Abstract
The decomposition rate of 1,1-diamino-2,2-dinitroethylene (FOX-7) at 200°C in a wide range of changes in the conditions of the experiment and methods of sample preparation is measured. The nature of the first stage of decomposition is established and the reason the reaction stopped at this stage is found.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
INTRODUCTION
Experimental study of the thermal decomposition of 1,1-diamino-2,2-dinitroethylene FOX-7 (I) was carried out mainly by the differential scanning calorimetry (DSC) method in an inert gas flow (see review [1]). At the same time, it was found that the decomposition proceeds in two stages, which in the DSC curves correspond to two heat release peaks, strongly differing in temperature (220 and 270°C). It was proved in [2] that the appearance of two peaks are not related to any impurity reactions or with the formation of products that could decompose at elevated temperatures. Such a double character of the reaction during the decomposition of explosives had previously never been observed, even in the case of polyfunctional compounds with a complex structure [3–6]. In the work [2], the first peak (in which 60% of the heat is released) was explained by the presence in the crystals of substance I, an amorphous substance with a high decomposition rate. However, in an earlier work [7], it was shown that in closed vessels the termination of the reaction depends on the ratio of the mass of sample m to the volume of vessel V and can be observed at small conversions. The inhibition effect itself was explained in [7] by the reversibility of the first stage of decomposition, which consists of splitting I into HNO3 and the corresponding nitrile oxide. It can be noted, however, that the possibility of attaching HNO3 to nitrile oxides, especially in vapors and at high temperatures, has not been proven. In [8], a new effect was discovered: after a short period (~20 min) of heating at a temperature of about 200°C, the crystals of compound I in a stream of argon acquire new properties. After cooling them to room temperature and subsequent heating, no α → β and β → γ phase transitions are observed, as usual, and the first decomposition peak is absent. In [8], it was assumed that during preliminary heating a polymorphic transition occurs with the formation of a new, δ-modification, thermally and phase-stable at temperatures ranging from room temperature to 270°C. In the same work, it was found that substance I is volatile and even at atmospheric pressure it readily sublimates at 200°C from the microscope’s support, and the nonvolatile residue (assumed δ-modification) changes color. The color change can be due to chemical conversion. However, the possible connection between sublimation, decomposition reactions, and changes in the properties of crystals was not discussed in [8], although it was in this work that the simultaneity of these phenomena was first discovered. Note that even before the appearance of [8], it was shown [2] that the same properties, that is, disappearance of the first peak of heat release at 220°С and the absence of a transition to the α-modification upon cooling, belong to the product formed after three days of heating I in an argon flow at 185°C (40% weight loss), and the structure of this product corresponds to the γ-phase.
No thermal effects that should accompany the phase transition were observed in [8]. Thus, the assumption of the γ → δ phase transition has not been confirmed. However, even if we assume that the δ‑modification exists, it cannot be the reason the decay stops. It is impossible to explain why the high-temperature modification, the formation of which proceeds with the absorption of heat, i.e., with an increase in the heat content of a substance, it becomes, judging by the difference in the temperatures of the appearance of the peaks, more stable than the preceding γ-phase by 10s to 100s of factors. It is also unclear why the compound, which is highly volatile at 200°C, completely loses the ability to sublimate even when heated to 270°C after the polymorphic transition.
At the same time, such indisputable facts as high volatility I, confirmed in the detailed work [9], and the inevitable fast reaction of decomposition of a substance in the gas phase, require more detailed consideration as the probable reasons for the appearance of the first stage. Therefore, it is not surprising that the following hypothesis was put forward in [10] as one of the possible explanations for the first stage: the decomposition of I proceeds mainly in the gas phase. The reason for the decrease in the thermal decomposition rate is the formation of a dense film on the outer surface of the crystals as a result of some secondary reactions involving the products of gas-phase decomposition. The film prevents sublimation. However, in this study, this hypothesis was rejected, and preference was given to the phase transition.
In relation to the low probability of the explanations of the first stage, proposed in the literature, in this paper, a further study of the decomposition of the substance I was undertaken in order to test, primarily, the hypothesis of the self-decelerating decay in vapor as the cause of the first stage. Unlike most other works, the study was carried out not in open systems in an inert gas flow but in closed vessels and mainly under isothermal conditions. Compared with the isothermal studies performed in [2, 7], the observation time for the reaction was increased by tens and hundreds of times, which made it possible to study both stages at the same temperature. Such methodological changes made it possible to obtain new information on the decomposition of compound I and ultimately determine the nature of the first decomposition stage.
