Abstract—
In the present study, close spatial relationships between peripheral serotoninergic nerve elements and the musculature of the body in planaria Polycelis tenuis, Schmidtea mediterranea, and Girardia tigrina have been demonstrated using immunocytochemical and histochemical methods and a confocal laser scanning microscopy. Such a localization of serotoninergic neurons and their fibers indicates an important role of serotonin in the regulation of the muscle function in planaria. Investigation of the mechanisms of muscle contraction in planaria have shown that depolarization caused by high concentration of potassium ions (15–100 mM) and serotonin (10–4 –10–9 M) induced contractions of isolated muscle fibers of Procerodes littoralis. Dihydropyridine calcium channel blockers, nicardipine, nitrendipine, and nifedipine, inhibited contractions of muscles induced by potassium and serotonin suggesting the dependence of the muscle contraction on the extracellular calcium. Thapsigargin and cyclopiazonic acid decreased the number of muscle cells contracting in response to potassium ions, but did not influence the induction of the contractions by serotonin. Thus, the muscle contraction caused by serotonin did not depend on the intracellular calcium. The results provide evidence of the presence of different types of receptors and ion channels mediating the muscle contraction in planaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In turbellaria (planaria), free-living flatworms, musculature maintains the shape of the body and also participates in various types of motor activity, namely, moving, swimming, searching for prey and capturing it, and performing reproductive behavior. Planaria also use musculature to uptake food through the muscular pharynx. Planaria are used in the study of regeneration and asexual reproduction due to their outstanding regenerative capability; they are an important object in stem cell biology [1, 2]. In addition, planaria are a convenient model for studying the mechanisms of muscle contraction, as well as the search for new antiparasitic drugs [3].
Serotonin (5-hydroxytryptamine, 5-HT) belongs to biogenic amines; it is a low molecular weight nitrogenous compound widely distributed in the animal kingdom [4]. It is found in animals from different taxonomic groups, namely, mammals, crustaceans, insects, mollusks, and worms [5–7]. Serotonin as a neurotransmitter regulates hunger, body temperature, pain sensitivity; it also modulates mood, arousal, sexual behavior, hormone secretion, and plays a role in the immune response in vertebrates [8–10]. Stimulating effect on the muscles of the body stands out among the many properties of serotonin. It is known that serotonin causes contraction of the smooth muscles of the intestine, uterus, bronchi, blood vessels, and regulates the contractile function of skeletal muscles in mammals [9–13]. It also regulates the contraction of intestinal muscles in insects [14]. However, the presence of serotonin and its functional role in most invertebrates have not been sufficiently studied.
Parasitic species, belonging to the classes trematodes, cestodes and monogeneas, are the most studied among Platyhelminthes, as they are of economic and medical importance. Many species of trematodes and cestodes, being parasites of humans and farm animals, cause serious harm to human health and significant economic damage to economic activities (livestock, poultry and fisheries). Serotonin has been found in the nervous system of parasitic worms Schistosoma mansoni, Hymenolepis diminuta, Moniezia expansa, Mesocestoides vogae, Aspidogaster conchicola, Opisthiogliphe ranae, and other species [15, 16]. Free-living representatives of flatworms, planaria, are the least studied in this respect.
In this paper, the spatial location of serotoninergic nerve cells and myofilaments in planaria was studied to test a hypothesis on the regulatory role of serotonin in the functioning of their muscles. Immunohistochemical and physiological studies have been conducted to shed light on the role of serotonin in muscle contraction in planaria. Close spatial interaction of serotoninergic neurons and their processes with the body muscles of planaria Polycelis tenuis, Schmidtea mediterranea, and Girardia tigrina has been revealed. It has been shown that serotonin, as well as excess of potassium ions (K+), induced muscle contraction in the planarian Procerodes littoralis.
The studies are of theoretical and practical interest; they reveal some regulatory mechanisms of excitation and contraction of muscles in planaria. The obtained data can be used in the development of new antiparasitic drugs, the target of which is the musculature of parasitic worms.
