
In the activities of Prof. Borisov on the theoretical consideration of a set of issues related to modeling air waves from explosions, two stages can be conventionally distinguished:
– at the first stage (until the mid-1990s), explosions of spherical clouds were considered and the wave parameters were described either by simple parametric formulas or were determined based on the solution of one-dimensional problems;
– at the second stage, explosions of complex heterogeneous systems with an extended time of energy release were considered; the problems were solved in a multidimensional setting.
The early works of A.A. Borisov [36, 37] showed that the use of the TNT method (for example, in the version presented in [33]) for estimating the parameters of shock waves from detonating gas clouds is unacceptable and erroneous. This is illustrated in Fig. 5, which shows the dependence of the excess pressure in the shock wave front on the distance during the detonation of a spherical hydrogen-oxygen cloud of a stoichiometric composition (data from [38, 39]). The same figure shows data on the parameters of shock waves from an explosion of an equivalent charge of TNT [33]. It can be seen that there is a noticeable overestimation of the pressure when using the TNT method.
Dependence of excess pressure on distance during detonation of a stoichiometric hydrogen–oxygen mixture in a spherical cloud with a radius of 1.5 m in a free volume. FAM detonation (experiment), approximation of experimental data from works [37, 38]; FAM detonation (calculation), calculation [35]; condensed explosive detonation, dependence for TNT equivalent charge explosion [33].
To replace the formulas for calculating the TNT equivalent, parametric relationships were proposed to estimate the parameters of pressure waves both in the compression phase [36] and in the rarefaction phase [37]. Figure 5 shows the calculation results for one of these dependences [35]. As can be seen from this figure, this approach is in much better agreement with the observed experimental data. These ratios continue to be used to this day [40]. An example of such use is shown in Fig. 6, where the dependences of the dimensionless pressure (ΔP/P0, where P0 is the initial pressure in the medium through which the wave propagates) on the dimensionless distance \({r \mathord{\left/ {\vphantom {r {{{{\left( {{E \mathord{\left/ {\vphantom {E {{{P}_{0}}}}} \right. \kern-0em} {{{P}_{0}}}}} \right)}}^{{1{\text{/}}3}}}}}} \right. \kern-0em} {{{{\left( {{E \mathord{\left/ {\vphantom {E {{{P}_{0}}}}} \right. \kern-0em} {{{P}_{0}}}}} \right)}}^{{1{\text{/}}3}}}}},\) where E is the amount of released energy) at various burning rates, from 50 m/s to detonation, are shown.
At the same time, approaches were developed for the computer simulation of the propagation of pressure waves during combustion and detonation of gas clouds [41–43]. In [43], a method was proposed for calculating the parameters of pressure waves during the combustion of spherical clouds at any speed, from low-velocity deflagrations to detonation. In [42], in particular, it was shown that during the detonation of overenriched FAM clouds of fuel assemblies above the surface, hydrodynamic instability develops at the lower edge of the cloud due to the interaction of hot products with a shock wave reflected from the surface. This leads to the admixture of air into the products, afterburning of the detonation products, and additional energy release, which as a result enhances the air shock wave.
In the 1990s and 2000s, a scientific group led by Prof. Borisov intensively carried out mathematical modeling of flows and reacting two-phase mixtures, both premixed flows and flows injected into air that reacted as they mixed with air. The densities of such mixtures were considered in the range of 2 to 100 kg/m3, which is much less than the density of mixtures based on condensed explosives, but more than the usual densities of FAM. Since at such densities the air will not be sufficient for complete oxidation, the system included a solid oxidizer in the form of particles of ammonium nitrate and ammonium perchlorate. Aluminum was considered as a fuel component of such systems. The characteristics of pressure waves generated during explosions of both spherical charges and mixtures injected into the air were studied, and the energy equivalents of such explosions were estimated [44–48]. It was shown that the spatial expansion of the products and their afterburning when air was added at a greater distance from the epicenter of the explosion contributed to the generation of more powerful waves [48–51]. Figure 7 shows the calculated dependences of the overpressure on the distance for various variants of the explosion of heterogeneous charges weighing 800 g [51]. For comparison, a line with a drop in pressure in the shock wave from an equivalent charge of TNT is shown (line 1 in Fig. 7). Figure 7 shows that in the case of a compact reaction of a heterogeneous charge containing large particles (slow combustion), the parameters of the shock wave are close to the level of pressures created by the explosion of a TNT charge (lines 1 and 4 in Fig. 7). In the case of smaller particles, the reaction in the expanding cloud is faster, resulting in higher pressures compared to the explosion of TNT (lines 1 and 2 in Fig. 7). Finally, even higher pressures are achieved when the fuel component burns out at some distance from the initial location of the charge when air is mixed into the products (lines 1 and 3 in Fig, 7).