EXPERIMENTAL
Compound I was synthesized according to the method described in [11]. Two samples were used: small, with particles of ~ 0.1 mm; and large, with particles of ~1 mm. In both cases, recrystallization was carried out from acetonitrile.
Calorimetric measurements in the scanning mode were performed on a device developed at the Institute of Problems of Chemical Physics, Russian Academy of Sciences (IPCP RAS), which allows accurate measurements of heat release in sealed ampules with a volume of ~2.0 cm3 at a heating rate of 1 to 5 deg/min.
Gauge measurements are made with a high pressure unit. A glass ampule (0.5–5.0 cm3), equipped with a crescent membrane with a volume of 0.3 cm3 with an arrow, was placed in a split metal capsule with a window for observing the deflection of the arrow and the cover, which was connected with a pump, a pressure gauge, and a compressed air cylinder using copper pipes and valves. The pressure in the ampule was measured by the compensation method.
RESULTS AND DISCUSSION
In accordance with the set task, after describing the observed facts or phenomena, an analysis of their connection with gas-phase or solid-phase reactions is immediately given, and their correspondence with the mechanism of decomposition of substance I and general laws of reactions in the gas and solid phases is discussed.
Differential Scanning Calorimetry Study
The DSC curves of decomposition in sealed ampules show, as in the decomposition in an inert gas flow, two peaks of heat release (Fig. 1). However, there are two significant differences between data obtained under open and closed conditions. The heat release peaks in the sealed ampule are very close to each other, mainly due to the shift of the second peak towards low temperatures. The maximums of the heat released from the first and second peaks lie in the sealed ampule at 228 and 250°C instead of 220 and 270°C as in open systems. This can be explained by the fact that the decay of I in the second stage is an autocatalytic process, the rate of which depends on the pressure of the gaseous products. In addition, in a closed volume in the first stage of decomposition, no more than 2% of the total heat is released, instead of 40–60% in the flow.
In closed vessels, it is also observed that the heat release in the first stage is almost independent of the mass of the substance taken. This result means that the first stage is not related to any reaction involving the solid. Consequently, the reaction takes place in the gas phase.
The second observation, showing the gas-phase nature of the reaction in the first stage, concerns the change in the color of the sample. When heating a test tube containing 200 mg of substance I, up to 200°C at atmospheric pressure, a rapid coloration of the end surface of the sample in a beige color is visually observed. The color slowly spreads into the interior and along the walls of the tube. The lower layers of the substance remain yellow for a long time. Observations of this kind have been carried out many times in the study of thermal decomposition in closed vessels described below. With small (up to 5 mg) weighed portions, when the crystals lie on the bottom of the vessel in a thin layer, they all lose their yellow color immediately after warming up to 200°C. When rubbing such darkened crystals, a yellow powder is formed. This means that the color change only affects the thin outer layer of the crystal. In samples with a mass of 20 to 30 mg, heated in a vessel with a volume of 5 cm3 at 200°C for 6 hours, there is always a part of the crystals that does not change its original color.
Coloring of only the crystal surface and only the outer surface of the sample indicates the occurrence of a chemical reaction, not in the solid phase but in vapors. In order to explain why this reaction stops, it can be assumed, as suggested in [10], that the decomposition of vapors produces low volatile compounds, which are deposited on the surface of crystals in the form of a film. This film modifies the crystal surface and prevents sublimation. The vapors released into the volume of the vessel decompose quickly, and the reaction stops.
The formation of a film can explain not only the termination of the reaction but also the second feature of the heated sample, which served as the base for the hypothesis of the polymorphic transition γ → δ in [8]. This feature consists, as noted, in the absence of a rapid γ → α transition when the heated sample is cooled to room temperature. The phase transition requires large fluctuations of the free volume to release the translational motion of the molecules. Therefore, it usually begins in large defects lying on the outer surface of the crystal. Covering the surface, the film deactivates its active regions and inhibits the slowest stage of the phase transition—the formation of nuclei of a new phase—delaying, at least for some time, the phase transition. Perhaps that is why the hypothetical high-temperature δ-modification is simply a γ-form covered with a film of products.