MATERIALS AND METHODS
The species of planaria Polycelis tenuis, Schmidtea mediterranea, and Girardia tigrina were used for the determination of serotonin in the nervous system of planaria. Research was carried out in ICB RAS (Pushchino, Russia). Physiological studies on the planarian Procerodes littoralis were carried out at Queen’s University, Belfast, UK. Frozen sections of P. tenuis and S. mediterranea and whole mount preparations of G. tigrina of 9–10 mm long were prepared for histochemical detection of muscles and immunocytochemical determination of serotoninergic nerve components. The samples were fixed with 4% paraformaldehyde (MP Biomedicals, USA) in 0.1 M phosphate buffer (PBS, pH 7.4; Helicon, Russia) for 4 h at room temperature; the subsequent procedures were performed at 4°C. To prepare the whole mount preparations, the samples were washed in PBST containing PBS with 0.3% Triton X-100 (Sigma), 0.1% sodium azide and 0.1% bovine serum albumin (Amresco, USA). The samples were placed in a solution of primary polyclonal (whole serum) rabbit unconjugated antibodies against serotonin (Immunostar, USA, cat. no. 20080, concentration 1 : 500; or Sigma, USA, cat. no. 5545, concentration 1 : 1000) for 48 h, washed in PBS and incubated in a medium containing secondary FITC-conjugated swine anti-rabbit antibodies (Daco, Denmark, cat. no. F-025, dilution 1: 40, 48 h) or secondary goat anti-rabbit conjugated with Alexa Fluor488 antibodies (Molecular Samples, USA, cat. no. A1108, dilution 1 : 100, 48 h). For the preparation of frozen sections, the fixed material was placed in a 10% sucrose solution (Helicon, Russia) for 3–5 days; the frozen sections were prepared using an embedding medium Tissue Tek (Tissue Tek, USA) on the Shandon Cryomatrix cryotome (Termoelectron Corporation, USA) at a temperature of –18…–20°C. The slices were collected on poly-L-lysine-treated slide glasses (Polysine, Menzel-Glaser, Germany), dried in air for 1 h and stored at –20°C. The preparations were washed in PBST (3 times for 5 min in a horizontal position) and stained in a wet chamber at 4°C with antibodies against serotonin (Immunostar, USA, 1 : 1000 or Sigma, 1 : 1000) for 48 h. After washing in PBS (3 times for 5 min), the preparations were treated with secondary immunoglobulins (Daco, Denmark, 1 : 50) for 24 h and again washed in PBS (5 min 3 times). To identify the musculature of the body, the preparations were stained with TRITC-(tetramethylrhodamine isothiocyanate)-labeled phalloidin (Sigma, 1 : 200) for 6–12 h. Finally, the preparations were washed with PBS, mounted in a 75% glycerol solution (Helicon, Russia), and covered with a cover glass.
Negative controls included samples incubated in the solution without primary antibodies (1) and in nonimmune serum instead of the immune one (2).
Microscopy. The stained sections were examined with a Leica DM6000 B fluorescence microscope (Leica Mycrosystems, Germany) equipped with a DC300F digital camera (Leica Mycrosystems, Germany) at the Optical Microscopy and Spectrophotometry core facilities, ICB RAS, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences” (Pushchino). The filter of transmitted light for bright field examination (BF) was used, as well as filters for the excitation of fluorescence with wavelengths in the range of 450–490 nm for localization of fluorochromes FITC (fluorescein isothiocyanate) and Alexa488 (I3), and with wavelengths in the range of 515–560 nm for localization of fluorochrome TRITC (tetramethylrhodamine isothiocyanate) (N2.1) were used.
The whole mount preparations of planaria were analyzed with Leica TCS SP5 confocal laser scanning microscope (Leica Mycrosystems, Germany). The micrographs from confocal laser scanning microscope were presented in the form of a maximal projection of 12–32 consecutive optical sections obtained by scanning through a tissue of 30–60 μm thick and summed (to obtain a total image if necessary) with a maximal fluorescence intensity using the image analysis software supplied with the confocal microscope. Micrographs obtained with the conventional fluorescence and confocal microscopes were saved as TIFF files. The sizes of the images varied depending on the microscope and magnification (for example, from 1024 × 1024 to 4096 × 4096 pixels). Five to seven preparations of each species of planaria were used for the analysis.
Physiology. Isolated muscle fibers of planaria were used for the study of muscle contraction. The method of isolation of individual muscle fibers of parasitic worms was successfully used earlier [17]. Muscle fibers were obtained by enzymatic digestion. The tissue homogenate was prepared from 25 individuals (P. littoralis) with a thin scalpel and placed in an incubation medium (IM) containing 13.6 mM CaCl2, 13.4 mM KCl, 458 mM NaCl, 9.8 mM MgCl2 · 6H2O, 13.6 mM Na2SO4, 10 mM HEPES, 10 mM D-glucose, and 1% antibiotic/antimicotic solution (GibcoBRL, UK) with 0.11 mg/mL collagenase (type 1A, from Clostridium hystoliticum, Sigma), 0.15 mg/mL protease (type XIV, from Streptomyces griseus, Sigma), and 0.15 mg/mL dithiothreitol (Sigma); the samples were left to stand for 12 h at 4°C.
The resulting mixture of tissue fragments and medium was stirred on a magnetic stirrer for 10 min and gently passed through a thin tip of the pipette. The suspension was placed in a 15 mL plastic tubes (Falcon) and centrifuged at 28 g for 5 min (at 4°C). The supernatant was discarded and the sediment was resuspended in the enzyme-free IM for 15 min (4°C). The suspension of muscle cells was placed in 50 mm Petri dishes (Falcon) (3 mL/plate) and left for 30–90 min at 4°C before the examination.