Calculated pressure at the front of the blast wave for an explosion of a charge weighing 800 g, depending on the distance: 1, TNT, calculation according to the Sadovsky formula; 2, a mixture of ammonium nitrate: aluminum composition 50 : 50, particle size of both components, 1 µm (fast burning, burning time 0.5 ms), turbulent mixing is not taken into account; 3, the conditions are the same as in calculation 2 except that turbulent mixing is turned on; 4, aluminum particles with a diameter of 3 µm, ammonium perchlorate particles with a diameter of 10 µm (slow combustion).
The action of shock waves was modeled separately, in particular, the rise of dust during the passage of a shock wave over its layer [52]. The emissivity of clouds of high-temperature products formed during the explosion of heterogeneous mixtures was also studied [53].
Models of heterogeneous reacting flows developed for open spaces were also used in solving problems in a cluttered space, and in particular, in developing issues related to pulsed detonation engines. The work of Borisov et al. [29] showed the advantages of the detonation thermodynamic cycle in terms of its efficiency, taking into account the actual composition of the products. In relation to such promising use of the detonation cycle in real structures, a number of problems were solved for modeling processes in the corresponding installations. It was shown by calculation that with the multipoint injection of fuel into a tube simulating a real design, good combustion completeness is achieved in an acceptable time frame, which makes it possible to create the necessary thrust force [54–57].
SELF-IGNITION AND DETONATION PROCESSES OF GAS AND HETEROGENEOUS SYSTEMS IN VARIOUS FLOWS
The ignition delay method should be regarded as very useful in kinetic measurements. In the laboratory of “explosive processes in gases and two-phase media,” under the guidance of Prof. Borisov, a technique was developed for estimating the critical energy of detonation initiation in the free volume of the reacting gas from ignition delays [58]. Subsequently, a series of experimental and theoretical studies of the kinetics of the self-ignition process of various fuels was carried out. Much attention was paid to studying the effect of small active additives on the ignition of fuels [59–66]. Such additives (promoters), decomposing during the reaction, give active radicals that accelerate self-ignition. For the first time, analytical methods were used to study the process of fuel ignition on the example of model systems that include the main stages of the chain reaction mechanism: nucleation, branching, continuation, chain termination, and promoter decomposition, taking into account the heat release of the reaction. The results of the studies showed that, contrary to the popular notion that the faster the promoter decomposes during the reaction the more efficiently it accelerates the ignition of the fuel, it was proved that, in fact, the efficiency of the promoter depends on the ratio of the rate constants of the elementary stages. In other words, for various fuels, there may be promoters with the optimal decomposition rate [58] that provide the maximum reduction in the ignition delay period. An analytical study of the kinetics of chain-thermal ignition makes it possible to obtain the general patterns of the process and, thereby, makes it possible to target the search for the optimal promoters.
However, to solve specific practical problems, reliable detailed kinetic mechanisms are needed. Based on the detailed oxidation mechanisms of the simplest propellants H2 and CO, Borisov et al. proposed a kinetic scheme for the ignition and combustion of hydrocarbons from C1 to C3 [67, 68]. A feature of the proposed kinetic mechanism is that it describes not only the initial stage of combustion in the form of an ignition delay but also a later stage of burnout, at which the formation of combustion products occurs. Some calculation results are shown in Fig. 8. Such a mechanism gives an idea of the time of the energy release in the process of combustion of gases.
