Abstract
In this paper, we study the energetic capabilities of four N-dinitro- and N-trinitromethyl derivatives of nitroazoles described in the literature as potential components of composite solid propellants. Using the thermodynamic calculations, some of the considered compounds are shown to have good potential for the creation of solid propellants with improved energy characteristics based on them. The quantitative dependences of the energetic parameters of the fuel on the properties of the studied oxidizer, the aluminum fraction in the composition, and the type and the content of binder are established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the past decade, the search for new energy-intensive compounds that can be used to create various energetic materials (EMs) has been sharply intensified [1–9]. In most of these studies, explosive substances (explosives), in which one energy-intensive compound is used and its fraction is close to 100%, were selected as the target EMs. Composite solid rocket propellants (CSRPs) are a fundamentally different type of EM. In addition to a highly efficient individual energy-intensive compound, at least one other component is required in the CSRP’s composition—a polymer binder in an amount sufficient to ensure the necessary physical and mechanical properties of the finished solidified fuel charge and to ensure the required level of rheological properties of the unsolidified fuel mass. At least two components are used in the CSRP’s compositions, and the energetic properties of the formulation are determined both by the characteristics of the main component and by the entire formulation [10–12]. As a result of the optimal selection of components and their ratio in the formulation of the CSRP’s composition, it is possible to achieve the maximal attainable values of the energy indicators.
At present, N-trinitromethyl-azoles [13–27], which are poorly studied derivatives of N–H heterocycles, are being actively studied as promising EM components. The trinitromethyl moiety bound to the nitrogen atom of the heterocycle increases the oxygen balance and positively contributes to the enthalpy of formation. The contribution to the enthalpy of formation upon replacement of the N–H fragment by N–C(NO2)3 was determined experimentally (141.4 and 148.1 kJ/mol) using the 3,4- and 3,5-dinitro-1-trinitromethyl-1H-pyrazoles compounds as an example [28]. With a combination of high enthalpies of formation, a satisfactory oxygen balance, and high density, these compounds may be of interest as potential CSRP components. Earlier, within this subclass of compounds, only some N-trinitromethyl derivatives of dinitropyrazoles were considered as CSRP components (oxidizing agents) [25, 28]. In this paper, we present a brief review of N‑trinitromethyl derivatives of other energetic azoles and estimate the prospect of using them as CSRP components for some of them using thermodynamic calculations.
PROBLEM FORMULATION AND RESEARCH METHODS
1 Brief Review of N-Trinitromethyl Derivatives of Energetic Azoles and Selection of Objects of Study
In this study, we calculated the energy characteristics of the composites for CSRP containing compounds I–IV as the main component [18, 23]. Figure 1 shows the structural formulas of compounds I–IV and their names in the caption to Fig. 1. Table 1 shows the properties of compounds I–IV.
In this study, we focused on N-dinitromethyl and N-trinitromethyl derivatives with one heterocycle in the molecule. As the number of nitrogen atoms in the cycle grows, their energetics should certainly increase, while their thermal stability and sensitivity to mechanical stress should simultaneously deteriorate. Therefore, we chose extreme examples: on the one hand, the least energetically loaded N-trinitromethyl derivatives of 2- and 4-nitroimidazoles (I and II), and on the other hand, the high-enthalpy N-dinitromethyl derivative of nitrotetrazole (III) and its hydroxylammonium salt (IV).
The additional interest in N-trinitromethyl derivatives of azoles was initiated by the publication in 2018 of [25], in which a bis-N-trinitromethyl derivative of tetranitrobipyrazole (V) was synthesized and proposed as an oxidizing agent of the CSRP. Due to the unique combination of properties, compound V in some CSRP compositions is significantly superior to the known oxidizing agents AP (NH4ClO4) and ADNA (NH4N3O4) [25]. The achievement of [25] appears especially striking against the background of the failure of the attempt to synthesize compound V in [24]; moreover, [24] was published in the same journal almost simultaneously with [25]. Instead of compound V, compound VI with one N-trinitromethyl group was obtained in [24]. Compound VI could also be considered as a CSRP component; however, it is obvious even without calculations that it is inferior to compound V.

