Abstract
The energy capabilities of five real-life high-enthalpy derivatives of 1,2,4,5-tetrazine N-oxides as components composite solid rocket propellants (CSRPs), which do not contain metallic fuel, are considered. Various ways of reducing the combustion temperature of CSRPs to an acceptable value, if necessary, are studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
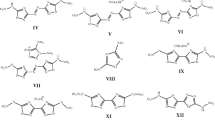
The aim of this study is to study the possibility of using some derivatives of 1,2,4,5-tetrazine N-oxides (I–V [1–6], Fig. 1) as components of composite solid rocket propellants (CSRPs). All five compounds have actually been synthesized and their structures have been proven by various methods, including X-ray diffraction analysis.
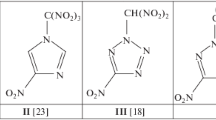
Structural formulas of compounds I–V: I, 6-aminotetrazolo[1,5-b][1,2,4,5]tetrazine2,5-dioxide [1]; II, 3,6-diazido-1,2,4,5-tetrazine 1,4-dioxide [1]; III, 6-aminotetrazolo[1,5-b][1,2,4,5]tetrazine 5-oxide [2]; IV, 3-amino-6-nitro-1,2,4,5-tetrazine 1,5-dioxide [3–5]; V, 6-amino-3-nitro[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazine 7-oxide [6].
1,2,4,5-Tetrazine N-oxides (s-tetrazine N-oxides) are a relatively poorly studied subclass of high energy heterocycles [1–13], the first representatives of which were described in 1993 [3]. Over the past 5–6 years, the intensity of works in this direction has sharply increased: during this period, eight articles were published, including 4 in 2019.
The main interest in s-tetrazine N-oxides is related to the prospect of their use as explosives. This is facilitated by the reduced proportion of carbon and hydrogen due to the high proportion of nitrogen and oxygen, high enthalpies of formation, and indicators α (the coefficient of providing the molecule with oxygen) higher than s-tetrazine. s-Tetrazine N-oxides were not previously considered as potential components of CSRPs.
The obvious gain in the elemental composition during the oxidation of s-tetrazines into mono- and di-N-oxides is accompanied by an additional and completely unexpected bonus: a decrease in the sensitivity of their azide derivatives (or tautomeric bicyclic tetrazolo[1,5-b][1,2,4,5]tetrazines) to mechanical stress. Thus, the oxidation of 3-amino-6-azido-1,2,4,5-tetrazine (VI-a), which exists in the crystal in the bicyclic form VI-b, into compound III (reaction (1)) leads to a sharp decrease in impact sensitivity (IS) from 1.5 to 10 J [2].

The deeper oxidation of compound III by the powerful oxidizing agent HOF goes through the tetrazole cycle with the formation of compound I (the last stage of reaction (1)), the IS = 6 J [1] of which, although it increased in comparison with compound III (IS = 10 J [1]), decreased in comparison with compound VI by a factor of four. It is curious that oxidation in the tetrazole cycle ends, for compound I, the possibility of reverse azido-tetrazole tautomerism, an example of which is the interconversion of tautomers VI-a and VI-b (reaction (1)).
A similar decrease in IS was observed upon the oxidation of 3,6-diazido-1,2,4,5-tetrazine (VII) (IS < 1 J [1]), which is extremely sensitive to impact, friction, and electrostatic sparks [14], to compound II (IS = 1.5 J [1]) (reaction (2)). Molecules II and VII in crystals have the azide form [1, 13].

For completeness, we added compounds IV [3–5] and V [6] to compounds I–III. Compound IV has a higher value α (0.8), since there is a nitro group in its molecule and not one but two nitrogen atoms in the cycle are oxidized. For the first time, compound IV was synthesized with a yield of 7% in 1993 by oxidizing of 3,6-diamino-1,2,4,5-tetrazine (VIII) with trifluoroperacetic acid [3]. Later, the yield was increased to 50% by taking 3-amino-6-nitro-1,2,4,5-tetrazine (IX) (reaction (3)) [5].

When using a stronger reagent (HOF) to oxidize compound VIII a mixture of compound IV with its structural isomer, 3-amino-6-nitro-1,2,4,5-tetrazine 1,4-dioxide (X) (70 : 30), was obtained, and the oxidation of the other starting compound, 3,6-diamino-1,2,4,5-tetrazine 1-oxide (XI), gave an equal ratio of isomers IV and X [4] (reaction (4)).