The formation of a protective film is a unique property of substance I related to the peculiarities of its structure and the mechanism of decomposition. To understand how the film is formed, it is necessary to consider the mechanism of the reaction.
Mechanism of the Reaction
The most likely decomposition mechanism of compound I is a sequence of elementary processes

According to quantum chemical calculations [12], out of the five possible primary stages of decomposition of compound I (Fig. 2) path d of isomerization in the aci-form (nitronic acid) through a 1.5-sigmatropic hydrogen shift has the lowest activation energy. This regularity will be retained for all unsaturated mononitro compounds having hydrogen-containing substituents such as CH3, OH, and NH2 in the cis-position to the nitro group. Admittedly, in the case of substance I, another primary reaction, intramolecular oxidation with the formation of an oxazet-type structure (path c), has the same activation energy as reaction d.
The nitronic acid resulting from I is highly stable in the gas phase. In [12], not a single pathway was found for the conversion of nitronic acid with an activation energy lower than that of the first stage. Therefore, in this study, preference is given to the decomposition through a transition state of the oxazet type. However, it was shown in [13] that this reaction also cannot proceed, since it is completely reversible. The activation energy of the reverse process is only 10.5 kJ/mol versus 125.5 kJ/mol for the direct reaction. Therefore, in [13], another mechanism of the first stage was proposed: the structural enamino-imine rearrangement (path e), taking place with an activation energy of about 200 kJ/mol. Despite the high value of the latter, this decay path is considered the most probable. Apparently, this conclusion is correct for a homogeneous reaction in the gas phase. However, it should be taken into account that many substances in the liquid phase decompose by a different mechanism and much more easily than in the vapor phase.
From the literature (see brief reviews in [3, 14]), it is known that nitronic acids in the condensed state are relatively unstable compounds. Nitronic acids of the aromatic and heterocyclic types, isolated in a free state, decompose at a temperature of 100°C. Due to the possibility of rapid isomerization in the aci-form, the rate of decomposition of liquid nitromethane is 5 and more orders of magnitude higher than in the vapor phase [15].
With a large difference in the reaction rates in the liquid and gas phases, vapor decomposition, as a rule, proceeds heterogeneously, in the layers adsorbed on the walls of the reaction vessel. In this case, the reaction rate, proportional to the amount of adsorbate, will depend on the vapor pressure and adsorption capacity of the surface and its size.
Thus, taking into account the high probability of the rapid heterogeneous decomposition of nitronic acids, it can be assumed that the decomposition of compound I proceeds both in the vapor phase and in the solid state according to reaction (1).
Note that the decomposition mechanism (1) was first proposed in the experimental work [7], in which, based on it, the laws of combustion of I were explained. The same work presents an analysis of the mass spectrometric studies of substance I carried out in [16], and it is shown that nitrile oxide II is one of the primary products of the fragmentation of I with an electronic shock.
Based on the information given in [17] on the transformation of nitrile oxides, it is possible to imagine the secondary processes that occur after reaction (1). At high temperatures, nitrile oxides are characterized by monomolecular isomerization in the isocyanate:

In the presence of amino compounds, isocyanates quickly attach to amino groups to form urea derivatives. Therefore, between compounds II and III, the following reaction may take place:

with the formation of a heavier compound IV, which is also capable of further transformations with the participation of the nitrile oxide group. In addition to isomerization (2), at high temperatures, the abstraction of an oxygen atom with the formation of nitrile is possible:

Oxadiazole is formed in the presence of nitrile:

The rate of reaction (4) is much higher than that of reactions (2) and (3), so it may turn out that compound V is one of the decomposition products.
Compounds of type IV and V, having low volatility, will be deposited on the surface of crystals I, and possibly, fixing on it due to the formation of hydrogen bonds. These compounds contain conjugated C=N bonds; therefore, the film formed by them has a color. Note that a compound with conjugated C=N bonds was discovered in [10] by IR spectroscopy in the final decomposition products of compound I at 215°C. Thus, within mechanism (1), the formation of a film finds a natural explanation.
It should be noted that the film, modifying the surface, prevents only the sublimation of vapors. The decomposition products, as well as the gases desorbed from the inner parts of the crystal, can freely pass through the film through the micropores formed under the pressure of these gases.