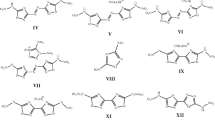
Not many N-trinitro- and N-dinitromethyl azoles derivatives are known in the literature [13–27]. The structural formulas of most of them (in addition to I–VI) are presented below: nitropyrazoles VII–IX; 1,2,4-triazoles X–XII; 1,2,3- and 1,2,4-bis-triazoles XIII and XIV; 1,2,3-triazole-1-oxides XV and XVI; and tetrazoles XVII–XIX.

Some of these compounds have excessively low melting points to serve as solid CSRP components: (VII) 37°C [14], (X) 58–59°C [14], (XII) 63°C [26, supporting inf.], (XV) 38–40°C [22], and (XVI) 68–70°C [22]). Dinitropyrazoles VIII and IX already studied as CSRP components in [28], although their melting points are also on the verge of being permissible (80 and 81°C [17]). The melting point of 1,2,4-triazole XI is somewhat higher (99–100°C [14]). High-enthalpy tetrazoles XVII–XIX with two trinitromethyl and/or dinitromethyl groups could be the best; however, there is a limitation in thermal stability for them. Thus, compound XIX, the compound most loaded with oxygen and energy, is characterized only by the NMR spectra; it was isolated in the solid form by evaporation of the solution at room temperature and immediately decomposed [27]. Based on the elemental composition, density (1.921 and 1.831 g/cm3 at 20°C), and melting point (141 and 138°C), bitriazoles XIII and XIV could compete with compound V but the enthalpy of formation is unknown for them. In the near future, it is planned to measure the enthalpy of their formation (or calculate it if it is not possible to measure it) and, using thermodynamic calculations, determine the possibility of their use as potential CSRP components.
2 Calculation Method
One of two typical binders was taken as the binder: a conventional hydrocarbon binder (HB, C18.96H34.64N19.16O29.32, the standard enthalpy of formation is \(\Delta H_{{\text{f}}}^{^\circ }\) = –393 kJ/kg, and density is ρ = 0.92 g/cm3 [29]) and an active binder (AB, C72.15H119.21O0.68, the standard enthalpy of formation is \(\Delta H_{{\text{f}}}^{^\circ }\) = –757 kJ/kg, and ρ = 1.49 g/cm3 [29]).
In this paper, we studied the energy characteristics of both binary CSRP compositions (a binder and one of the studied compounds I–IV) and more complex compositions additionally containing aluminum (Al) as a metallic fuel (energy component, ρ = 2.7 g/cm3) and/or ammonium dinitramide (ADNA) (NH4N3O4, \(\Delta H_{{\text{f}}}^{^\circ }\) = –1129 kJ/kg, ρ = 1.82 g/cm3, α = 2) [11] as an additional oxidant. Compositions without metal and with ammonium perchlorate (AP), (NH4ClO4, \(\Delta H_{{\text{f}}}^{^\circ }\) = – 2495 kJ/kg, ρ = 1.95 g/cm3, α = 2) and octogen (HMX) (\(\Delta H_{{\text{f}}}^{^\circ }\) = 295 kJ/kg, ρ = 1.9 g/cm3, α = 0.67), one of the most effective CSRP oxidants among the available compounds, are also considered. It should be noted that due to the qualitative difference in the oxygen supply coefficient of α (2.00 versus 0.667), ADNA in binary formulations combines better with HB, and HMX combines better with AB [26].