The authors of [4] managed to isolate the minor isomer X using column chromatography (yields not shown) and even determine its structure. The density of compound X turned out to be higher compared to compound IV (1.972 and 1.919 g/cm3, respectively). Judging by the rather high melting point with decomposition (168°C at a heating rate of 2°C/min [4]), compound X surpasses compound IV also in terms of thermal resistance (the decomposition temperature was 110°C according to the DSC data [3, 5]). Probably due to low yield and small amount of compound X extracted, we failed to measure its sensitivity to mechanical stress. There is not even a calculated enthalpy \(\Delta H_{f}^{^\circ }\) of compound X or calculated detonation parameters in the literature. In this study for calculating the energy of CSRP compositions with compound X, its \(\Delta H_{f}^{^\circ }\) was accepted by us as equal to \(\Delta H_{f}^{^\circ }\) of compound IV [5]. Our immediate plans include the task of calculating \(\Delta H_{f}^{^\circ }\) of this extremely interesting compound by modern quantum chemical methods in the same basis with isomer IV and some other similar structures for a correct comparison of the efficiency of s‑tetrazine N-oxides as potential components of CSRP.
Compound V appears similar to I, formally molecule V is obtained by replacing the grouping N → O in the azole ring of molecule I on C–NO2. The calculated enthalpy \(\Delta H_{f}^{^\circ }\) of compounds V (744 kJ/mol [6]) raises some doubts in us (it seems overstated), since it is significantly higher than that of compound I (576 kJ/mol [1]), and tetrazoles have higher enthalpy heterocycles than triazoles. Perhaps this is due to the different calculation methods in [1] and [6]. Nevertheless, for the thermodynamic calculations, we used these published data on \(\Delta H_{f}^{^\circ }\) [1, 6] (Table 1), but in the future we plan to recalculate \(\Delta H_{f}^{^\circ }\) of compounds I and V together with compounds IV and X on the same basis and it is more correct to compare these four potential components of CSRP.
The properties of compounds I—V and X are presented in Table 1.
FORMULATION OF THE PROBLEM AND METHOD OF THE CALCULATION STUDIES
One of the two typical binders was taken to assemble the CSRP compositions: (i) a hydrocarbon binder (HB) (C72.15H119.21O0.68; standard enthalpy of formation \(\Delta H_{f}^{^\circ }\) = –393 kJ/kg; and density ρ = 0.92 g/cm3 [15]); and (ii) an active binder (AB) (C18.96H34.64N19.16O29.32; \(\Delta H_{f}^{^\circ }\) = –757 kJ/kg; and ρ = 1.49 g/cm3) [15].
We studied the energy characteristics of not only binary compositions of CSRP (a binder and one of the investigated compounds I—V) but also more complex compositions containing an additional oxidizing agent: ammonium perchlorate (AP) (NH4ClO4; \(\Delta H_{f}^{^\circ }\) = –2495 kJ/kg; ρ = 1.95 g/cm3; α = 2.25) or HMX (\(\Delta H_{f}^{^\circ }\) = 295 kJ/kg; ρ = 1.9 g/cm3; α = 0.67). We also considered the possibility of increasing the energy of the composition through the use of a mixed binder AB + HB at various AB : HB ratios.
Binary CSRPs based on the oxidizing agents AP and ADN (ammonium dinitramide, NH4N3O4, ADN, \(\Delta H_{f}^{^\circ }\) = –1129 kJ/kg; ρ = 1.82 g/cm3; α = 2.0 [16]) were selected as reference comparison compositions. It should be noted that due to the qualitative difference in the values α (2.00 versus 0.667) in the case of ADN, the most energy-intensive formulations are provided with the HB, while in the case of HMX, with the AB [17].
Specific impulse Isp calculations and temperatures in the combustion chamber Tc (at a pressure in the chamber and at the nozzle exit of 4.0 and 0.1 MPa, respectively) was carried out using the TEPPA code for calculating high-temperature chemical equilibriums [18]. The effectiveness of the components under study was analyzed according to the algorithm described in [19–22]. To compare the ballistic efficiency of compositions with different densities, when they are used in engines with different volume-mass characteristics, we used the so-called effective impulse values Ief (n) at different stages of rocket systems (n is the step number) [23].