Reliable information about the kinetics of the heat release of a chemical reaction is extremely important in solving problems related to the propagation of detonation waves, both in gaseous and heterogeneous media. Under the guidance of Prof. Borisov, studies have been carried out that make it possible to apply the results of the one-dimensional modeling of detonation waves with the global kinetics of chemical reactions to the solution of some practical problems [68].
In contrast to gas mixtures, the kinetics of energy release in heterogeneous mixtures cannot be limited by the use of well-defined kinetic mechanisms containing elementary steps, since it depends on many physical and chemical phenomena, the influence of which cannot be neglected. For this reason, empirical expressions that determine the global rate of heat release in such mixtures are extremely useful [41]. Based on the analysis of the physical processes occurring in heterogeneous reacting media, semiempirical relations were obtained for estimating the value of ignition delays in fuel-air mixtures with both solid and liquid particles, and global relations were proposed for determining the rate of energy release in aerosols [41, 68].
In the 1950s and 1960s, it became known that in shock tubes and rapid compression devices intended for kinetic studies, ignition is of a focal nature; i.e., ignition always begins in separate centers and only then does the combustion wave cover the entire volume. In his dissertation [1], Prof. Borisov showed that the occurrence of ignition centers (hot spots) long before the ignition of the entire volume is most likely related not to the peculiarities of the course of the exothermic reaction itself but with the peculiarities of the method of heating the mixture to a temperature at which the initiation reaction begins to noticeably proceed. This confirms the point of view about the gas-dynamic nature of the formation of early foci, for which the ignition delay is somewhat lower than its average value.
Later, in the experimental studies carried out under the guidance of Prof. Borisov, the focal nature of the ignition of hydrocarbons was demonstrated at low temperatures in static installations. Figure 9 shows high-speed video footage that clearly demonstrates the initiation of ignition centers and the development of a flame front in a mixture of pentane and air of a stechnometric composition.
Further experimental studies of the ignition of reacting gases in vortex flows confirmed the assumptions of A.A. Borisov expressed in his dissertation work [1]. He developed and created under his leadership an installation with a tangential inlet of a combustible gas mixture into a heated and evacuated reactor. This ensured the rotation of the gas in the reactor. The experiments showed that in the center of the vortex, due to the emergence of centripetal forces, the lightest ones are concentrated, i.e. the hottest portions of the gas. This is the reason for the formation of hot spots in real devices, in which the ignition delay time is traditionally measured [69–72]. The phenomenon of self-ignition of a reacting gas in a vortex flow must be taken into account when creating safe operating conditions for technical devices in which the formation of vortices of combustible gas mixtures is possible. Figures 10 and 11 show high-speed video footage from the autoignition of a rotating reactant gas. We note that the temperature of the reactor is much lower than the ignition temperature of the fuel.
In the last years of his life, Professor A.A. Borisov showed great interest in the nonclassical little-studied field of combustion science. Under his leadership, the combustion of superrich air and oxygen mixtures of methane and the associated petroleum gases was studied [73, 74]. The term superrich means that the fuel content in such mixtures exceeds the upper flammable limit under normal conditions. Such mixtures are capable of burning only under conditions of elevated pressures and temperatures. This is an understudied area of combustion, since such combustion modes have not been used until recently.
The process of noncatalytic partial oxidation of light hydrocarbons in combustion modes is a promising direction in the development of gas chemical technologies, the production of liquid hydrocarbon fuels and other petrochemical products, and methods for producing hydrogen for developing hydrogen energy. However, for the development of a noncatalytic method for producing synthesis gas, it is necessary to solve a number of scientific and technological problems.
