Abstract
Sigma-1 receptors are ubiquitous multifunctional ligand-regulated molecular chaperones in the endoplasmic reticulum membrane with a unique history, structure, and pharmacological profile. Sigma-1 receptors bind ligands of different chemical structure and pharmacological action and modulate a wide range of cellular processes in health and disease, including Ca2+ signaling. To elucidate the involvement of sigma-1 receptors in the processes of Ca2+ signaling in macrophages we studied the effect of sigma-1 receptor ligands, phenothiazine neuroleptics chlorpromazine and trifluoperazine, on Ca2+ responses induced by inhibitors of endoplasmic Ca2+–ATPases thapsigargin and cyclopiazonic acid, as well as by disulfide-containing immunomodulators Glutoxim and Molixan in rat peritoneal macrophages. Using Fura-2AM microfluorimetry we showed for the first time that chlorpromazine and trifluoperazine inhibit both phases of Ca2+ responses induced by Glutoxim, Molixan, thapsigargin, and cyclopiazonic acid in rat peritoneal macrophages. The data obtained indicate the participation of sigma-1 receptors in a complex signaling cascade caused by Glutoxim or Molixan and leading to an increase in intracellular Ca2+ concentration in macrophages. The results also indicate the involvement of sigma-1 receptors in the regulation of store-dependent Ca2+entry in macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ca2+ is a universal second messenger acting in cells of microorganisms, plants, and animals (Berridge et al., 1998; Carafoli and Krebs, 2016). Changes in the transport and intracellular Ca2+concentration, [Ca2+]i, play a key role in triggering and regulating general and specialized cellular functions such as proliferation, growth, secretion, contraction, nerve impulse transmission, immune response, etc. (Berridge et al., 2000, 2003). In cells of the immune system (lymphocytes, mast cells, macrophages), Ca2+ ions work at all stages of cell life, including development, activation, differentiation, production of cytokines, and, finally, cell death (Vig and Kinet, 2009; Trebak, Kinet, 2019).
Sigma-1 receptors, which have a unique history, structure, and pharmacology and modulate a wide range of cellular processes in health and disease, are important participants in the processes of Ca2+ signaling in cells (Su et al., 2010, 2016; Rousseaux and Greene, 2016; Penke et al., 2018; Schmidt and Kruse, 2019; Aishwarya et al., 2021). The International Union of General and Clinical Pharmacology included sigma receptors in the list of receptors only in 2013 as ligand-regulated nonopioid intracellular receptors (Alexander et al., 2013).
Sigma-1 receptors are unique multifunctional ligand-regulated molecular chaperones localized in the endoplasmic reticulum membrane at the border with mitochondria (mitochondria-associated endoplasmic reticulum membrane (MAM)) (Su et al., 2010, 2016; Rousseaux and Greene, 2016; Schmidt and Kruse, 2019; Delprat et al., 2020; Aishwarya et al., 2021). In addition, they can translocate to the plasmalemma and interact with ion channels and other receptors, as well as also being found in the nuclear envelope, where they are involved in the regulation of transcription (Su et al., 2016). These receptors are expressed in various cell types, including cells of the immune system (Rousseaux and Greene, 2016; Penke et al., 2018; Aishwarya et al., 2021).
The sigma-1 receptor was first cloned in 1996 from guinea-pig liver (Hanner et al., 1996) and human placental choriocarcinoma cells (Kekuda et al., 1996). It turned out that the sigma-1 receptor is a protein with a molecular weight of 25 kDa, containing 223 amino acids. The amino-acid sequence of the human sigma-1 receptor is unique and has no homologues among other mammalian proteins (Hanner et al., 1996; Ossa et al., 2017). In 2016, the three-dimensional structure of the human sigma-1 receptor was first established in Kruse’s laboratory using crystallography methods (Shmidt et al., 2016; Kruse, 2017). This receptor was found to be a trimer consisting of three identical protomers. Each protomer contains one transmembrane domain (Shmidt et al., 2016, 2018; Alon et al., 2017; Ossa et al., 2017; Shmidt and Kruse, 2019).
Sigma-1 receptors have a very broad pharmacolo-gical profile. Their ligands are compounds of different chemical structure and pharmacological action: antidepressants (fluvoxamine, sertraline, imipramine), neuroleptics (haloperidol, chlorpromazine), analgesics (pentazocine), anxiolytics (afobazole), anticonvulsants (phenytoin), antitussives (dextromethor-phan, carbetapentane) and antihistamines (chlorphenamine), narcotic drugs (methamphetamine and cocaine), and drugs used in the treatment of neurodegenerative diseases (amantadine, memantine, donepezil) (Cobos et al., 2008; Maurice and Su, 2009; Chu and Ruoho, 2016; Vavers et al., 2019; Voronin et al., 2020). Of the common structural features of the ligands, the cationic amino group and at least one aromatic ring should be noted. Typical neuroleptics (haloperidol, fluphenazine, chlorpromazine, trifluoperazine) have a high affinity for sigma-1 receptors (Tam and Cook, 1984).