These values characterize the ballistic efficiency of the fuel at the corresponding stages of the three-stage rocket systems.
To ensure satisfactory physical and mechanical characteristics of CSRP and the rheological properties of the uncured fuel mass, the compositions must contain a sufficient amount of polymer binder, which is usually achieved with a volume content of the binder of at least 18–19 vol %. For a correct comparison, all CSRP compositions considered in this study have approximately the same volume fraction of the binder, 18.0 ± 0.1 vol %.
RESULTS AND DISCUSSION
Binary Formulations: Test Compound + Binder (AB or HB)
Compounds I—III and V have low coefficients for providing the molecule with oxygen (α from 0.2 to 0.5); therefore, they are better combined with the AB. Compound IV and its isomer X (α = 0.8) can be combined with both the AB and HB [24]. Since it was shown in [17] that with increasing \(\Delta H_{f}^{^\circ }\) of the oxidizing agent, the advantage of HB over AB is increasingly manifest, and compound II with a very high enthalpy of formation (\(\Delta H_{f}^{^\circ }\) = 4935 kJ/kg) can also be tried in compositions with HB too. It is not excluded that a certain optimum is also possible in compositions with a binder, which is a mixture of the AB with the HB due to the higher hydrogen content in the HB than in the AB [24]. The calculated characteristics of the binary compositions are presented in Table 2. For comparison, there are also parameters of the binary compositions: HMX + AB and ADN + HB.
From Table 2 it can be seen that in binary compositions with AB compounds II and V significantly outperform HMX (by 19.1 and 10.9 s, respectively) in terms of the values of the specific impulses Isp; and in terms of the effective impulse, Ief (3), the superiority is about the same (at 19.5 and 10.4 s, respectively). Compounds IV and X with the AB are slightly better than HMX (by Isp, both by 0.8 s; and for Ief (3), by 1.5 and 2.6 s, respectively). Compounds IV and X have identical elements content (structural isomers) and \(\Delta H_{f}^{^\circ }\) (our assumption), but compound X has a higher density (1.972 g/cm3) than compound IV (1.919 g/cm3). The superiority of compound X in terms of Ief (3) over compound IV (1.1 s) demonstrates the influence of the density of the main component on the ballistic efficiency of CSRP compositions, even for the third stage of the rocket complex. In the first and second stages, this advantage would be even higher. Compounds I and III with the AB (not to mention the HB) did not show a good result, which is explained by the lower values of coefficient α (0.4 and 0.2, respectively). It should be noted that compounds with low α (below 0.5) are impractical to use as independent oxidants, and even in binary compositions with the AB, they require the introduction of an additional oxidizing agent, for example, AP [25].
Compound II in the composition with the HB is superior to the ADN + HB composition in terms of Isp and Ief (3) by 1.5 and 0.5 s, respectively, although α in compound II is significantly lower than that of ADN (0.5 versus 2). This is the result of the extremely high difference between the enthalpies of formation of compounds II and ADN. However, the binary II + HB composition still performs significantly (by ~20 s) worse than the II + AB composition; i.e., even such a high \(\Delta H_{f}^{^\circ }\) (4935 kJ/kg) does not allow the component with α = 0.5 to become more efficient with the HB than with the AB. Compositions II + AB with the maximum values Isp and Ief (3) with the AB content of about 18 vol % have an unacceptably high Tc (~4050 K).
The layout of compound IV with the HB did not give good results, which is to be expected from a component with α = 0.8 and value \(\Delta H_{f}^{^\circ }\) at the level of 1300 kJ/kg. Thus, in binary compositions, the best result is demonstrated by compound II paired with the AB, which is not surprising for a compound with value \(\Delta H_{f}^{^\circ }\) of about 5000 kJ/kg.
CSRP Compositions Based on Compound II and Possible Ways of Reducing the Temperature in the Combustion Chamber (Tc) to Technologically Permissible Values (3700–3800 K)
Separately, we should dwell on compound II and compositions based on it. As noted above, the binary composition II + AB at 18 vol % of the binder showed very high energy indicators; however, the temperature in the combustion chamber at the same time reached an unacceptably high value (4047 K, Table 2). Therefore, it is necessary to reduce Tc, moving it to technologically permissible values (3700–3800 K), since it is almost impossible to find structural materials for manufacturing a combustion chamber and a jet nozzle at Tc > 3800 K. Tc can be decreased in different ways.