Under the guidance of Professor Borisov, in installations of constant volume, adiabatic compression, and a flow reactor based on rocket technologies, the production of synthesis gas during the combustion of superrich methane-air and methane-oxygen mixtures was experimentally studied. Various ways of igniting these mixtures are considered, flame propagation velocities are measured, and the composition of the products of the process is studied. The influence of the combustion process modes on the yield of the condensed product (soot) has been studied and its properties have been investigated. Ways have been found to minimize the soot output. The experimental data obtained during the combustion of superrich mixtures in various devices are compared. It is shown that the main regularities established in a reactor of one type can be used to organize the process in other reactors. The results of these studies are the scientific base for the development of technology for the production of synthesis gas in noncatalytic combustion modes.
STUDY OF CHEMICAL TRANSFORMATIONS AND THEIR ROLE IN PROMOTING THE SELF-IGNITION OF PERSPECTIVE FUELS
Measuring the values of the ignition delay makes it possible to obtain only the total kinetic characteristics, by which we can qualitatively judge the mechanism and rates of chemical reactions [75]; however, with a sufficiently well-studied kinetic mechanism, from the measured temperature dependences of the ignition delay, we can determine the temperature dependence of the rate constants of the leading reactions or their ratio. The dissociation rate constant of molecular chlorine was measured in this way [75, 76]. Direct spectroscopic measurements of NO2, carried out in [77, 78], made it possible to determine the value of the rate constant for the decomposition of nitromethane, CH3NO2 → CH3 + NO2, which was confirmed by recent measurements [79] and theoretical calculations [80]. The values of the decay rate constant of N2O turned out to be so reliable that they were completely confirmed by more laborious and expensive measurement techniques [83, 84]. Using the decay of N2O as a source of oxygen atoms, the authors of [85] measured the rate constant k1 of the key ignition reaction of hydrocarbons:
All subsequent measurements by various methods of the rate constant of reaction (1) confirmed the accuracy of the value determined in [85] of k1 [86]. Review [65] formulated the main concepts of the mechanism of self-ignition and promotion of perspective fuels. Based on their own research and extensive data from the literature, the authors of [65] consider an important branch of the science of combustion, namely, modeling the complex set of phenomena occurring in a non-isothermal and non-adiabatic reacting media. It is shown that based on mathematical modeling it is possible to successfully solve a whole range of scientific and technical problems. These include the optimization of combustion conditions in order to minimize the emission of environmentally dangerous substances, develop conditions for the economically profitable conversion of fuel into combustion products, and increase their efficiency. The methodology presented in [65] makes it possible not only to study the kinetics of chemical reactions but also to determine their role in studying and predicting the combustion processes of various promising fuels. The approaches developed based on this methodology made it possible to determine the contributions of heat release from various elementary reactions to the total rate of heat release during the flame propagation of hydrogen–air mixtures [87].
In [88], small additions of propylene and isopropyl alcohol were studied on the flame propagation velocity of hydrogen–air mixtures in the predetonation mode, on the transition from deflagration to detonation, and on the intensity of combustion. It is shown that the difference in the effectiveness of these additives on combustion is determined primarily by their ability to terminate the reaction chains. Flame consumption of additives H2 occurs practically only as a result of their reactions with active intermediate products of hydrogen combustion, in which these particles are replaced by low-active radicals. In [89], the promoting effect of a small addition of molybdenum hexacarbonyl on the self-ignition of a hydrogen-air mixture was experimentally studied. It was shown that, at temperatures below 1000 K, the molybdenum atoms formed during the rapid thermal decomposition of Mo(CO)6, by interacting with O2 to form oxygen atoms, significantly reduced the period of the observed ignition delay.
CONCLUSIONS
The long-term work of the team under the guidance of Professor A.A. Borisov and his closest colleagues have shown that based on scientific experiments, supplemented by mathematical modeling, it is possible to successfully solve a number of fundamental, scientific, and technical problems. In particular, it is shown that in the existing theoretical models of detonation propagation limits, the independence of concentrations at the limit on the tube diameter is not confirmed; therefore, their addition is necessary. Based on the experiments and analysis carried out, it was concluded that, at least for a limited range of the change in the initiation energy and tube diameter, we can consider detonation limits as a characteristic of the mixture alone. It has also been established that the limit is always preceded by an area of unstable modes with a pulsating velocity.