The specific impulse Isp and the temperature in the combustion chamber Tc (at pressures in the chamber and at the nozzle exit of 4.0 and 0.1 MPa, respectively) were calculated using the TEPRA program for calculating high-temperature chemical equilibria [30]. The effectiveness of the studied components was analyzed according to the algorithm described in [31, 32]. To compare the ballistic efficiency of compositions with various densities when used in engines with various volume-mass characteristics, the so-called reduced effective impulse Ief(n) were used at various stages of rocket systems (n is the number of the stage) [33].
These values characterize the ballistic efficiency of the fuel at the corresponding stages of the rocket systems.
Compositions containing aluminum have losses in the real Isp value due to the formation of a condensed phase in the combustion products, and the value of these losses is estimated at 0.22% of the Isp value for each 1% of aluminum [29]. Therefore, to compare the effectiveness of compositions with various aluminum contents, the values of the effective pulses are used taking into account these losses. \(I_{{{\text{ef}}}}^{*}(n)\) is estimated by the formula \(I_{{{\text{ef}}}}^{*}(n)\) = Ief(n) – 0.0022Isp [Al], where [Al] is the percentage content of aluminum in the composition.
To ensure the satisfactory physical and mechanical characteristics of the CSRP and the rheological properties of the uncured fuel mass, the compositions must contain a sufficient amount of a polymer binder. Satisfactory performance is usually achieved when the volumetric content of the binder is not lower than 18–19 vol %. For a correct comparison, all CSRP compositions considered in this study have approximately the same volume fraction of the binder, 18.0–18.2 vol %.
RESULTS AND DISCUSSION
1 Binary Formulations: Test Compound + AB or HB
As noted above, the CSRP energy properties are determined both by the characteristics of the main component and by the entire formulation. This paper is aimed at studying the possibility of using compounds I–IV for the development of CSRP compositions. Of these, compound III is of the greatest interest as CSRP oxidants since it has sufficiently high values of the enthalpy of formation (\(\Delta H_{{\text{f}}}^{^\circ }\) = 1421.9 kJ/kg), density (ρ = 1.97 g/cm3) and oxygen supply coefficient (α = 1.33). Compounds I and II have a low value of the coefficient α (0.89) and hence it is better to combine them with AB. Compounds III and IV have higher α values (1.33 and 1.17). They can be combined with both AB and HB [34]. The parameters of the optimized formulations are presented in Table 2. For comparison, the parameters of two exemplary binary compositions—octogen (HMX) + AB and ADNA + HB, as well as the composition V + HB [25] are given there.
Based on the data in Table 2, in terms of Isp in binary compositions with AB, compounds I and II outperform HMX by 2 and 1 s, respectively. Compounds I and II with HB did not show good results, while compounds III and IV with HB outperform ADNA by 6.1 and 1.5 s, respectively. HB is generally more compatible with oxidants than AB. In binary compositions, compound III demonstrates the best result, which is not surprising for a compound with a coefficient of α = 1.33. In terms of the effective impulse Ief (3), compound III surpasses both the compositions ADNA + HB and HMX + AB (by 9.2 and 5.2 s, respectively). The composition 90.7% III + 9.3% HB with Ief (3) = 259.0 s is the best of the binary compositions based on compounds I–IV. It is somewhat (by 0.6 s) inferior to the composition 91% V + 9% HB; however, its temperature in the combustion chamber is 100 K lower (Tc = 3500 and 3600 K). Compound IV is inferior to compound III in terms of Isp and Ief(3), which can be explained by the lower parameter values (\(\Delta H_{{\text{f}}}^{^\circ }\) = 802.4 kJ/kg, ρ = 1.87 g/cm3, and α = 1.17).
2 CSRP Compositions: Test Compound + AB + Al and Test Compound + HB + Al
As expected, the addition of aluminum to the oxidant + AB and oxidant + HB compositions can increase the specific impulse values. The dependence of the effective impulse at the third stage with allowance for two-phase losses \(I_{{{\text{ef}}}}^{*}\)(3) on the Al content in compositions based on compounds I–IV was studied. Compounds I and II have a coefficient of α = 0.89. As for binary formulations, this means that they can be combined not only with AB or HB but also in more complex compositions with a mixture of AB and HB [34]. The parameters of the optimized formulations are summarized in Table 3; for comparison, it also lists the values for binary compositions without metal and for reference compositions with HMX and ADNA [35].