First, in binary composition II + AB, it is possible to increase the AB content in excess of the required 18% by volume by reducing the proportion of compound II (method A), but at the same time with decreasing Tc (Table 3) to acceptable values (3700–3800 K), the values of the target parameters Isp and Ief also decrease (3). Nonetheless, at the same time, the values of Isp and Ief (3) remain very high (263.4–265.6 s for Isp and 264.7–267.5 s for Ief (3)). To bring down Tc to the maximum permissible value 3800 K, the mass fraction of the AB should be increased to 25.25% (74.75% II + 25.25% AB), which corresponds to the volume fraction of the binder in 30.1 vol %, and values Isp and Ief (3) will decrease to 265.6 and 267.5 s, respectively. To further reduce Tc and drop it up to 3700 K, the mass fraction of the AB should be increased to 30.7%. In the composition 69.3% II + 30.7% AB, the value of Isp and Ief (3) will already be 263.4 and 264.7 s, and the volume fraction of the binder will exceed the minimum allowable value of 18% by an even greater margin to reach 36.1 vol % (Table 3), which should have a positive effect on improving the rheological parameters of the uncured fuel mass.
Second, paired with compound II, we can use a mixed AB + HB binder at a constant volumetric content (18 vol %) (method B). Partial replacement of AB with HB allows us to reduce Tc to 3800 K at the ratio of AB : HB = 2.4 : 1 at the cost of decreasing values Isp and Ief (3) to 263.1 and 265.2 s, respectively. The same Tc is reduced to 3700 K with an even lower mass fraction of АB (АB : HB = 1.4 : 1), and the values Isp and Ief (3) decrease to 260.4 and 262.2 s, respectively. Nevertheless, even these values are rather high for CSRP compositions without metal (Table 4).
Third, an additional low-enthalpy oxidant, AP, can be added to the composition due to compound II, keeping the volumetric content of the AB at the level of 18 vol % (method C). In principle, it is possible to use the addition of the oxidizing agent ADN to reduce Tc, but in [26] it was shown that the addition of AP reduces Tc most efficiently. Thus, for the composition 74.75% II + 14.65% AB + 10.6% AP, Tc = 3800 K, and this is a permitted value. At the same time Isp = 266.4 s and Ief (3) = 269.7 s. The introduction of 16.9% AP, while maintaining 18.0 vol % of the binder, lowers the temperature to 3700 K, further decreasing the values Isp and Ief (3) to 264.0 s and 267.3 s, respectively (Table 5).
Thus, comparing compositions based on II, reduced to the same permissible temperatures in the combustion chamber by the three methods described above (A–C), for example, when Tc = 3800 and 3700 K (Table 6), it can be argued that the third method (dilution with AP) leads to the smallest loss in values Isp and Ief (3). This means lowering the temperature in the combustion chamber to acceptable values with less energy loss is best done by diluting compound II by AP. The other two ways of reducing Tc (increasing the mass fraction of the AB in the binary composition II + AB and dilution of AB with a hydrocarbon binder) are much less effective. However, each of the described methods of lowering the temperature in the combustion chamber has its own advantages and disadvantages. Method B (Table 6) (dilution of AB with a hydrocarbon binder) loses in terms of energy to method C (4.5 s in Ief (3) when Tc to 3800 K), but facilitates the work with the uncured composition and improves the physical and mechanical properties of the cured product, and there is no HCl in the combustion products. The same should be said about method A (increasing the mass fraction of the AB to 30.1 vol %); however, here the loss to method C is somewhat less (about 1 s). Obviously, from the energy point of view, the best way to reduce value Tc is method C; method A is inferior to it, and method B is the least effective. If it comes to the real development of such compositions, the choice will need to be made between methods A and C, depending on the intended purpose of such a fuel.
CSRP Compositions: Test Compound + AB + AP
Since most of the studied components (I–III, V) have low values of α, the energy characteristics of formulations containing, in addition to components I–V and a binder, an oxidizing agent (AP), were studied. Earlier it was shown that such a technique can give a positive result at sufficiently high (approximately 700–1400 kJ/kg) values \(\Delta H_{f}^{^\circ }\) of the main component (but not as high as components I–III, V) and with low values of α (0.2–0.54) [27]. Formulations based on HMX + AP + AB [27] are taken for comparison. Figures 2 and 3 show the dependences of Ief (3) and Tc on the content of compounds I–V or HMX in the composition of CSRPs with the active binder (18 vol %) and AP (the rest).