A microperfusion system coupled to inverted microscope Nikon Eclipse TE200 (Japan) was used for the visual observation of muscle contraction. Test substances were injected in close proximity of the muscle cell membrane using an injector and a thin glass micropipette (tip diameter, 5 μm) under the microscope. Responses to the application of substances were investigated in a freshly prepared culture of the muscle cells of planaria. The contraction of the muscle fiber was observed on a monitor screen connected to the microscope. The data were presented as the percentage of muscle fibers that contracted within 30 s after the administration of the test substance. Only immobile muscle cells, which did not contract spontaneously, were used in all experiments. An incubation medium without additives was introduced before using the test substances (negative control); after applying the test substances, a medium with a high (20 mM) content of potassium ions (K+) was applied to the cells (positive control).
One hundred randomly selected muscle cells taken from four Petri dishes were used in one series of experiments. The experiments were repeated at least three times. The effect of serotonin (Sigma) and a medium with a high content of potassium ions (K+, 15–90 mM) in conjunction with some ion-channel antagonists was studied. Calcium channel blockers nicardipine, nitrendipine, nifedipine (dihydropyridines), as well as methoxyverapamil and diltiazem (all substances at concentrations of 10 and 100 μM) were used to study the role of extracellular calcium in the muscle contraction. Inhibitors of Ca2+-ATPase of the sarcoplasmic reticulum, cyclopiazonic acid and thapsigargin, were used to study the participation of intracellular calcium in serotonin-induced muscle contraction.
In all cases, antagonists were added to Petri dishes ten min before the start of the experiment (testing). The Student’s t-test was used for statistical processing of the results. The results of physiological studies were partially published previously in the form of abstracts [18].
RESULTS
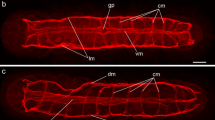
Histochemical examination: musculature. In planaria S. mediterranea, P. tenuis, and G. tigrina, phalloidin staining was found in the musculature of the body wall (Figs. 1a, 1b), as well as in specialized organs (intestine and pharynx, Figs. 1c, 1d) and reproductive system of the planarian P. tenuis, reproducing in a sexual way. The wall of the body of planaria consists of an outer ring (Fig. 1a, short thick arrows) and internal longitudinal muscle fibers (Fig. 1a, long thin arrows), composing compactly packed layers of muscles. There are few loosely spaced diagonal muscle fibers between them (Figs. 1a, 1b, arrowheads). Transverse muscle strands, consisting of several fibers, connect the dorsal and ventral sides of the body. The dorso-ventral muscle fibers are more or less evenly spaced and penetrate the entire body of the planaria (Fig. 1d, arrowheads). The muscles of the pharynx (Fig. 1c, long arrows), having the form of a tube located in the central part of the body, are represented by the circular and longitudinal muscle layers. The muscles located at the base of the pharynx and attaching the pharynx to the muscles of the planarian body, the anchor muscles of the pharynx, have been found (Fig. 1c, short arrows). Phalloidin staining was detected in the thin muscular filaments bordering the branches of the gut of the planaria. The muscles of the intestine contain, at least, circular (Fig. 1d, long arrows) as well as longitudinal muscle fibers (not shown).
Histochemical staining of the muscles of the body of planaria Girardia tigrina (a), Schmidtea mediterranea (b) and Polycelis tenuis (c and d): circular (short thick arrows), longitudinal (long thin arrows) and diagonal (arrowheads) muscle fibers in the body wall of planaria (a, b); pharyngeal muscles (Ph, long arrows); Phc, pharyngeal cavity and anchor muscles (Am) of the pharynx (c, short arrows); intestinal musculature (d), circular fibers (long arrows, intestinal cavity, IC), and, at the top, muscle fibers connecting the ventral and dorsal sides of the body of planaria (arrowheads). (a) Confocal laser scanning microscopy. (b–d) Conventional fluorescence microscopy. Scale bars: (a), 50 μm; (b), 40 μm; (c) and (d), 100 μm.
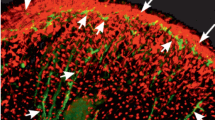
Immunocytochemical examination: serotonin. In planaria S. mediterranea and P. tenuis, positive serotonin staining was found in neurons and fibers of the central nervous system, namely, the brain ganglion, nerve trunks extending from the brain ganglion and extending along the sides of the body, as well as in the transverse commissures connecting the nerve trunks. Serotoninergic nerve components were also found in the central nervous system of the planarian G. tigrina [19]. The present study demonstrated an abundance of serotoninergic nerve elements, the neurons (Figs. 2a, 2c, and 2d, arrowheads) and their fibers (Figs. 2a, 2b, and 2d, thin arrows) in the peripheral parts of the nervous system in three studied species of planaria, S. mediterranea, P. tenuis and G. tigrina. A characteristic feature of the localization of serotoninergic nerve elements in the peripheral parts of the planarian nervous system was their location near the muscle fibers (Figs. 2a and 2b). This arrangement of serotonin neurons and their fibers may indicate the participation of serotonin in the regulation of muscle function in planaria.