The low volatile compounds, which are deposited on the surface, are formed, apparently, in a small concentration. The main decomposition products in the vapor phase are CO2, HCN, N2O, NO2, HOCN, and NO [1], resulting from the oxidation of compound I and its decomposition products by nitrogen dioxide. The latter is formed from HNO3 by the reaction
The decomposition rate constant of 100% HNO3 is 109 times greater than the rate constant of the homogeneous reaction in the gas phase. For this reason, the decomposition of HNO3 vapors proceeds, as with nitronic acids, heterogeneously on the walls of the reaction vessel [3]. By analogy with the oxidation of amines with oxygen, it can be assumed that the reaction with NO2 occurs along the nitrogen atom, and the higher the basicity of the amine the higher the rate of the reaction. For this reason, the decay products will react with NO2 faster than with I.
The described mechanism of film formation makes it possible to explain and predict some of the phenomena that have been or can be observed experimentally. Sublimation of the substance proceeds from the surface of the crystals, regardless of where they are located, on top or inside the bulk sample. However, the free volume between crystals inside the sample is too small, and the nonvolatile products formed during vapor decomposition (with a small, as noted above, yield) are insufficient for the formation of a film. The lack of heavy products can be the reason that the crystals inside the bulk sample are not covered with a film of products and do not change their original yellow color for a long time.
These crystals will be a constant source of vapors of the substance. However, it can be very difficult for the vapors to escape into the volume of the vessel . The vapor flow in narrow passages between crystals should follow the laws of capillary diffusion, and the rate of this process will primarily depend on the vapor pressure Pvp, the pressure of gases in the vessel, and on the mass and shape of the sample.
During the condensation of nonvolatile products from the gas phase, a large amount of heat is released on the surface of the crystals. Therefore, local heating of the surface is possible. Heating can result in a variety of phenomena; in particular, it will contribute to an increase in the rate of processes such as sublimation, decomposition in the gas phase, and film formation.
Gauge Measurements
In the gauge measurements, a high adsorption capacity of compound I, which manifests itself in the strong retention of atmospheric gases and water by crystals, was observed. When a vessel evacuated under standard conditions (15 min at room temperature) is heated to the temperature of the experiment, these gases create an initial pressure P0, which is fixed during preheating. The quantity P0 is poorly reproduced and is weakly dependent on m/V. Thus, in the case of heating up to 200°C at m/V = 10–3 g/cm3, value P0 varies in the range 50–100 Torr, and at m/V = 0.10 g/cm3 it usually lies in the range 150–300 Torr. Preliminary pumping at 110°C for 15 minutes has little effect on the result. When heated to 180–200°C together with the impurity gases, the vapors of the substance itself are released into the vessel. When cooled, they condense simultaneously with water and the adsorption of other gases; therefore, it is impossible to accurately determine the vapor pressure from the gauge measurements. We can only say with confidence that at 200°C value Pvp does not exceed 50 Torr. After heating in a vacuum at a temperature of 180 to 200°C for 10–20 minutes, a weight loss of the substance is observed, but P0, i.e., the desorption of the adsorbed gases may even increase. Apparently, polymorphic transitions facilitate the desorption of impurities from the inner layers of the crystal. Thus, the decomposition of compound I is forced to be carried out in the presence of a small amount of air and water vapor. In order to avoid crossing the decomposition curves, in the figures, the initial value of P0 is not given.
Decomposition at a Temperature of 200°C
A temperature of 200°C is optimal for studying the decay of the compound I in isothermal conditions. At this temperature, a complete kinetic curve can be obtained with a clear separation of the first and second stages and minimal impact of the secondary processes that lead to an increase in the rate of decomposition of the solid.
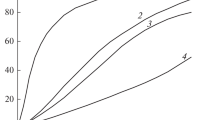
In Fig. 3 in the coordinates Vg – t (Vg is the volume of gaseous products released from 1 g of the substance reduced to normal conditions), the kinetic curves of the complete decomposition, obtained with weighed portions of 2 to 3 mg and m/V ∼ 10–3 g/cm3, are shown. Curve 1 is obtained under the standard conditions for the preparation of the experiment. At the very beginning, there is rapid gas evolution, which proceeds at a decreasing rate and stops after a few hours. This section corresponds to the first peak of the heat released under linear heating conditions. The conversion in the first section is 20% of the total gas evolution (curve 1, Fig. 2) and decreases with increasing m/V (Fig. 4), in the way that it should occur in the case of the decomposition of a certain portion of the substance released into the gas phase.