Acting as chaperones, sigma-1 receptors interact with target proteins (ion channels, plasmalemma receptors, etc.) and modulate many cellular processes, including Ca2+ signaling (Su et al., 2010, 2016; Schmidt and Kruse, 2019; Pontisso and Combettes, 2021). In the plasmalemma, they interact with voltage-dependent Ca2+-, Na+-, and K+-channels, proton-activated ion channels (ASICs), NMDA receptors, G-protein coupled receptors (muscarinic acetylcholine receptors, μ-opioid and D1- and D2-dopamine receptors), and other target proteins (Su et al., 2010, 2016; Schmidt and Kruse, 2019). In the membrane of the endoplasmic reticulum, the sigma-1 receptor interacts with the type 3 inositol-1,4,5-triphosphate receptor, with another molecular chaperone, BiP protein (binding immunoglobulin protein) (Hayashi and Su, 2007), and STIM1 Ca2+-sensor protein (Srivats et al., 2016). It was found that, when interacting with inositol-1,4,5-triphosphate receptors, sigma-1 receptors modulate Ca2+ signaling in cells: Ca2+ mobilization from the stores (Hayashi et al., 2000; Wu and Bowen, 2008) and Ca2+ entry from the external medium (Monnet, 2005; Hayashi and Su, 2007; Pontisso and Combettes, 2021). They participate in the regulation of the store-dependent Ca2+entry in cells (Brailoiu et al., 2016; Rosado, 2016; Srivats et al., 2016; Berlansky et al., 2021).
We have previously shown for the first time that the sigma-1 receptor antagonist neuroleptic haloperidol (a derivative of butyrophenone) significantly inhibits both phases of Ca2+ responses caused by disulfide-containing immunomodulators Glutoxim® (disodium salt of oxidized glutathione with d-metal in nanoconcentration) and Molixan® (a complex of Glutoxim and inosine nucleoside) (Krutetskaya et al., 2017) and endoplasmic Ca2+-ATPase inhibitors thapsigargin and cyclopiazonic acid (CPA) (Krutetskaya et al., 2018b) in rat peritoneal macrophages.
To confirm the involvement of sigma-1 receptors in the regulation of Ca2+ signaling in macrophages, it seemed appropriate to investigate the effect of other, structurally different, sigma-1 receptor ligands on Ca2+ responses induced by Glutoxim and Molixan, as well as thapsigargin (Thastrup et al., 1989) and CPA (Goeger et al., 1988), in rat peritoneal macrophages, which was the subject of this study.
The sigma-1 receptor ligands chlorpromazine (CP, aminazine, thorazine) (Itzhak et al., 1990; Hayashi, Su, 2004) and trifluoperazine (TFP, triftazine, stelazine) (Schuster et al., 1995; Hanner et al., 1996), belonging to the first generation typical neuroleptics (antipsychotic agents) of the phenothiazine series and having a long history of clinical application for the treatment of schizophrenia and other mental diseases (Dilsaver, 1993; Ayano, 2016), were used in our expe-riments.
MATERIALS AND METHODS
Isolation and Cultivation of Rat Peritoneal Macrophages
Experiments were carried out on cultured resident peritoneal macrophages of Wistar rats. The keeping of animals and all manipulations were performed in accordance with the regulations and requirements of the Order no. 267 of June 19, 2003, of the Ministry of Health of the Russian Federation “On Approval of the Rules of Laboratory Practice in the Russian Federation.”
Resident macrophages were isolated from the peritoneal cavity of rats weighing 180–250 g according to the traditional method (Conrad, 1981; Randriamampita, Trautmann, 1987). Immediately after isolation, the cells were spherical and 10–20 µm in diameter. The cell suspension was placed in culture dishes with quartz glasses (10 × 10 mm) and cultured for 1–3 days at 37°C in medium 199 (pH 7.2) containing 20% bovine serum, glutamine (3%), penicillin (100 U/mL), and streptomycin (100 mg/mL). The α‑naphthylesterase test confirmed that at least 96% of the cells in the monolayers were macrophages (Monahan et al., 1981).