In the I + AB composition without Al, the effective impulses Ief(3) and \(I_{{{\text{ef}}}}^{*}\)(3) are 254.8 s (for compositions without metal, the values of Ief(3) and \(I_{{{\text{ef}}}}^{*}\)(3) are the same). The addition of 1% aluminum to the I + AB composition does not change the value of the impulse Ief(3). The maximal value of Ief(3) = 261.0 s is achieved at 16% Al; however, the two-phase losses turn this gain into a loss, while the combustion temperature rises sharply (Tc = 4064 K). A similar picture is observed when aluminum is added to the composition II + AB. In other words, no aluminum is needed. This is even more pronounced in the compositions of compounds III and IV with AB.
However, the optimized composition with compound III, 84.85% III + 9.15% HB + 6% Al, with an index of \(I_{{{\text{ef}}}}^{*}\)(3) = 259.8 s outperforms the reference composition 78.4% ADNA + 9.6% HB + 12% Al by 6 s. This composition with compound III, albeit, only slightly (by 0.4 s), is superior to the optimized composition with compound V [25], 88% V + 9% HB + 3% Al. Compositions with compounds III and V have sufficiently high Tc values, especially in comparison with the reference composition based on ADNA (3695 and 3700 K versus 3407 K); however, this is within the acceptable range. For compound IV, the maximal value of \(I_{{{\text{ef}}}}^{*}\)(3) = 256.8 s, which is 3 s better than that for the composition with ADNA.
Thus, we may conclude that compositions with aluminum and HB as a binder based on oxidants III and IV are of interest for creating a CSRP. These results are shown in Fig. 2 that shows the dependence of the effective impulse \(I_{{{\text{ef}}}}^{*}\)(3) on the Al content in compositions based on compounds I and II with AB and HB, as well as based on compounds III and IV with AB at a volume binder content of about 18%.
3 CSRP Compositions: Test Compound + AB + ADNA + Al and Test Compound + HB + ADNA + Al
All the studied compounds I–IV have a sufficiently high value of the coefficient α (0.89–1.33). Therefore, according to the preliminary estimates, compositions with them may not need an additional oxidizing agent. Nevertheless, it was decided to try to partially replace the studied oxidants with ammonium salt of dinitratic acid (ADNA) with an even higher value of the oxygen supply coefficient for the molecule (α = 2) with the addition of Al to some of the compositions.
The addition of ADNA to compositions based on compounds I and II did not increase the energy potentials of the compositions. For the optimized composition based on compound II, the \(I_{{{\text{ef}}}}^{*}\)(3) value is only higher by 0.1 s than that for the reference composition with ADNA, 78.4% ADNA + 9.6% HB + 12% Al. For the composition 65.65% III + 9.35% AB + 20% ADNA + 5% Al, the impulse value of \(I_{{{\text{ef}}}}^{*}\)(3) = 258.0 s, which is by 4.2 s higher than that of the reference composition, was obtained (Table 4). The optimized composition based on compound IV, 39.5% IV + 9.5% HB + 40% ADNA + 11% Al, showed a good result of \(I_{{{\text{ef}}}}^{*}\)(3) = 255.3 s, which is 1.5 s higher than that for the reference ADNA composition (Table 4).