Immunocytochemical staining of serotonin (green) and histochemical staining of muscles (red) in planaria Girardia tigrina (a), Schmidtea mediterranea (b) and Polycelis tenuis (c, d). Serotoninergic nerve fibers (thin arrows) and neuron bodies (arrowheads) in close proximity to muscle fibers. (a) Confocal laser scanning microscopy. (b–d) Conventional fluorescence microscopy. Scale bars: (a), 40 μm; (b), 50 μm; (c), 200 μm and (d), 100 μm.
Physiological studies. Physiological studies of the effect of serotonin on the muscle fibers isolated from the planarian P. littoralis were conducted to test the assumption on serotonin involvement in the regulation of muscle function. It was found that potassium ions at high concentrations, as well as serotonin caused the contractions of muscle fibers of the planarian (Figs. 3a and 3b). The excess of potassium ions in the medium (K+) caused a contraction of muscle cells starting from a concentration of 15 mM (about 20%) and induced a contraction of about 80% of muscle fibers at a concentration of 40 mM; the contraction curve reached a plateau at concentrations of potassium ions in the range of 40–80 mM (Fig. 3a). Nicardipine at concentrations of 10 and 100 μM, as well as nitrendipine and nifedipine at concentrations of 100 μM reduced the number of cells contracting in response to high concentrations of potassium ions (Table 1). The data confirm the presence of calcium ion channels in the membrane of the muscle cell of the planarian.
Physiology of muscle contraction of the planaria Procerodes littoralis. (a) The dependence of the intensity of muscle contractions on the concentration of potassium ions (in mM, abscissa); the percentage of muscle fiber contractions (ordinate); at each point of the graph, the number of repetitions of experiments N was from 4 to 20 for different points of the graph; the number of cells in the series n = 100. (b) The dependence of muscle contractions (ordinate) on the decimal logarithm of serotonin concentration (5-HT) (abscissa); the number of repeated experiments N was from 6 to 16 for different points of the graph; the number of cells in one series n = 100. The error bars show standard deviations.
Serotonin caused contractions of muscle fibers of P. littoralis in a dose-dependent manner (Fig. 3b) at concentrations from 10–4 to 10–9 M, when administered near the cell membrane. Dihydropyridine blockers of calcium (Ca2+) channels of L-type significantly (p < 0.001) reduced the number of muscle cells contracting in response to serotonin administration (Fig. 4); the order of the inhibitory activity was as follows: nicardipine > nitrendipine > nifedipine. Diltiazem, a benzothiazepine Ca2+ channel blocker, at concentrations of 10 and 100 µM had no significant effect on serotonin-induced muscle contractions. Methoxyverapamil (10 and 100 µM), a phenylalkylamine Ca2+ channel blocker, did not inhibit serotonin-induced contractions of the muscle cells of the planarian (Fig. 4). Thapsigargin and cyclopiazonic acid (10–4–10–8 M) significantly reduced the number of muscle fibers contracting in response to high potassium concentrations (30 mM, Fig. 5a) but had no significant effect (at 10–5 and 10–7 M) on serotonin-induced muscle contraction (Fig. 5b).
Percentage of contractions of isolated muscle fibers (ordinate) in response to serotonin (5-HT) (10–5 M) (N = 15, the number of cells in one series n = 100) after 10 min of incubation of the cells with dihydropyridine blockers: nicardipine, 10 µM (N = 4) and 100 µM (N = 4); nitrendipine, 10 µM (N = 4) and 100 µM (N = 4); nifedipine, 10 µM (N = 9) and 100 µM (N = 4); and methoxyverapamil, 10 µM (N = 4) and 100 µM (N = 3) and diltiazem, 10 µM (N = 6) and 100 µM (N = 4). The error bars show standard errors of the mean (SE). ***, the differences from the control (5-HT, 10–5 M) are valid at p < 0.001.
(a) The effect of thapsigargin and cyclopiazonic acid on the contractions of isolated muscle fibers of planaria caused by an excess of K+ ions (30 mM). Ordinate indicates the percentage of contracted muscle fibers in response to the potassium ions; abscissa, a decimal logarithm of the concentration of thapsigargin and cyclopiazonic acid added to the culture of muscle cells for 10 min before the experiment. Curve 1, control: mean percent of muscle contractions in response to the introduction of the solution with a high content of potassium ions (30 mM) (N = 18); at the bottom are shown the empirical curves reflecting the responses of muscle fibers after their pre-incubation with cyclopiazonic acid (curve 2) and thapsigargin (curve 3), constructed by Fit spline analysis with the GraphPad Prizm 3.02 statistical software (GraphPad Software, Inc.). The number of repeated experiments at each point of the two lower curves was from 3 to 7. Error bars show standard errors of the mean (SE). (b) Number of contractions of the isolated muscle cells of planaria induced by 10–5 M serotonin (column 1) after preliminary cultivation with 10–5 M thapsigargin (column 2) and 10–5 M cyclopiazonic acid (column 3). Error bars show standard deviations (SD).