The experiments were carried out at a temperature of 22–24°C 1–2 days after the start of cell cultivation. Quartz glasses with cells were placed in an experimental chamber filled with physiological solution of the following ionic composition (mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES-NaOH, pH 7.3–7.4. The calcium-free medium differed in that it contained 1 mM EGTA and did not contain CaCl2. The studied agents were added to macrophages in a calcium-free medium. To initiate Ca2+ entry into the cells, 2 mM Ca2+ were introduced into the external medium.
Measurement of [Ca2+]i
A Fura-2AM fluorescent probe (Sigma-Aldrich, United States) was used. Macrophages were incubated for 45 min in physiological solution containing 2 μM Fura-2AM at 22–24°C. Glasses with stained cells were washed with physiological solution and transferred to the experimental chamber fixed on the table of Leica DM 4000B fluorescent microscope (Leica Microsystems, Germany). The fluorescence of the object was excited at wavelengths of 340 and 380 nm through the microscope objective. Narrow-band optical filters were used to isolate the corresponding parts of the spectrum. The emission was recorded at a wavelength of 510 nm using a specialized Leica DFC340FX video camera. The experiment was controlled using the ImageJ image processing system (Micro-Manager 1.4 plug-in).
The result of the measurements was the ratio of the fluorescence intensity of Fura-2AM when irradiated with light with a wavelength of 340 nm to the fluorescence intensity when irradiated with light with a wavelength of 380 nm (F340/F380), where F340 is the fluorescence intensity of Fura-2AM associated with Ca2+ and F380 is the fluorescence intensity of Fura-2AM not associated with Ca2+, reflecting changes in [Ca2+]i in cells during measurements (Bruce and Elliott, 2000; Xie et al., 2002). To avoid photobleaching, measurements were taken every 20 s, irradiating the object for 2 s. A 10x objective with an 8 mm aperture was used in the experiments. [Ca2+]i values were calculated using Grynkiewicz equation (Grynkiewicz et al., 1985). Statistical analysis was carried out using Student’s t-test. Data are presented as mean and standard deviation. Each registration was obtained for a group of 40-50 cells. The figures show the results of typical experiments from six to eight independent ones. Differences were considered significant at p ≤ 0.05.
Reagents Used
All reagents were purchased from Sigma-Aldrich (United States). Stock solutions of Fura-2AM (1 mM), CPA (10 mM), and thapsigargin (0.5 mM) were prepared in dimethyl sulfoxide. The drugs Glutoxim and Molixan were provided by PHARMA-VAM (St. Petersburg). Stock solutions of Glutoxim (50 mg/mL), Molixan (50 mg/mL), TFP (2 mg/mL), and CP (25 mg/mL) were prepared in water.
RESULTS AND DISCUSSION
The Effect of Chlorpromazine and Trifluoperazine on Ca2+ Responses Induced by Disulfide-Containing Immunomodulators
Pharmacological analogues of oxidized glutathione (Glutoxim and Molixan) are used as immunomodulators and cytoprotectors in the complex therapy of bacterial, viral and oncological diseases (Borisov et al., 2001; Sokolova et al., 2002; Antushevich et al., 2013; Tolstoy et al., 2019). These drugs have a complex effect on the processes of redox regulation in cells, but the subtle biophysical mechanisms of their action are far from being fully understood.
In the present work, control experiments showed that incubation of macrophages for 20 min with 100 μg/mL Glutoxim (Fig. 1a) or 100 μg/mL Molixan (Fig. 2a) in a calcium-free medium causes a slowly growing increase in [Ca2+]i, reflecting Ca2+ mobilization from intracellular Ca2+ stores. On average, twenty minutes after the addition of agents, [Ca2+]i increased from the basal level of 90 ± 18 to 135 ± 18 nM (n = 7; p < 0.05) for Glutoxim and 134 ± 20 nM (n = 6; p < 0.05) for Molixan. Upon introduction of 2 mM Ca2+ into the external medium, a further increase in [Ca2+]i was observed, reflecting the store-dependent Ca2+ entry into the cytosol (Figs. 1, 2). On average, the increase in [Ca2+]i during Ca2+ entry was 223 ± 22 nM (n = 7; p < 0.05) and 202 ± 20 nM (n = 6; p < 0.05) for Glutoxim and Molixan, respectively.