4 CSRP Compositions: Test Compound + AB + HB + Al
In this subsection, four-component CSRP compositions that, in addition to the main component, include both binders and aluminum are considered. In terms of the energetics, such compositions are optimal for compounds I and II, for which the coefficient is α = 0.89 [34]. For these compositions, the results should be compared according to the value of the effective impulse \(I_{{{\text{ef}}}}^{*}\)(3), since it is necessary to take into account the effect of the two-phase losses, which was mentioned in the Calculation Method subsection. It is correct to compare these CSRP compositions with the 78.4% ADNA + 9.6% HB + 12% Al composition, in which the level of \(I_{{{\text{ef}}}}^{*}\)(3) = 253.8 s is reached at Tc = 3407 K and 18.0 vol. % HB. The parameters of the optimized formulations are summarized in Table 5. In such a four-component composition for compound I, the optimal result (\(I_{{{\text{ef}}}}^{*}\)(3) = 255.1 s) is achieved for the formulation 83.5% I + 13.5% AB + 1% HB + 2% Al (at Tc = 3558 K). This is better by 1.3 s than that for the 78.4% ADNA + 9.6% HB + 12% Al composition. For compound II, the optimized composition 75.92% II + 13.53% AB + 0.55% HB + 10% Al provides parameters \(I_{{{\text{ef}}}}^{*}\)(3) = 253.1 s and Tc = 3873 K. This result is somewhat worse (by 0.7 s) than that for the reference composition 78.4% ADNA + 9.6% HB + 12% Al.
5 CSRP Compositions: Test Compound + AB + AP for Compounds I and II and Test Compound + AB or HB + AP for Compounds I–IV
We studied the energy characteristics of the formulations containing ammonium perchlorate (AP) as an additional oxidant. These compositions were compared with the composition based on HMX in the same way as the DAzFNF or DNFNF oxidants were studied in [35]. Figures 3–6 show the dependences based on the calculated data, which allow comparing the energetic capabilities of the compositions based on compounds I–IV with AP and AB and similar compositions with HMX, one of the most effective available CSRP oxidants.
As can be seen in Fig. 3, the additional oxidizing agent PA in the I or II + АB compositions is harmful. The binary compositions I or II + AB have practically the same Ief(3) parameters as the optimized composition HMX + AP + AB (254.8 s). However, the temperatures in the combustion chamber for compounds I and II (3506 and 3491 K) significantly exceed this parameter for the composition with HMX (3231 K) (Fig. 4), while they do not exceed the permissible temperature level (3700–3800 K) [36].
As can be seen in Fig. 5, an additional oxidizing agent of AP in the III or IV + HB compositions is not needed; as its fraction decreases down to zero, the Ief(3) parameter monotonically increases. The binary composition III + AB in terms of Ief(3) significantly exceeds the optimized composition HMX + AP + AB (254.8 s). However, the temperatures in the combustion chamber for compounds I and II (3506 and 3491 K) significantly exceed this parameter for the composition with HMX (3231 K) (Fig. 4). Figure 5 shows that for the composition with HB, the Ief(3) values for compound III exceed the Ief(3) values for the HMX + AP + AB composition for all the considered compositions, while the energy potentials of I and II with HB are noticeably inferior to the composition with HMX. For compositions where there are restrictions on the content of organic explosives (explosives), those compounds, with which high impulses can be obtained with a relatively low content in the CSRP composition, have an advantage [35]. An analysis of the obtained data showed that compounds I–IV in compositions with PA do not have any noticeable advantages over those with HMX since, in terms of Isp and Ief(3), they do not outperform HMX for the considered compositions (30–90% of the high-enthalpy component). However, compounds III and IV with a 50–70% fraction in the CSRP composition can compete with the HMX + AP + AB composition.
The obtained data allow affirming that compounds III and IV could be promising oxidizing agents for CSRP formulations. However, they are thermally much less stable and more sensitive to mechanical stress than HMX. Therefore, the practical application of compounds III and IV as CSRP components appears very problematic.