This is followed by an accelerating process with the maximum rate shifted towards the end of the reaction. This stage corresponds to the second peak of heat released in the DSC curves. At the initial stage of the second stage (1–2% decomposition), the rate does not depend on m/V in the range of values m/V (1–30) × 10–3 g/cm3. This independence is characteristic of a solid-phase process. A slight increase in the slope of the linear section from the very beginning is observed only when m/V = 0.16 g/cm3. Apparently, at such large values of m/V, the acceleration of the solid-phase decomposition begins at a very early stage of the reaction. During the acceleration phase, the rate depends on the ratio m/V increasing with its increase (Fig. 4). This means that the acceleration is caused by the action of gaseous products.
Curve 2 in Fig. 3 has a different form, obtained after the preliminary heating of the sample in a vacuum at 200°C for 20 min. After this heating, the ampule with the substance was cooled to room temperature, sealed in a vacuum, and then heated to the temperature of the experiment. The first stage of decomposition on this curve is practically absent (the yield of the products at this stage decreases by a factor of about 10), which corresponds to the disappearance of the first peak on the DSC curves of the decomposition of compound I in an argon flow after preheating for 20 min at a temperature of about 200°C [8]. The result does not change if the heating time in the vacuum is reduced to 5 minutes or if the cycle of cooling–heating in a vacuum is repeated.
When heated in a vacuum, a film cannot form. After preheating, weight loss of the substance is observed, but the crystals remain yellow. This means that the film is formed at the time when the vessel that has been cooled and sealed in a vacuum is heated to the temperature of the experiment.
The question arises as to why preliminary heating in a vacuum suppresses the first stage or at least reduces its conversion. During heating, in addition to partial sublimation, volatile impurities are removed from the sample surface. It can be assumed that the adsorbed impurities (air and especially water) prevent the contact of condensing products with the crystal surface and interfere with the formation of a film. Therefore, cleaning the surface of the adsorbed gases facilitates the formation of a film, which can occur even before the experimental temperature is reached and the corresponding vapor pressure is established.
As can be seen from Fig. 3, the increase in pressure with time for the heated sample is less pronounced than for the initial one. An obvious reason for this is the absence of the first stage of decomposition in the heated sample. It is possible that during the first stage, which is observed only for an unheated sample, under the action of oxidizing gases, a partial decomposition of the solid occurs and products accumulate, which increase the defectiveness of the crystals, and hence the rate of decomposition.
It should be noted that effective suppression of the gas-phase reaction using preliminary heating in a vacuum is observed only at low values of m and m/V. Therefore, in general, preheating cannot be used to eliminate the first stage.
From the analysis of curve 2 in Fig. 3, it follows that the total gas evolution ∆Vg∞ in the second stage of decomposition, i.e., in the solid-phase decay, is 500 cm3/g or 3.3 moles per mole I. This value ∆Vg∞ is approximate, since in experiments with a sample of a substance of 2 to 3 mg, the determination accuracy m/V did not exceed 20%.
At a temperature of 200°C, the effect of the size of a particle on the kinetics of the decomposition of compound I was tested. Curve 1 in Fig. 3 is also reproduced on a sample with a particle size of ~1 mm. Only the stage of acceleration in this case is somewhat less pronounced. Samples ground in a mortar to reduce particle size when heated to 200°C give a flash at m ~ 20 mg. Ignition occurs in the vapor phase. The entire substance is burned, and the products are deposited on the walls of the vessel in the form of black dust. The flash is not observed if the weight of the crushed samples does not exceed 5 mg. In this case, during the first day, the reaction proceeds in accordance with curve 2 in Fig. 3; i.e., it has the same weakly pronounced first stage as in the sample heated in a vacuum. Since the flash, and vice versa, the almost complete absence of a reaction in the gas phase is related to the crushing of crystals and the mass of the sample, it can be assumed that the reason for these phenomena is the heating of the crystal surface during the condensation of the solid products of the gas-phase decomposition. When there are many crystals and their total surface is large, the heat from condensation is sufficient to stimulate sublimation and quickly warm the vapors to the flash point. When the sample is small, the effect is limited only by the increase in the vapor pressure and their nonexplosive decomposition, resulting in rapid film formation.