In other words, Figs. 2 and 3 demonstrate how the parameters Ief (3) and Tc are affected by the replacement of part of compounds I–V or HMX by PA in compositions with the AB. Figure 2 shows that for compounds II, IV, V, and X the introduction of PA is ineffective from the point of view of energy. There is an explanation for this: compounds II and V have such high \(\Delta H_{f}^{^\circ },\) that even at small values of α for these components (0.50 and 0.43), the introduction of low-enthalpy AP reduces \(\Delta H_{f}^{^\circ }\) of the whole composition to such an extent that this drop is not compensated by the exothermic oxidation reaction of components II or V (and AB) with AP. For compound II, the introduction of PA turned out to be the most effective way to reduce the unacceptably high Tc (method C, Section 2). There is no need to cool composition V + AB in this way (method C, Section 2), since its Tc = 3570 K and thus it lies in the allowed range, despite the high value of Isp = 262 s. The same can be said about binary systems IV or X + 15 wt % AB. Taking into account the rather high value of α of compounds IV and X (0.8), they are almost optimal in terms of the oxygen content. The curves for compound X in Figs. 2 and 3 are not shown. They practically repeat the course of the curves for compound IV, but are located slightly higher.
For component I, the values of α and \(\Delta H_{f}^{^\circ }\) are significantly lower than for components II and V; therefore, the introduction of an additional oxidizing agent (for example, AP) in the compositions with compound I is entirely appropriate. The optimized 60.55% I + 14.45% AB + 25% PA composition outperforms binary composition I + AB by 3.3 s in terms of Isp and 3.2 s in terms of Ief (3). The temperature in the combustion chamber of such a composition is 3355 K. This composition only slightly outperforms the optimized HMX + PA + AB composition in terms of Ief (3) (255.8 and 255.5 s). The curve for component I in Fig. 2 has the following feature: a weak dependence of Ief (3) on the content of component I in the fairly wide range of 45 to 65%, (marked in Fig. 2 by the vertical dashed lines A and B). HMX has the same feature (Fig. 2), but the range of weak dependence Ief (3) is shifted by ~5% towards the higher content of the component. Therefore, limiting the content of the organic high-enthalpy component due to its sensitivity to mechanical stress can make the advantage of component I more significant in comparison with HMX. Compound I and HMX are comparable in sensitivity to mechanical stress [1]. In particular, at a 40% content of the organic component, the composition based on compound I outperforms the HMX composition in terms of Ief (3) by 3 s.
Adding 41% AP to the composition based on compound III with AB significantly increases values Isp and Ief (3), by 8.6 and 9.4 s, respectively, which is entirely expected for a compound with α = 0.2. The maximum value Ief (3) = 252.7 s is achieved when the content of component III in the composition is 44%; moreover, Ief (3) barely changes about this optimum in the range 40—50%. As a consequence, at a 40% limitation of the proportion of the organic high-enthalpy component, compound III surpasses HMX as part of the CSRP with AB and PA in terms of Ief (3) by 3 s; and at the 35% limit, by 6 s. Compound III is less sensitive to mechanical stress than HMX [1, 2]. Therefore, its content in the CSRP composition may not need to be limited.
In compositions with all the studied components, except II, the value Tc does not exceed the permissible level (3700–3800 K) (Fig. 3).
Comparison of Optimized CSRP Compositions Based on Compounds I–V and X with Each Other and with Some Known Compositions
The data from the optimized compositions based on the I–V and X compounds with the addition of AP (or without it) at the volumetric content of the AB of about 18% and provided Tc < 3800 K are presented in Table 7. The optimized compositions ADN + HB and HMX + AP + AB, as well as the compositions based on two promising components—4,4',5,5'-tetranitro-2,2'-bis(trinitromethyl)-2H,2'H-3,3'-bipyrazole (XII) [28, 29] and [1,2,5]oxadiazolo[3,4-е][1,2,3,4]tetrazine-4,6-dioxide (FTDO) [30, 31] with HB (for ADN, FTDO, and oxidizer XII, the hydrocarbon binder is more advantageous than the active one)—are given as the reference compositions.

For clarity, the data on values Ief (3) from Table 7 are shown in the histogram (Fig. 4).