These studies are closely related to those formulated under the guidance of A.A. Borisov, based on his own research and extensive data from the literature, the basic concepts of the mechanism of self-ignition, and promotion of various fuels. An important section of the science of combustion is considered, namely, the modeling of a complex set of phenomena occurring in a nonisothermally and nonadiabatically reactive medium.
In the work done under the guidance of Professor A.A. Borisov, experimental confirmation of the focal nature of the ignition of hydrocarbons at low temperatures in static installations is important. In the works of Professor A.A. Borisov and his colleagues, mathematical modeling of the parameters of pressure waves plays a significant role. Mathematical modeling was carried out mainly for the air–gas, air–condensed suspension, and air–dust systems, including solid oxidizer sprays. The correction of the calculation of TNT equivalent was substantiated using the proposed parametric relationships for estimating the parameters of pressure waves both in the compression phase and in the rarefaction phase.
The contribution made by A.A. Borisov and his colleagues in the study of the effect of reactions occurring behind the LVD front on its stability cannot be denied. Based on the studies carried out, a theoretical stationary model of two-front detonation is proposed, which is in good agreement with the experiment.
REFERENCES
A. A. Borisov, Doctoral (Phys. Math.) Dissertation (Inst. Chem. Phys. Acad. Sci. USSR, Moscow, 1970).
A. A. Borisov, S. M. Kogarko, and A. V. Lyubimov, Zh. Prikl. Mekh. Tekh. Fiz., No. 3, 175 (1960).
A. A. Borisov, I. S. Zaslonko, and S. M. Kogarko, Zh. Prikl. Mekh. Tekh. Fiz., No. 6, 104 (1964).
A. A. Borisov, B. E. Gel’fand, S. A. Loban’, A. E. Mailkov, and S. V. Khomik, Khim. Fiz. 1, 848 (1982).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, USSR Inventor’s Certificate No. 1496469 (1989).
S. V. Khomik, S. P. Medvedev, A. A. Borisov, V. N. Mikhalkin, O. G. Maksimova, V. A. Petukhov, and A. Yu. Dolgoborodov, Russ. J. Phys. Chem. B 10, 298 (2016).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, USSR Inventor’s Certificate No. 1403793 (1988).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, USSR Inventor’s Certificate No. 1396765 (1988).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, Sov. Phys. Dokl. 32, 736 (1987).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, USSR Inventor’s Certificate No. 1522916 (1989).
A. A. Borisov, V. N. Mikhalkin, and S. V. Khomik, Khim. Fiz. 8, 798 (1989).
A. A. Borisov, S. V. Khomik, and V. N. Mikhalkin, Prog. Astronaut. Aeronaut. 133, 118 (1991).
A. A. Borisov, S. V. Khomik, V. N. Mikhalkin, and E. V. Saneev, Prog. Astronaut. Aeronaut. 133, 142 (1991).
A. A. Borisov, in Gaseous and Heterogeneous Detonations: Science to Applications, Ed. by G. D. Roy, S. M. Frolov, K. Kailasanath, and N. N. Smirnov (ENAS, Moscow, 1999), p. 3.
N. M. Kuznetsov, Sov. Phys. JETP 25, 199 (1967).
B. A. Khasainov, B. S. Ermolaev, A. A. Borisov, and A. I. Korotkov, Acta Astronaut. 6, 557 (1979).
B. Veyssiere, Prog. Astronaut. Aeronaut. 106, 522 (1986).
B. A. Khasainov and B. Veyssiere, Arch. Combust. 7, 333 (1987).
B. A. Khasainov, B. Veyssiere, and W. Ingignoli, in High-Speed Deflagration and Detonation. Fundamentals and Control, Ed. by G. Roy, S. Frolov, D. Netzer, and A. Borisov (ELEX-KM, Moscow, 2001), p. 163.