In samples taken before or after grinding, the first stage is generally weak if the decomposition is carried out at atmospheric air pressure. A simple explanation of this is that, due to the decrease in the diffusion coefficient in air, during the formation of the film, the vapors of compound I manage to fill the vessel only partially, up to a much lower pressure than Pvp.
The Effect of Mixing
As mentioned above, the hypothesis of the formation of a protective film admits direct verification: the reaction that slowed at the end of the first stage should resume after grinding the crystals or after stirring a sample that is sufficiently representative in weight. Due to the above-mentioned ambiguous behavior of the crushed crystals, the second method, i.e., mixing the sample with m ~ 20 mg, was used. In order to carry out a full cycle of research and not get into the region of acceleration, which at m/V = 0.01 g/cm3 occurs after 1000 min, the reaction was stopped not after the complete completion of the first stage (~300 min) but earlier, after 150 min. Stirring was performed by simply shaking the cooled vessel. The results are shown in Fig. 5. After the first and second stirring, a reproduction of the first stage is observed, albeit, with a slightly reduced gas evolution. After performing stirring twice, the remainder of the substance was ground in a mortar and 6 mg was loaded into the same vessel. The decomposition took place in the same amount of time as during the first heating, but with insignificant gas evolution. A similar result, i.e., a small increase in pressure, was observed earlier for small weighed portions of a ground sample.
Thus, we can reasonably believe that the first stage is the gas-phase decomposition I.
Kinetic Analysis of the First and Second Stages
We can see in Fig. 3 that at 200°C and m/V ∼ 10–3 g/cm3 the first and second stages are clearly separated, which makes it possible to carry out an independent kinetic analysis of each of them. The aim of this analysis is to compare the rates of reactions in the gas and solid phases and, most importantly, to clarify the possibility of determining the rate constant k1 of the first noncatalytic solid state decomposition. It can be assumed that below 100°C, i.e., in the real conditions of using explosives, the first stage disappears and thermal stability I will be entirely determined by the value of k1.
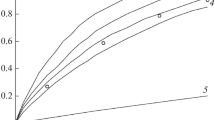
In Fig. 6, curves 1 and 2, taken from Fig. 3, are presented in the initial pressure–time coordinates. In these coordinates, it is convenient to analyze the first stage of decay, by presenting it on an enlarged scale. The initial section of curve 2 in Fig. 6, corresponding to the first stage of decay, is shown (together with the other data) on an enlarged scale in Fig. 7 (curve 5). This curve is strictly described by a first-order equation with a half-life τ1/2 = 40 min, which corresponds to the rate constant kgas = 2.9 × 10–4 s–1. The increase in pressure in the first stage (∆P1) is 85 Torr. The values of kgas and ∆P1, which are close to each other, are obtained by the decomposition of a sample with a large particle size and a sample purified by sublimation in a vacuum (curves 4, 6 in Fig. 7). The strict 1st order of the reaction indicates that vapors do not enter into the vessel during decomposition. This is consistent with the earlier conclusion that the film cuts off the vapors by the time the heating ends. The constancy of ∆P1 on curves 4–6 indicates the same initial vapor pressure. The vessels are probably filled with vapors to a pressure equal to Pvp. From the value of ∆P1 we can make a rough estimate of Pvp at 200°C. If we assume that the stoichiometric coefficient for gas evolution, f, when the vapor is decomposed is equal to three, then Pvp ≈ 42 Torr, and if f = 4, then Pvp ≈ 28 Torr.
Decomposition of compound I at 200°C. Complete kinetic curves 2 and 4 in coordinates P–t, corresponding to curves 1 and 2 (Fig. 3), and the second sections of these curves (1, 3) in coordinates η–t.
The first stage of decomposition of compound I at 200°C and the following values m/V (g/cm3) and preheating conditions (°C/min): (1) 0.1, without warming up (ww); (2) 0.03, ww; (3) 0.01, ww; (4) (△), 0.005 (crystals of 0.1 mm), ww; (5) (⚪), 0.00072, ww; (6) (◻), 0.0023 (sublimated sample), ww; (7) (▷), 0.0075, 180/30; (8) ( ), 0.00082, 200/20.5; (9) (☆), 0.001 (air pressure 1 atm), ww.
), 0.00082, 200/20.5; (9) (☆), 0.001 (air pressure 1 atm), ww.