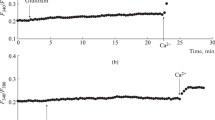
The effect of chlorpromazine (CP, 25 µg/mL) and trifluoperazine (TFP, 2 µg/mL) on the [Ca2+]i increase induced by Glutoxim in rat peritoneal macrophages. Here and in Figs. 2–4, the ratio of Fura-2AM fluorescence intensities at excitation wavelengths of 340 and 380 nm (F340/F380, arb. units) is along the ordinate axis; the abscissa axis is time. Stimulation conditions: (a) macrophages were incubated for 20 min in the presence of 100 μg/mL Glutoxim in a nominally calcium-free medium; then, Ca2+ entry was initiated by introducing 2 mM Ca2+ into the external medium; (b, c) macrophages were incubated for 10 min with CP (b) or for 15 min with TFP (c) in a calcium-free medium; then, 100 µg/mL Glutoxim was added; 20 min later, Ca2+ entry was initiated by introducing 2 mM Ca2+ into the external medium. Here and in Figs. 2–4, each recording was obtained for a group of 40–50 cells and represents a typical variant of six to eight independent experiments.
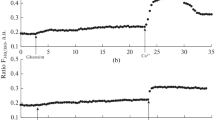
The effect of CP and TFP on the [Ca2+]i increase induced by Molixan (100 μg/mL) in rat peritoneal macrophages. (a–c) Conditions of preliminary stimulation in a calcium-free medium and subsequent initiation of Ca2+ entry are the same as those indicated in the legend to Fig. 1.
In our experiments, it was found for the first time that preincubation of peritoneal macrophages with 25 μg/mL CP for 10 min before the administration of 100 μg/mL Glutoxim led to a significant suppression of both Ca2+ mobilization from the stores (by 58.5 ± 4.6% , n = 7; p < 0.05) and the subsequent store-dependent Ca2+ entry into cells (by 59.1 ± 6.1%, n = 7; p < 0.05) induced by Glutoxim (Fig. 1b). Preincubation of cells with 2 μg/mL TFP for 15 min prior to administration of 100 μg/mL Glutoxim also caused suppression of the Ca2+ mobilization phase from the stores (by 36.2 ± 5.7%, n = 8; p < 0.05) and store-dependent Ca2+ entry into macrophages (by 60.7 ± 7.1%, n = 7; p < 0.05), caused by Glutoxim (Fig. 1c).
Similar results were obtained in experiments on the effect of CP and TFP on Ca2+ responses induced by 100 μg/mL Molixan in macrophages (Figs. 2b, 2c). Thus, suppression of the phase of Ca2+ mobilization from the stores averaged 43.2 ± 8.9% (n = 8; p < 0.05) and 63.3 ± 2.4% (n = 7; p < 0.05), while the suppression of the store-dependent Ca2+ entry into macrophages averaged 52.3 ± 9.1% (n = 8; p < 0.05) and 65.3 ± 5.0% (n = 7; p < 0.05) for CP and TFP, respectively.
The Effect of Phenothiazine Neuroleptics on Ca2+ Responses Induced by Inhibitors of Endoplasmic Ca2+ ATPase
In control experiments, we found that the addition of 0.5 μM thapsigargin to macrophages in a calcium-free medium caused a slight increase in [Ca2+]i, reflecting Ca2+ mobilization from the intracellular Ca2+ stores (Fig. 3a). On average, the increase in [Ca2+]i during the mobilization phase was 26 ± 7 nM (n = 7; p < 0.05). Subsequent addition of 2 mM Ca2+ to the external medium resulted in the store-dependent Ca2+ entry into the cytosol (Fig. 3a). On average, the increase in [Ca2+]i during Ca2+ entry was 160.2 ± 20.5 nM (n = 7; p < 0.05). We obtained similar results when using 10 µM CPA (Fig. 4a): on average, the increase in [Ca2+]i during the phase of Ca2+ mobilization from the stores, induced by CPA, was 37.8 ± 9.8 nM (n = 7; p < 0.05), while it was 150.2 ± 23.7 nM (n = 7; p < 0.05) during the store-dependent Ca2+ entry into macrophages (Fig. 4a).
The effect of CP (25 µg/mL) and TFP (2 µg/mL) on Ca2+ responses induced by thapsigargin (0.5 μM) in rat peritoneal macrophages. (a–c) Experimental conditions are the same as those indicated in the legend to Fig. 1.
The effect of CP (25 µg/mL) and TFP (2 µg/mL) on Ca2+ responses induced by cyclopiazonic acid (CPA, 10 μM) in rat peritoneal macrophages. (a–c) Experimental conditions are the same as those indicated in the legend to Fig. 1.