From Table 7 and Fig. 4, it can be seen that in their optimized formulations, component II is at the level of FTDO and together with component V, significantly outperforms not only HMX and ADN but also the oxidizing agent XII, and component V significantly underperforms FTDO. Components I, IV and X are at the level of HMX and outperform ADN but underperform the oxidizing agent XII. Finally, component III is significantly inferior in terms of ballistic efficiency to HMX and oxidizer XII, but slightly more efficient than ADN.
Until recently, the energy characteristics of CSRP compositions based on FTDO remained record-breaking, far behind other actually synthesized compounds. The appearance of compound II and [1,2,3,4]]tetra-zino[5,6-e][1,2,3,4]tetrazine1,3,6,8-tetroxide (TTTO) [31, 32] destroyed this monopoly. It should be noted that compound II and FTDO, in terms of their sensitivity to mechanical stress, are related to the initiating explosives and their use as CSRP components is problematic.
The advantage of components I, IV, and X over HMX is small if any. Therefore, given the complex synthesis of these compounds, the prospects for their practical use are also questionable.
Of all the considered compounds, compound V with a high level of thermal stability and low sensitivity to mechanical stress appears to be the most promising CSRP component. However, as noted in the introduction, a more careful calculation of the value \(\Delta H_{f}^{^\circ }\) of this compound and ideally an experimental measurement of this parameter are required.
CONCLUSIONS
In this study, it is shown that some derivatives of 1,2,4,5-tetrazine N-oxides can be considered as promising components of CSRPs. Compound II, due to itd high nitrogen content (two azide groups in the s-tetrazine di-N-oxide cycle in the molecule), high values of the standard enthalpy of formation and density with the content of the polymer binder in the composition of the formulation at least 18 vol %, can provide rather high impulse values. Thus, even with the restriction Tc < 3800 K for compositions with AB and AP, Isp and Ief (3) values of 266.4 and 269.7 s, respectively, can be obtained. The method of reducing Tc to 3800 K compositions with compound II by using the mixed AB + HB binder turned out to be somewhat less effective. Nevertheless, the achieved values Isp = 263.1 s and Ief (3) = 265.2 s significantly outperform the reference compositions based on DNAA and HMX in terms of energy characteristics.
REFERENCES
D. E. Chavez, D. A. Parrish, L. Mitchell, and G. H. Imler, Angew. Chem. Int. Ed. 56, 3575 (2017). https://doi.org/10.1002/anie.201612496
H. Wei, J. Zhang, and J. M. Shreeve, Chem. Asian J. 10, 1130 (2015). https://doi.org/10.1002/asia.201500086
M. D. Coburn, M. A. Hiskey, K.-Y. Lee, D. G. Ott, and M. M. Stinecipher, J. Heterocycl. Chem. 30, 1593 (1993). https://doi.org/10.1002/jhet.557030062.3
D. E. Chavez and M. A. Hiskey, J. Energ. Mater. 17, 357 (1999). https://doi.org/10.1080/07370659908201796
H. Wei, H. Gao, and J. M. Shreeve, Chem.-Eur. J. 20, 16943 (2014). https://doi.org/10.1002/chem.201405122
L. Hu, P. Yin, G. H. Imler, D. A. Parrish, et al., Chem. Commun. 55, 8979 (2019). https://doi.org/10.1039/C9CC04496E
C. J. Snyder, L. A. Wells, D. E. Chavez, G. H. Imler, and D. A. Parrish, Chem. Commun. 55, 2461 (2019). https://doi.org/10.1039/C8CC09653H
Y. Liu, G. Zhao, Q. Yu, et al., Org. Chem. 84, 16019 (2019). https://doi.org/10.1021/acs.joc.9b02484
G. Wang, Z. Fu, H. Yin, and F.-X. Chen, Propellants Explos. Pyrotech. 44, 1010 (2019). https://doi.org/10.1002/prep.201900014
D. E. Chavez, D. A. Parrish, and L. Mitchell, Angew. Chem. Int. Ed. 55, 8666 (2016). https://doi.org/10.1002/anie.201604115
A. B. Sheremetev, N. V. Palysaeva, and M. I. Struchkova, Mendeleev Commun. 20, 350 (2010). https://doi.org/10.1016/j.mencom.2010.11.017
D. E. Chavez, M. A. Hiskey, and D. L. Naud, Propell. Explos. Pyrotech. 29, 209 (2004). https://doi.org/10.1002/prep.200400050
H.-H. Licht and H. Ritter, J. Energ. Mater 12, 223 (1994). https://doi.org/10.1080/07370659408018652
M. H. V. Huynh, M. A. Hiskey, J. G. Archuleta, E. L. Roemer, and R. Gilardi, Angew. Chem. Int. Ed. 43, 5658 (2004). https://doi.org/10.1002/anie.200460708