D. Desbordes and H. N. Presles, Shock Wave Science and Technology Reference Library, Vol. 6: Detonation Dynamics, Ed. by F. Zhang (Springer, New York, 2012), p. 281.
B. Khasainov, F. Virot, H.-N. Presles, and D. Desbordes, Shock Waves 23, 213 (2013).
A. A. Borisov, B. A. Khasainov, B. Veiss’er, et al., Khim. Fiz. 10 (1), 369 (1991).
B. Veyssière, B. Khasainov, and A. Briand, Shock Waves 18, 307 (2008).
A. Briand, B. Veyssière, and B. Khasainov, Shock Waves 20, 521 (2010).
B. Khasainov, F. Virot, and B. Veyssière, Shock Waves 23, 271 (2013).
B. A. Khasainov, A. A. Borisov, B. S. Ermolaev, and A. I. Korotkov, in Proceedings of the International Symposium on Detonation, Ed. by J. M. Short (NSWC, Annapolis, MD, 1981), Vol. 7, p. 435.
B. A. Khasainov, A. V. Attetkov, and A. A. Borisov, Khim. Fiz. 15 (7), 53 (1996).
B. A. Khasainov, B. S. Ermolaev, H.-N. Presles, and P. Vidal, Shock Waves 7, 89 (1997).
S. M. Frolov, A. E. Barykin, and A. A. Borisov, Khim. Fiz. 23 (3), 17 (2004).
S. M. Frolov, V. S. Aksenov, P. A. Gusev, V. S. Ivanov, S. N. Medvedev, and I. O. Shamshin, Dokl. Phys. Chem. 459, 207 (2014).
S. M. Frolov, V. S. Ivanov, I. O. Shamshin, V. S. Aksenov, M. Yu. Vovk, I. V. Mokrynskij, V. A. Bruskov, D. V. Igonkin, S. N. Moskvitin, A. A. Illarionov, and E. Yu. Marchukov, Dokl. Phys. 65, 36 (2020).
S. M. Frolov and V. S. Ivanov, Russ. J. Phys. Chem. B 15, 318 (2021).
M. A. Sadovskii, in Physics of the Explosion, Collection of Articles (Akad. Nauk SSSR, Moscow, 1952), No. 1, p. 20 [in Russian].
S. I. Sumskoi, A. S. Sof’in, S. Kh. Zainetdinov, and A. A. Agapov, Russ. J. Phys. Chem. B 14, 625 (2020).
A. A. Agapov, V. S. Safonov, S. I. Sumskoi, and A. A. Shvyryaev, Bezopasn. Truda Prom-ti, No. 5, 36 (2020).
A. A. Borisov, B. E. Gel’fand, and S. A. Tsyganov, Fiz. Goreniya Vzrava 21 (2), 90 (1985).
B. E. Gel’fand, A. A. Borisov, and S. A. Tsyganov, Fiz. Goreniya Vzryva 25 (1), 136 (1989).
S. M. Kogarko, V. V. Adushkin, and A. G. Lyamin, Nauch.-Tekh. Probl. Goreniya Vzryva, No. 2, 22 (1965).
V. V. Adushkin, Yu. A. Gostintsev, and V. E. Fortov, Preprint (Inst. Probl. Chem. Phys. RAS, Chernogolovka, 1995).
A. A. Agapov, A. S. Sof’in, and S. I. Sumskoi, Bezopasn. Truda Prom-ti, No. 4, 27 (2020).
A. A. Borisov, A. P. Kasimov, V. V. Kosenkov, et al., Khim. Fiz. 15 (4), 126 (1996).
A. A. Borisov, B. E. Gel’fand, S. A. Gubin, S. I. Sumskoi, and V. A. Shargatov, Fiz. Goreniya Vzryva 24 (2), 124 (1988).
A. A. Borisov, B. E. Gel’fand, S. A. Gubin, V. V. Odintsov, and V. A. Shargatov, Khim. Fiz. 3 (5), 435 (1986).
A. A. Borisov, S. I. Sumskoi, and I. O. Shamshin, Khim. Fiz. 22 (3), 61 (2003).
A. A. Borisov, P. V. Komissarov, G. N. Sokolov, and G. V. Kaplyukov, in Combustion and Explosion, Ed. by S. M. Frolov (Torus, Moscow, 2010), No. 3, p. 161 [in Russian].