DISCUSSION
The development of a histochemical technique for identifying actin filaments using phalloidin, a toxin from the death cap mushroom Amanita phalloides, capable of irreversible binding to the fibrillar actin of a muscle cell, has led to significant progress in the study of the muscle fiber cytoskeleton. Conjugation of phalloidin with a fluorochrome (for example, TRITC or FITC) made it possible to study stained tissues using conventional fluorescence or confocal scanning microscopes [20, 21]. The presence of actin in planaria was demonstrated by Italian researchers in 1992 [22]. However, despite some progress in the study of musculature in representatives of flatworms, and, in particular, planaria, information about its structure and especially about its functioning, is limited for most known species. As an example, the anatomy of the muscular system has been studied in detail only in a few species of planaria, Dugesia japonica [23], G. tigrina, P. tenuis [21, 24], and S. mediterranea [25]. The morphology of the musculature of the planaria G. tigrina, S. mediterranea and P. tenuis used in this study has common features. The muscular wall of the body in these animals is highly ordered and regularly organized; that is, there are internal longitudinal fibers under the outer circular muscle fibers; together they make up compactly packed layers of muscles. Few and rarely located diagonal muscle fibers are localized between the longitudinal and circular layers of myofibrils. The arrangement of myofilaments in the body wall of G. tigrina is less dense compared to S. mediterranea. The most compact packed muscle fibers are in P. tenuis; however the arrangement of layers in G. tigrina does not differ from that of P. tenuis. Dorso-ventral muscle fibers more or less evenly penetrate the entire body of the planaria. The present study showed the conservatism of the general plan of the body musculature structure in the studied species of planaria.
Two neuropeptides, NPF and GNFFRFamide, were identified in the nervous system of the planarian P. littoralis using immunocytochemical method and specific antibodies. The spatial arrangement of peptidergic structures made it possible to describe the basic morphology of the central nervous system in this species of planaria [26, 27]. There is no information about the presence of serotonin in the nervous system of P. littoralis in the available literature. Serotoninergic cells and fibers were found in the nervous system of some other closely related species, for example, Crenobia alpina, Microstomum lineare, Castrella truncate, S. mediterranea, Dendrocoelum lacteum, and Planaria torva (for references see [19]). Serotonin neurons have been described in the pharynx [28] and the central nervous system [19, 29] of G. tigrina.
In the studies focused on the detection of serotonin in planaria, the interaction of serotoninergic nerve fibers and muscle filaments was either not studied at all, or muscle staining during the identification of serotoninergic nerve elements was not carried out. As an example, the close proximity of muscle and serotonin nerve fibers in the planarian S. mediterranea was mentioned in the study of Cebria [30]. The authors of the study [31] observed the penetration of thin serotonin-immunopositive nerve fibers into the muscles of the body in the planarian Bipalium kewense. In the present study, conducted with highly specific staining of tissues with antibodies to serotonin and histochemical staining of muscle filaments with fluorescently labeled phalloidin, a close interposition of serotoninergic nerve elements (cells and fibers) and body muscles has been demonstrated in three species of planaria, P. tenuis, G. tigrina and S. mediterranea. The obtained data may indicate an important role of the neurotransmitter serotonin in the regulation of functional activity of planarian muscles.
The role of serotonin in planaria is poorly understood. There are only a few studies in which serotonin was found to be involved in the regulation of muscle or locomotor activity. It was shown in these studies that serotonin stimulated the beating of cilia, covering the surface of the body of planaria, and promoted their movement in water [32]; it also caused specific movements (“folding” and “twisting”) when added to water [33]. In the literature concerning parasitic species of Platyhelminthes, the role of serotonin in the induction of motor activity of whole worms [34], as well as in muscle preparations or isolated muscle fibers [35, 36] was previously discussed. However, due to the objective difficulties of working with parasitic species, when a large number of animals are required for physiological experiments, the studies were irregular, and the exact mechanisms of action of serotonin on the muscles have not been determined.