G. H. Hechiporenko and D. B. Lempert, Khim. Fiz. 17 (10), 93 (1998).
D. B. Lempert, G. P. Dolganova, G. N. Nechiporenko, and L. N. Stesik, Khim. Fiz. 16 (9), 91 (1997).
A. V. Shastin and D. B. Lempert, Russ. J. Phys. Chem. B 10, 632 (2016). https://doi.org/10.1134/S1990793116040266
B. G. Trusov, in Proceedings of the 14th International Conference on Chemical Thermodynamics (NII Khim. SPbGU, St. Petersburg, 2002), p. 483.
D. B. Lempert and A. B. Sheremetev, Khim. Geterotsikl. Soedin. 52, 1070 (2016).
S. M. Aldoshin, D. B. Lempert, T. K. Goncharov, A. I. Kazakov, S. I. Soglasnova, E. M. Dorofeenko, and N. A. Plishkin, Russ. Chem. Bull. 65, 2018 (2016).
A. V. Shastin and D. B. Lempert, Russ. J. Phys. Chem. B 8, 716 (2014).
D. B. Lempert, E. M. Dorofeenko, and Yu. Shu, Russ. J. Phys. Chem. B 10, 483 (2016).
G. Ya. Pavlovets and V. I. Tsutsuran, Physicochemical Properties of Propellants and Rocket Fuels, The School-Book (MO, Moscow, 2009) [in Russian].
E. M. Dorofeenko, S. I. Soglasnova, G. N. Nechiporenko, and D. B. Lempert, Combust. Explos., Shock Waves 54, 698 (2018). https://doi.org/10.1134/S0010508218060096
D. B. Lempert, A. I. Kazakov, V. S. Sannikov, A. V. Nabatova, D. V. Dashko, and A. I. Stepanov, Combust. Explos., Shock Waves 55, 148 (2019). https://doi.org/10.1134/S0010508219020035
I. Yu. Gudkova, I. N. Zyuzin, and D. B. Lempert, Russ. J. Phys. Chem. B 14, 302 (2020). https://doi.org/10.1134/S1990793120020062
I. N. Zyuzin, A. I. Kazakov, D. B. Lempert, I. A. Vatsadze, L. S. Kurochkina, and A. V. Nabatova, Combust. Explos., Shock Waves 55, 327 (2019). https://doi.org/10.1134/S0010508219030109
I. L. Dalinger, K. Yu. Suponitsky, T. K. Shkineva, D. B. Lempert, and A. B. Sheremetev, J. Mater. Chem. A 6, 14780 (2018). https://doi.org/10.1039/C8TA05179H
I. N. Zyuzin, I. Yu. Gudkova, and D. B. Lempert, Russ. J. Phys. Chem. B 14, 804 (2020).https://doi.org/10.1134/S1990793120050140
A. M. Churakov, S. L. Ioffe, and V. A. Tartakovsky, Mendeleev Commun. 5, 227 (1995). https://doi.org/10.1070/MC1995v005n06ABEH000539
D. B. Lempert, E. M. Dorofeenko, and S. I. Soglasnova, Omsk. Nauch. Vestn., Ser. Aviats.-Raketn. Energ. Mashinostr. 2 (3), 58 (2018). https://doi.org/10.25206/2588-0373-2018-2-3-58-62
M. S. Klenov, A. A. Guskov, O. V. Anikin, et al., Angew. Chem. Int. Ed. 55, 11472 (2016). https://doi.org/10.1002/anie.201605611
Funding
The study was supported by the Institute of Problems of Chemical Physics, Russian Academy of Sciences as part of a state assignment (state registration number АААА-А19-119101690058-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zyuzin, I.N., Gudkova, I.Y. & Lempert, D.B. Energy Abilities of Certain Derivatives of 1,2,4,5-Tetrazine N-Oxides as Components of Solid Composite Rocket Propellants. Russ. J. Phys. Chem. B 15, 611–621 (2021). https://doi.org/10.1134/S1990793121040138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121040138