A. A. Borisov, A. A. Sulimov, B. S. Ermolaev, et al., in Combustion and Explosion, Ed. by S. M. Frolov (Torus, Moscow, 2009), p. 78 [in Russian].
P. V. Komissarov, A. A. Borisov, B. A. Khasainov, B. S. Ermolaev, and A. A. Sulimov, Gorenie Vzryv 11 (2), 126 (2018).
I. O. Shamshin, Cand. Sci. (Phys. Math.) Dissertation (MIFI Univ., Moscow, 2003).
A. A. Borisov, S. I. Sumskoi, I. O. Shamshin, et al., Khim. Fiz. 21 (5), 97 (2002).
I. O. Shamshin, in Combustion and Explosion, Ed. by S. M. Frolov (Torus, Moscow, 2010), No. 3, p. 169 [in Russian].
A. A. Borisov, A. A. Sulimov, M. K. Sukoyan, P. V. Komissarov, O. Shamshin, R. Kh. Ibragimov, and Yu. M. Mikhailov, Russ. J. Phys. Chem. B 3, 936 (2009).
A. A. Borisov, P. V. Komissarov, S. I. Sumskoi, et al., Khim. Fiz. 22 (12), 48 (2003).
A. A. Borisov, S. I. Sumskoi, and P. V. Komissarov, Khim. Fiz. 21 (11), 52 (2002).
A. A. Borisov, A. E. Mailkov, S. I. Sumskoi, et al., Khim. Fiz. 22 (6), 87 (2003).
A. A. Borisov, S. I. Sumskoi, A. E. Mailkov, et al., Application of Detonation to Propulsion ICPCD-2004 (Torus, Moscow, 2004) [in Russian].
A. A. Borisov, A. E. Mailkov, S. I. Sumskoi, et al., Khim. Fiz. 22 (8), 68 (2003).
A. E. Mailkov, M. A. Silakova, A. E. Barykin, et al., Khim. Fiz. 24 (2), 68 (2005).
A. A. Borisov, V. M. Zamanskii, V. V. Lisyanskii, G. I. Skachkov, and K. Ya. Troshin, Khim. Fiz. 5, 1683 (1986).
A. A. Borisov, V. M. Zamanskii, V. V. Lisyanskii, G. I. Skachkov, and K. Ya. Troshin, Khim. Fiz. 6, 101 (1987).
A. A. Borisov, V. M. Zamanskii, V. V. Lisyanskii, G. I. Skachkov, and K. Ya. Troshin, Khim. Fiz. 8, 1640 (1989).
A. A. Borisov, V. M. Zamanskii, V. V. Lisyanskii, and K. Ya. Troshin, Khim. Fiz. 11, 1235 (1992).
A. A. Borisov, G. I. Skachkov, K. Ya. Troshin, and V. M. Zamanskii, Khim. Fiz. 15 (8), 54 (1996).
A. A. Borusov, V. V. Lisyanskii, G. I. Skachkov, K. Ya. Troshin, and V. M. Zamanskii, Proc. Symp. Combust. 22, 903 (1989).
A. A. Borisov, G. I. Skachkov, and K. Ya. Troshin, in Proceedings of the International Solloquium on Advanced Somputation Analysis Combustion. ENAS (Torus, Moscow, 1997).