Studies conducted on planaria, close relatives of parasitic worms, which are introduced into laboratory culture and available in large quantities for experiments, may shed light on the mechanisms of muscle contraction in flatworms. A technique for producing viable isolated muscle fibers of planaria was developed at Queen’s University of Belfast (UK). This procedure made it possible to investigate the direct effect of the tested substances on a single muscle cell without the influence of the surrounding tissues and nerve impulses. The study of Moneypenny and co-authors [37] was the first to demonstrate the inducing effect of serotonin on the contraction of a single planarian muscle cell. The data presented here is a continuation of the ongoing research on muscle contraction and serotonin receptor identification in planaria. The obtained results confirmed that high concentrations of potassium ions and serotonin cause contractions of muscle fibers of P. littoralis. The studies have shown that both serotonin-induced and depolarization-induced muscle contraction depends on extracellular calcium because it is blocked by dihydropyridine calcium channel blockers (nicardipine, nitrendipine, and nifedipine). Thapsigargin and cyclopiazonic acid, specific inhibitors of Ca2+-ATPase of the endoplasmic reticulum membrane, causing passive outflow of Ca2+ from intracellular depots into the cytosol, significantly reduced the number of muscle contractions in response to high concentrations of potassium ions (depolarization), but did not affect serotonin-induced contractions. Thus, the contraction caused by serotonin was independent of intracellular calcium stores. The results of this study indicated that there were calcium ion channels in planaria (they have not been identified by the time of the experiments yet), which have some properties of calcium channels of vertebrates. The absence of the inhibitory effect of calcium blockers, diltiazem and metoxyverapamil, on the contractions of the muscle cells of planaria may indicate that the calcium channels of planaria differ from the calcium channels of vertebrates. The stimulating effect of serotonin on the musculature of planaria, which depends on extracellular calcium, but does not depend on intracellular calcium stores, indicates the existence of specific serotonin receptors, the mechanism of action of which requires further study.
Some pharmacological and molecular biological studies in addition to physiological studies also indicate the presence of serotonin receptors in free-living and parasitic flatworms [38–43]. It has been suggested that cyclic AMP is mediating a physiological action of serotonin in planaria [38]; however, the receptors themselves at the molecular level at that time had not been identified. Four G protein coupled receptors, named 5HTLpla1–4, were identified in the planarian D. japonica later; their nucleotide sequences have been fully established. These sequences had significant homology with the human 5HT1A serotonin receptor and the Drosophila5HTdrol receptor of Drosophila [39]. Another receptor of planaria, DjSER-7, demonstrated high affinity for serotonin when injected into clawed frog oocytes [40]. Recently, a large number of heptaspiral receptors associated with G proteins have been identified in the genomic databases of a parasitic trematode S. mansoni and a free-living planaria S. mediterranea, of which 24 of S. mansoni and 66 of S. mediterranea are considered as putative aminergic receptors [41, 42]. A decrease in motor activity of adult schistosomes (S. mansoni) has been shown with a decrease in the expression of one of the serotonin receptors (5HTR7) as a result of RNA interference [43]. Thus, our immunocytochemical and physiological studies, along with our previous results [37] and available literature [39, 43] suggest that serotonin receptors of planaria are localized on the membrane of muscle cells and mediate the myostimulatory effect of serotonin. The study of the mechanisms of action of serotonin and the type of receptors through which this biogenic amine exerts its effect in flatworms, planaria, continues. Studies conducted on these relatively simple organisms provide fundamental information about the nature of muscle excitation and contraction and its regulatory mechanisms in animals that are at the origin of the evolutionary development of all Bilateria.
REFERENCES
Baguna J. 1981. Planarian neoblasts. Nature. 290, 14–15.
Grohme M.A., Schloissnig S., Rozanski A., Pippel M., Young G.R., Winkler S., Brandl H., Henry I., Dahl A, Powell S., Hiller M., Myers E., Rink J.Ch. 2018. The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature.554, 56–61. https://doi.org/10.1038/nature25473
Nogi T., Zhang D., Chan J.D., Marchant J.S. 2009. A novel biological activity of praziquantel requiring voltage-operated Ca channel beta subunits: Subversion of flatworm regenerative polarity. PLoS Negl. Trop. Dis. 3 (6), e464.
Page I.H. 1976. The discovery of serotonin. Perspect. Biol. Med.20 (1), 1–8.
Khozhai L.I., Puchkov V.F., Otellin V.A. 1995. The influence of the serotonin deficiency on the embryonic development of mammalians. Ontogenez (Rus.). 26 (5), 350–355.
Vleugels R., Verlinden H., Vanden Broeck J. 2015. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter.2, e314. https://doi.org/10.14800/nt.314
Ivashkin E.G., Khabarova M.Yu., Melnikova V.I., Kharchenko O.A., Voronezhskaya E.E. 2017. Local serotonin-immunoreactive plexus in the female reproductive system of hermaphroditic gastropod mollusc Lymnaea stagnalis.Invertebrate Zoology.14 (2), 134–139.
Baganz N.L., Blakely R.D. 2013. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 4 (1), 48–63.
Plieger T., Melchers M., Vetterlein A., Görtz J., Kuhn S., Ruppel M., Reuter M. 2017. The serotonin transporter polymorphism (5-HTTLPR) and coping strategies influence successful emotion regulation in an acute stress situation: Physiological evidence. Int. J. Psychophysiol.114, 31–37.