CONCLUSIONS
We showed that some N-trinitromethyl derivatives of nitroazoles containing a trinitromethyl group could be considered as promising components of CSRPs. Their characteristics, such as the high values of the standard enthalpy of formation, the coefficient of the oxygen supply to the molecule, and the density when the polymer binder’s content in the formulation is not less than 18 vol % can provide for the compositions sufficiently high values of effective impulses Ief(3) and \(I_{{{\text{ef}}}}^{*}\)(3) (up to 261.6 and 255.1 s, respectively), which are better, in terms of the energy characteristics, than the reference compositions based on ADNA and HMX. Some of the considered compounds in compositions with AP can compete with HMX when they are present in a CSRP in an amount of 50 to 70%.
REFERENCES
A. A. Larin, N. V. Muravyev, A. N. Pivkina, et al., Chem.-Eur. J. 25, 4225 (2019). https://doi.org/10.1002/chem.201806378
H. Xiong, G. Cheng, Z. Zhang, and H. Yang, New J. Chem. 43, 7784 (2019). https://doi.org/10.1039/C9NJ00955H
C. He, H. Gao, G. H. Imler, D. A. Parrish, and J. M. Shreeve, J. Mater. Chem. A 6, 9391 (2018). https://doi.org/10.1039/C8TA02274G
I. N. Zyuzin, Russ. J. Org. Chem. 51, 174 (2015).https://doi.org/10.1134/S1070428015020050
I. N. Zyuzin, K. Yu. Suponitskii, and I. L. Dalinger, Khim. Geterotsikl. Soedin. 53, 702 (2017).https://doi.org/10.1007/s10593-017-2112-y
E. C. Koch, Propell. Explos. Pyrotech. 41, 526 (2016). https://doi.org/10.1002/anie.201600068
H. Wei, C. He, J. Zhang, and J. M. Shreeve, Angew. Chem., Int. Ed. Engl. 54, 9367 (2015). https://doi.org/10.1002/anie.201503532
Y. Tang, H. Gao, L. A. Mitchell, D. A. Parrish, and J. M. Shreeve, Angew. Chem., Int. Ed. Engl. 55 (9), 3200 (2016). https://doi.org/10.1002/anie.201600068
G. N. Nechiporenko, D. B. Lempert, and S. I. Soglasnova, Khim. Fiz., No. 3, 69 (2005).
D. B. Lempert, Chin. J. Explos. Propell. 38 (4), 1 (2015).
D. Lempert, G. Nechiporenko, and G. Manelis, Cent. Eur. J. Energ. Mater. 3 (4), 73 (2006).
G. K. Khisamutdinov, V. L. Korolev, I. Z. Kondyukov, et al., Russ. Chem. Bull. 42, 1559 (1993).
T. I. Godovikova, S. A. Vozchikova, E. L. Ignat’eva, L. I. Khmel’nitskii, and B. L. Korsunskii, Khim. Geterotsikl. Soedin. 39, 548 (2003).
T. P. Kofman, G. Yu. Kartseva, E. Yu. Glazkova, and K. N. Krasnov, Russ. J. Org. Chem. 41, 753 (2005).
R. S. Stepanov, L. A. Kruglyakova, and A. M. Astakhov, Russ. J. Gen. Chem. 77, 1933 (2007).
V. V. Semenov and S. A. Shevelev, Mendeleev Commun. 20, 332 (2010). https://doi.org/10.1016/j.mencom.2010.11.010
I. L. Dalinger, I. A. Vatsadze, T. K. Shkineva, et al., Chem. Asian J. 10, 1987 (2015). https://doi.org/10.1002/asia.201500533
X. X. Zhao, S. H. Li, Y. Wang, et al., J. Mater. Chem. A 4, 5495 (2016). https://doi.org/10.1039/C6TA01501H
I. L. Dalinger, K. Yu. Suponitsky, A. N. Pivkina, and A. B. Sheremetev, Propell. Explos. Pyrotech. 41, 789 (2016). https://doi.org/10.1002/prep.201600050
I. L. Dalinger, A. V. Kormanov, K. Yu. Suponitsky, N. V. Muravyev, and A. B. Sheremetev, Chem. Asian J. 13, 1165 (2018). https://doi.org/10.1002/asia.201800214
T. Liu, X. Qi, K. Wang, et al., New J. Chem. 41, 9070 (2017). https://doi.org/10.1039/C7NJ01917C
V. V. Semenov, S. A. Shevelev, A. B. Bruskin, A. Kh. Shakhnes, and V. S. Kuz’min, Khim. Geterotsikl. Soedin. 53, 728 (2017).