V. M. Zamanskii and A. A. Borisov, Itogi Nauki Tekh., Kinet. Katal. 19, 156 (1989).
A. A. Borisov, G. I. Skachkov, and K. Ya. Troshin, Khim. Fiz. 18 (9), 45 (1999).
A. A. Borisov, V. M. Zamanskii, V. V. Lisyanskii, et al., Khim. Fiz. 7 (5), 665 (1988).
A. A. Borisov, O. I. Mel’nichuk, A. R. Kasimov, et al., J. Phys. IV Coll. C4 Suppl. J. Phys. III 5, C4-129 (1995).
A. A. Borisov, N. M. Rubtsov, G. I. Skachkov, and K. Ya. Troshin, in Combustion and Explosion, Ed. by S. M. Frolov (Torus, Moscow, 2011), No. 1, p. 4 [in Russian].
A. A. Borisov, N. M. Rubtsov, G. I. Skachkov, and K. Ya. Troshin, Russ. J. Phys. Chem. B 6, 517 (2012).
A. A. Borisov, V. A. Smetanyuk, K. Ya. Troshin, and I. O. Shamshin, Gorenie Vzryv 9 (1), 4 (2016).
K. Ya. Troshin, I. O. Shamshin, V. A. Smetanyuk, and A. A. Borisov, Russ. J. Phys. Chem. B 11, 952 (2017).
V. G. Sister, A. A. Borisov, K. Ya. Troshin, et al., Khim. Fiz. 25 (1), 61 (2006).
I. V. Bilera, A. A. Borisov, A. B. Borunova, Yu. A. Kolbanovskii, Yu. M. Korolev, I. V. Rossikhin, and K. Ya. Troshin, Pet. Chem. 50, 338 (2010).
A. A. Borisov, S. M. Kogarko, and G. I. Skachkov, Nauch.-Tekh. Probl. Goreniya Vzryva, No. 1, 15 (1965).
A. A. Borisov, S. M. Kogarko, and G. I. Skachkov, Fiz. Goreniya Vzryva 3, 10 (1965).
A. A. Borisov, C. M. Kogarko, and G. I. Skachkov, Kinet. Katal. 7, 589 (1966).
A. A. Borisov, I. S. Zaslonko, and S. M. Kogarko, Fiz. Goreniya Vzryva, No. 3, 387 (1968).
N. M. Kuznetsov, Yu. P. Petrov, and S. V. Turetskii, Kinet. Catal. 53, 1 (2012).
R. S. Zhu, P. Raghunath, and M. C. Lin, J. Phys. Chem. A 117, 7308 (2013).
A. A. Borisov, Kinet. Katal. 9, 482 (1968).
A. A. Borisov and G. I. Skachkov, Kinet. Katal. 13, 42 (1972).
M. Rohrig, E. L. Petersen, D. F. Davidson, and R. K. Hanson, Int. J. Chem. Kinet. 28, 599 (1996).
S. Javoy, R. Mevel, and C. E. Paillard, Int. J. Chem. Kinet. 41, 357 (2009).
A. A. Borisov, E. V. Dragalova, V. M. Zamanskii, V. V. Lisyanskii, and G. I. Skachkov, Kinet. Katal. 22, 305 (1981).
A. M. Tereza and E. K. Anderzhanov, Russ. J. Phys. Chem. B 13, 626 (2019).
V. V. Azatyan, Z. S. Andrianova, A. A. Borisov, and A. N. Ivanova, Kinet. Catal. 53, 641 (2012).
V. V. Azatyan, A. A. Borisov, A. G. Merzhanov, et al., Fiz. Goreniya Vzryva 41 (1), 3 (2005).
P. A. Vlasov, V. N. Smirnov, and A. M. Tereza, Russ. J. Phys. Chem. B 10, 456 (2016).
ACKNOWLEDGMENTS
The authors express their sincere gratitude to S.V. Khomik for his help in preparing the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the Memory of Professor A.A. Borisov
Rights and permissions
About this article
Cite this article
Mikhalkin, V.N., Sumskoi, S.I., Tereza, A.M. et al. Ignition, Combustion, and Detonation of Gas-Phase and Heterogeneous Mixtures (Review). Russ. J. Phys. Chem. B 16, 629–641 (2022). https://doi.org/10.1134/S1990793122040261
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122040261