From Fig. 7 it can be seen that with an increase in m/V the 1st order of the initial stage is distorted. This is due to the contribution from the solid-phase process and an increase in the duration of the first stage due to the diffusion of vapors from the inner layers of the sample. As noted above, the crystals inside the sample are not covered with a film as quickly as outside. At m/V = 0.01 g/cm3, kgas can no longer be determined precisely.
For samples heated in a vacuum at elevated temperatures (curves 7, 8 in Fig. 7), the first stage of decomposition is weakly expressed. The quantity ∆R1 does not exceed 10 Torr. The same is observed if the decomposition is carried out at an air pressure of 1 atm. In both cases, as noted, the film is formed before the vapors fill the volume of the vessel to the pressure Pvp.
In order to consider the second stage of decomposition, we must first separate it from the first stage. To this end, we subtract the increase in pressure ∆P1 obtained in the first stage from curves 2 and 4 in Fig. 6. The values of ∆P1 (85 and 10 Torr for curves 2 and 4, respectively) are found by extrapolation to t = 0 of the linear sections of curves 2 and 4 in Fig. 6, describing the reaction after the end of the first stage. The curves obtained after subtracting ∆P1 reflect the pressure changes Psol due to decomposition in the solid phase. In coordinates η–t (η = Psol/Psol ∞) they are shown in Fig. 6. Curves 1 and 3 in Fig. 6 were obtained, respectively, in experiments without preliminary heating and with heating. They practically merge at the beginning when the noncatalytic reaction is dominant. Then, when the acceleration of the process begins, these curves diverge. The maximum rate during solid-phase decomposition has shifted towards the end of the reaction. The curves are not described by any simple equation. However, even if the rate law is unknown, the value of k1 can be determined in the presence of a complete kinetic curve from the dependence of the specific rate Wsp = (dη/dt)/(1 – η) on η by extrapolating Wsp to value η = 0.
Figure 8 shows the dependence Wsp on η for curves 1 and 3 in Fig. 6. This dependence is linear up to η = 40% for the initial sample and up to η = 80% for the heated sample. Then there is a sharp increase in Wsp. The presence of linear sections on the dependence curves Wsp(η) allows reliable extrapolation and finding the rate constant of the initial stage k1. For the original sample k1 = 2.6 × 10–7 s–1. For a heated sample, it has almost the same meaning. Comparison of the rate constants in the gas and solid phases gives the value of the ratio kgas/ksol ≈ 1000.
Dependence of the specific rate on the conversion of decomposition η for the second stage of decomposition of compound I at 200°C and the following values m/V (g/cm3): (1) 0.00072 (original sample); (2) 0.0008 (sample heated in a vacuum of 0.1 Torr for 20 min at 200°C). Curves 1 and 2 correspond to curves 1 and 3 in Fig. 6.
Thus, the concept of the reaction proceeding in the gas phase makes it possible to explain the variety of facts discovered in the study of the first stage: the actually observed formation of a film; inhibition of the first stage; the independence of the pressure increment of the products at this stage on the size of the crystals, as well as the method of obtaining and purifying the sample at the standard start of the experiment; a sharp decrease in gas evolution in the first stage after preliminary heating in a vacuum or at atmospheric air pressure; a flash during grinding; resuming the reaction after mixing the sample; and a very large difference in the rates of the first and second stages.
CONCLUSIONS
Decomposition I in closed vessels takes place in two stages, as well as in an inert gas flow. The first stage is a side process accompanying the solid-phase reaction, including the sublimation and decomposition of a substance in the gas phase. Vapor pressure I at 200°C is several tens of Torr, and the decomposition rate in the gas phase is about a thousand times higher than in the solid phase. A feature of the first stage is its ability to slow down itself. Upon the decomposition in vapors, nonvolatile compounds are formed, which are deposited on the surface of the crystals in the form of a dense film. The film, preventing sublimation, interrupts the source of the side reaction. At 200°C, the conditions are found under which the first and second stages are clearly separated and can be analyzed independently.
REFERENCES
A. J. Bellamy, Struct. Bond. 125, 1 (2007). https://doi.org/10.1007/430_2006_054
U. Ticmanis, M. Kaiser, G. Pantel, et al., in Proceedings of the 35th International Annual Conference ICT (ICT, Karlsruhe, 2004), p. 70/1.