Okaty B.W., Commons K.G., Dymecki S.M. 2019. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 20 (7), 397–424.https://doi.org/10.1038/s41583-019-0151-3
Gershon M.D. 2004. Review article: Serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 20 (7), 3–14.
Sung D.J., Noh H.J., Kim J.G., Park S.W., Kim B., Cho H., Bae Y.M. 2013. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp. Mol. Med. 45, e67. https://doi.org/10.1038/emm.2013.116
Zavaritskaya O, Lubomirov L.T., Altay S., Schubert R. 2017. Src tyrosine kinases contribute to serotonin-mediated contraction by regulating calcium-dependent pathways in rat skeletal muscle arteries. Pflugers Arch. 469 (5–6), 767–777. https://doi.org/10.1007/s00424-017-1949-3
French A.S., Simcock K.L., Rolke D., Gartside S.E., Blenau W., Wright G.A. 2014. The role of serotonin in feeding and gut contractions in the honeybee. J. Insect. Physiol.61, 8–15. https://doi.org/10.1016/j.jinsphys.2013.12.005
Hrchkova G., Halton D.W., Maule A.G., Show Ch., Johnston C.F. 1994. 5-Hydroxytryptamine (serotonin)-immunoreactivity in the nervous system of Mesocestoides corty tetrathyridia (Cestoda: Cyclophyllidea). J. Parasitol. 80 (1), 144–148.
Terenina N. B., Gustavsson M. K. S. 2014. Funktsionalnaya morfologiya nervnoi sistemy paraziticheskikh ploskikh chervrey (trematodi, cistidi) (The functional morphology of the nervous system of parasitic flatworms (trematodes, cestodes)). Moscow: KMK Tovarishchestvo nauchnykh izdanii.
Blair K.L., Day T.A., Lewis M.C., Bennett J.L., Pax R.A. 1991. Studies on muscle cells isolated from Schistosoma mansoni: A Ca2+-dependent K+ channel. Parasitol. 102, 251–258.
Totten M., Kreshchenko N., Day T., Marks N., Halton D.W., Maule A.G. 2002. Signal transduction mechanisms mediating muscle contraction in platyhelminth. In: Proceedings of the 10th International Congress of Parasitology. Vancouver, Canada. p. 117.
Kreshchenko N. D. 2016. Immunocytochemical identification of serotonergic neurons in planaria Girardia tigrina. Biol. membrany (Rus.). 33 (5), 353–362.
Wulf E., Deboben A., Bautz F.A., Faulstich H., Wieland T. 1979. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc. Natl. Acad. Sci. USA. 76, 4498–4502.
Kreshchenko N. D. 2017. Some details of the morphological structure of planarian muscles identified by fluorescent and confocal laser scanning microscopy. Biofizika (Rus.). 62 (2), 347–354.
Pascolini R., Panara F., Di Rosa I., Fagotti A., Lorvik S. 1992. Characterization and fine-structural localization of actin- and fibronectin-like proteins in planaria (Dugesia lugubris s.1). Cell. Tiss. Res. 267, 499–506.
Orii H., Ito H., Watanabe K. 2002. Anatomy of the planarian Dugesia japonica. I. The muscular system revealed by antisera against myosin heavy chain. Zoological science. 19, 1123–1131.
Bueno D., Baguñà J., Romero R. 1997. Cell-, tissue-, and position-specific monoclonal antibodies against the planarian Dugesia (Girardia) tigrina.Histochem. Cell. Biol. 107, 139–149.
Cebrià F. 2016. Planarian body-wall muscle: Regeneration and function beyond a simple skeletal support. Front. Cell Devel. Biol. 4, 8. https://doi.org/10.3389/fcell.2016.00008
Reuter M., Gustafsson M.K.S., Sahlgren C., Halton D.W., Maule A.G. Shaw Ch. 1995. The nervous system of Tricladida. I. Neuroanatomy of Procerodes littoralis (Maricola, Procerodidae): An immunocytochemical study. Invert. Neurosci.1, 11–122.
Mäntylä K., Reuter M., Halton D.W., Maule A.G., Brennan G.P., Shaw C., Gustafsson M.K.S. 1998b. The nervous system of Procerodes littoralis (Maricola, Tricladida). An ultrastructural and immunoelectron microscopical study. Acta Zoologica. 79, 1–8.
Kreshchenko N.D., Reuter M., Sheiman I.M., Halton D.W., Johnston R.N., Shaw Ch., Gustafs-son M.K.S. 1999. Relationship between musculature and nervous system in the regenerating pharynx in Dugesia tigrina (Plathelminthes). Invert. Reprod. Dev.35 (2), 109–125.
Reuter M., Gustafsson M.K., Sheiman I.M., Terenina N., Halton D.W., Maule A.G., Shaw C. 1995b. The nervous system of Tricladida. II. Neuroanatomy of Dugesia tigrina (Paludicola, Dugesiidae): An immunocytochemical study. Invert. Neurosci. 1, 133–143.