X. Yin, J. Li, G. Zhang, et al., Chem. Plus Chem. 54, 787 (2018). https://doi.org/10.1002/cplu.201800305
Y. Tang, C. He, G. H. Imler, D. A. Parrish, and J. M. Shreeve, J. Mater. Chem. A 6, 5136 (2018). https://doi.org/10.1039/C7TA11172J
I. L. Dalinger, K. Yu. Suponitsky, T. K. Shkineva, D. B. Lempert, and A. B. Sheremetev, J. Mater. Chem. A 6, 14780 (2018). https://doi.org/10.1039/C8TA05179H
G. Zhao, D. Kumar, Ping Yin, et al., Org. Lett. 21, 1073 (2019). https://doi.org/10.1021/acs.orglett.8b04114
Q. Yu, G. H. Imler, D. A. Parrish, and J. M. Shreeve, Org. Lett., No. 12, 4684 (2019). https://doi.org/10.1021/acs.orglett.9b01565
A. I. Kazakov, I. L. Dalinger, I. N. Zyuzin, D. B. Lempert, N. A. Plishkin, and A. B. Sheremetev, Russ. Chem. Bull. 65, 2783 (2016).
G. N. Nechiporenko and D. B. Lempert, Khim. Fiz. 17 (10), 93 (1998).
B. G. Trusov, in Proceedings of the 14th International Conference on Chemical Thermodynamics (SPb. NII Khim., St. Petersburg, 2002), p. 483.
D. B. Lempert and A. B. Sheremetev, Khim. Geterotsikl. Soedin. 52, 1070 (2016).
S. M. Aldoshin, D. B. Lempert, T. K. Goncharov, et al., Russ. Chem. Bull. 65, 2018 (2016).
G. Pavlovets and V. Tsutsuran, Physicochemical Properties of Propellants and Rocket Fuels (Voenizdat MO RF, Moscow, 2009) [in Russian].
E. M. Dorofeenko, S. I. Soglasnova, G. N. Nechiporenko, and D. B. Lempert, Combust. Explos., Shock Waves 54, 698 (2018). https://doi.org/10.1134/S0010508218060096
D. B. Lempert, A. I. Kazakov, V. S. Sannikov, A. V. Nabatova, D. V. Dashko, and A. I. Stepanov, Combust. Explos., Shock Waves 55, 148 (2019). https://doi.org/10.1134/S0010508219020035
I. Yu. Gudkova, I. N. Zyuzin, and D. B. Lempert, Russ. J. Phys. Chem. B 14, 302 (2020). https://doi.org/10.31857/S0207401X20030061
Funding
This study was supported by the Institute of Problems of Chemical Physics, Russian Academy of Sciences (IPCP RAS) (topic no. 0089-2014-0019 “Creation of high-energy materials and technologies for developed and advanced systems”), the Presidium of the Russian Academy of Sciences “Development of solid fuels and combustibles for gas generators of ramjet engines of hypersonic aircraft and research of heat and mass transfer and combustion in gas generators” as part of program 56 “Fundamentals of breakthrough technologies in the interests of national security.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Zyuzin, I.N., Gudkova, I.Y. & Lempert, D.B. Energetic Capabilities of N-Dinitro- and N-Trinitromethyl Derivatives of Nitroazoles as Composite Solid Propellant Components. Russ. J. Phys. Chem. B 14, 804–813 (2020). https://doi.org/10.1134/S1990793120050140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793120050140