G. B. Manelis, G. M. Nazin, Yu. I. Rubtsov, et al., Thermal Decomposition and Combustion of Explosives and Propellants (Nauka, Moscow, 1996) [in Russian].
V. V. Nedel’ko, V. V. Zakharov, B. L. Korsunskii, T. S. Larikova, N. V. Chukanov, M. S. Kiselev, and P. I. Kalmykov, Russ. J. Phys. Chem. B 7, 113 (2013). https://doi.org/10.7868/S0207401X13030060
V. V. Nedel’ko, V. V. Zakharov, B. L. Korsunskii, B. L. Korsounskii, T. S. Larikova, N. V. Chukanov, and A. V. Shastin, Russ. J. Phys. Chem. B 9, 885 (2015). https://doi.org/10.1134/S1990793115060172
V. V. Zakharov, N. V. Chukanov, I. N. Zyuzin, V. V. Nedel’ko, and B. L. Korsunskii, Russ. J. Phys. Chem. B 13, 62 (2019). https://doi.org/10.1134/S0207401X19020146
V. P. Sinditskii, A. I. Levshenkov, V. Yu. Egorshev, et al., in Proceedings of the 30th International Pyrotechnics Seminar, Saint-Malo, 2003, Vol. 1, p. 299.
N. V. Garmasheva, I. V. Chemagina, V. P. Filin, et al., in Proceedings of the 36th International Annual Conference ICT (ICT, Karlsruhe, 2005), p. 87/1.
N. N. Volkova, A. V. Shastin, Z. G. Aliev, et al., in Advances in Special Chemistry and Chemical Technology, Proceedings of the All-Russia Conference Dedicated to 80 Years of Foundation of the Chemical Engineering Faculty of Mendeleev Russian Chemical University (RKhTU im. D. I. Mendeleeva, Moscow, 2015), p. 142.
V. V. Zakharov, N. V. Chukanov, N. N. Dremova, et al., Propell. Explos. Pyrotech. 41, 1006 (2016). https://doi.org/10.1002/prep.201600071
A. I. Astrat’ev, D. V. Dashko, A. Yu. Mershin, A. I. Stepanov, and N. A. Urazgil’deev, Russ. J. Org. Chem. 37, 729 (2001). https://doi.org/10.1023/A:1012568305472
G. M. Khrapkovskii, A. G. Shamov, E. V. Nikolaeva, and D. V. Chachkov, Russ. Chem. Rev. 78, 903 (2009). https://doi.org/10.1070/RC2009v078n10ABEH004053
V. G. Kiselev and N. P. Gritsan, J. Phys. Chem. A 118, 8002 (2014). https://doi.org/10.1021/jp507102x
H. Feuer, The Chemistry of the Nitro and Nitroso Groups (Wiley, New York, 1969).
G. M. Khrapkovskii, P. N. Stolyarov, V. P. Dorozhkin, et al., Khim. Fiz. 9, 648 (1990).
H. Oestmark, H. Bergman, U. Baum, et al., in Proceedings of the 32nd International Annual Conference ICT on Energetic Materials: Ignition, Combustion and Detonation (Inst. of ICT, Karlsruhe, 2001), p. 26/1.
L. I. Khmel’nitskii, S. S. Novikov, and T. I. Godovikova, Chemistry of Furoxans. Reactions and Applications (Nauka, Moscow, 1996) [in Russian].
ACKNOWLEDGMENTS
The authors express thank V.G. Prokudin for studying the decomposition of FOX-7 in closed vessels by DSC.
Funding
The study was supported by the Institute of Problems of Chemical Physics, Russian Academy of Sciences (IPCP RAS) on topic 0089-2019-0005 “Fundamental and problem-oriented research in the field of creating energy condensed systems (ECS) for various purposes” (registration number AAAA-A19-119100800130-0) with financial support from the program of the Presidium of the Russian Academy of Sciences “Promising physicochemical technologies for special purposes.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nazin, G.M., Dubikhin, V.V., Goncharov, T.K. et al. Decomposition Kinetics of 1,1-Diamino-2,2-Dinitroethylene (Fox-7). 1. Nature of the First Stage of Decomposition. Russ. J. Phys. Chem. B 15, 483–493 (2021). https://doi.org/10.1134/S1990793121030283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121030283