Cebrià F. 2008. Organization of the nervous system in the model planarian Schmidtea mediterranea: An immunocytochemical study. Neurosci. Res. 61, 375–384.
Fernandes M.C., Alvares E.P., Gama P., Silveira M., 2003. Serotonin in the nervous system of the head region of the land planarian Bipalium kewense.Tissue Cell. 35, 479–486.
Sakharov D.A., Golubev A.I., Malyutina L.V., Kabotyanski E.A., Nezlin L.P. 1988. Serotoninergic control of ciliary locomotion in a turbellarian flatworm. In: Neurobiology of invertebrates: Transmitters, modulators and receptors. Budapest: Akadémiai Kiadó. p. 479–491.
Farrell M.S., Gilmore K., Raffa R.B., Walker E.A. 2008. Behavioral characterization of serotonergic activation in the flatworm Planaria. Behav. Pharmacol. 19 (3), 177–182.
Hrchkova G., Velebny S., Halton D.W., Maule A.G. 2002. Mesocestoides corti (syn.M. vogae): Modulation of larval motility by neuropeptides, serotonin and acetylcholine. Parasitol.124, 409–421.
Graham M.K., McGeown J.G., Fairweather I. 1999. Ionic mechanisms underlying spontaneous muscle contractions in the liver fluke, Fasciola hepatica.Amer. J. Physiology.277, R374–R383.
Blair K.L., Bennet J.L., Pax R.A. 1993. Serotonin and acetylcholine: Further analysis of praziquantel-induced contraction of magnesium-paralysed Schistosoma mansoni.Parasitol.107, 387–395.
Moneypenny C.G., Kreshchenko N., Day T.A., Moffett C., Halton D.W., Maule A.G. 2001. Physiological effects of platyhelminth FMRFamide-related peptides and classical transmitters on dispersed muscle fibres of the turbellarian, Procerodes littoralis.Parasitol. 115, 281–288.
Creti P., Capasso A., Grasso M., Parisi E. 1992. Identification of a 5-HT receptor positively coupled to planarian adenilate cyclase. Cell. Biol. Inter. Rep. 16 (5), 427–432.
Saitoh O., Yuruzume E., Nakata H. 1996. Identification of planarian serotonin receptor by ligand binding and PCR studies. Neuroreport. 8, 173–178.
Nishimura K., Unemura K., Tsushima J., Yamamuchi Y., Otomo J., Taniguchi T., Kaneko S., Agata K., Kitamura Y. 2009. Identification of a novel planarian G-protein-coupled receptor that responds to serotonin in Xenopus laevis oocytes. Biol. Pharm. Bull. 32(10), 1672–1677.
Zamanian M., Kimber M.J., McVeigh P., Carlson S.A., Maule A.G., Day T.A. 2011. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea.BMC Genomics. 12, 596. http://www.biomedcentral.com/1471-2164/12/596
Zamanian M., Agbedanu P.N., Wheeler N.J., McVeigh P., Kimber M.J., Day T.A. 2012. Novel RNAi-mediated approach to G protein-coupled receptor deorphanization: Proof of principle and characterization of a planarian 5-HT receptor. PLoS One. 7 (7), e40787.
Patocka N., Sharma N., Rashid M., Ribeiro P. 2014. Serotonin signaling in Schistosoma mansoni: A serotonin–activated G protein-coupled receptor controls parasite movement. PLOS Pathogens.10 (1), e1003878.
ACKNOWLEDGMENTS
The author thanks Prof. A. G. Maule and Prof. D.W. Halton of Queen’s University of Belfast (Northern Ireland, UK) for the opportunity to work in the laboratory, as well as to Dr. A. Mousley for assistance in the technique of the muscle cell cultivation of planaria and valuable recommendations. Fluorescence microscope with digital camera (Leica Mycrosystems, Germany) was provided by the Optical Microscopy and Spectrophotometry core facilities, ICB RAS (Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences”, Pushchino). The work on the physiology of muscle contraction was supported by a Royal Society Fellowship Program, Great Britain. Immunocytochemical studies on the identification of serotonin in the nervous system of planarian were supported by the Russian Foundation for Basic Research (project no. 18-04-00349a).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author states that there is no conflict of interest.
All procedures were performed in accordance with the European Communities Council Directive (November 24, 1986; 86/609/EEC) and the Declaration on humane treatment of animals. The Protocol of experiments was approved by the bioethics committee of the Institute of Cell Biophysics, RAS.
Additional information
Translated by E. Puchkov
Rights and permissions
About this article
Cite this article
Kreshchenko, N.D. Investigation of the Physiological Role of Serotonin in the Muscle Function in Planaria. Biochem. Moscow Suppl. Ser. A 14, 81–90 (2020). https://doi.org/10.1134/S1990747820010067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820010067