In our experiments, it was found for the first time that the preincubation of macrophages with 25 µg/mL CP in nominally calcium-free medium for 10 min before the administration of 0.5 µM thapsigargin causes a significant suppression of both phases of Ca2+ responses induced by thapsigargin (Fig. 3b). Thus, suppression of the phase of Ca2+ mobilization from the stores was 59.3 ± 8.2% (n = 7; p < 0.05), while the inhibition of store-dependent Ca2+ entry was 68.2 ± 10.4% (n = 7; p < 0.05). Similar results were obtained in experiments using 10 µM CPA (Fig. 4b). The suppression of Ca2+ mobilization from the stores amounted to 40.2 ± 9.1% (n = 7; p < 0.05), and the suppression of the store-dependent Ca2+ entry was 63.4 ± 11.5% (n = 7; p < 0.05).
Another phenothiazine neuroleptic, TFP, also significantly suppressed both phases of Ca2+ responses induced by thapsigargin or CPA. It was shown that preliminary incubation of cells with 2 µg/mL TFP for 10 min before the introduction of 0.5 µM thapsigargin (Fig. 3c) caused suppression of the phase of Ca2+-mobilization from the stores (by 59.0 ± 9.4%, n = 7; p < 0.05) and inhibition of the store-dependent Ca2+ entry into macrophages (by 73.5 ± 11.2%, n =7; p < 0.05), caused by thapsigargin (Fig. 3c). Similar data were obtained in experiments using 10 µM CPA (Fig. 4c). The suppression of Ca2+ mobilization from the stores amounted to 40.1 ± 9.7% (n = 7; p < 0.05), and the suppression of the store-dependent Ca2+ entry was 60.4 ± 10.8% (n = 7; p < 0.05). This confirms our earlier data that the preincubation of cells with TFP leads to suppression of the store-dependent Ca2+ entry induced by inhibitors of endoplasmic Ca2+–ATPase thapsigargin and CPA, in rat peritoneal macrophages (Krutetskaya et al., 2018a).
Thus, in the present work, we have shown for the first time on rat peritoneal macrophages that the sigma-1 receptor ligands, phenothiazine neuroleptics CP and TFP, suppress both phases of Ca2+ responses caused by Glutoxim or Molixan, as well as thapsigargin and CPA, in peritoneal macrophages. The results are consistent with the data of studies by other authors, who found that sigma-1 receptor ligands, CP and TFP, suppress Ca2+ mobilization from the stores and subsequent store-dependent Ca2+ entry, induced by ATP or thapsigargin in human leukemia cells (HL-60 line) (Harper et al., 1997; Harper and Daly, 1999). It has also been shown that CP inhibits the store-dependent Ca2+ entry induced by bradykinin or thapsigargin in rat pheochromocytoma cells (PC12 line) (Choi et al., 2001), as well as preincubation of cells with TFP leads to significant suppression of store-dependent Ca2+ entry caused by thapsigargin in human embryonic kidney cells (HEK-293 line) (Wang et al., 2015). It was also found that sigma-1-receptor antagonists (compounds BD1063 and BD1047) inhibit store-dependent Ca2+ entry induced by histamine in human saphenous vein endothelial cells (Amer et al., 2013), while BD1063 significantly suppresses the store-dependent Ca2+ entry caused by thapsigargin in human mammary adenocarcinoma cells (MCF7 line) (Gasparre et al., 2017). In addition, it is known that CP and TFP inhibit voltage-dependent Ca2+ channels in various cell types. Thus, CP reversibly and dose-dependently blocks L- and T‑type voltage-dependent Ca2+ channels in mouse neuroblastoma cells (N1E-115 line) (Ogata and Narahashi, 1990; Ogata et al., 1990), R-type voltage-dependent Ca2+ channels in human neurons (McNaughton et al., 2001), and L-type Ca2+ channels in rat pheochromocytoma cells (PC12 line) (Ito et al., 1996), while TFP blocks L-type voltage-dependent Ca2+ channels in rat smooth muscle cells (Nakazawa et al., 1993) and Helix aspersa neurons (Cruzblanca et al., 1998).
The results of this and our earlier works (Krutetskaya et al., 2017, 2018c) on the suppression by sigma-1 receptor ligands of Ca2+ responses induced by Glutoxim and Molixan in macrophages indicate the involvement of sigma-1 receptors in the complex signaling cascade triggered by Glutoxim or Molixan and leading to an increase in [Ca2+]i in rat peritoneal macrophages. The results also indicate that the combined use in clinical practice of the drugs Glutoxim or Molixan and phenothiazine neuroleptics CP and TFP is undesirable.
Our data also suggest the involvement of sigma-1 receptors in the regulation of the store-dependent Ca2+ entry induced by disulfide-containing immunomodulators and inhibitors of endoplasmic Ca2+–ATPase in rat peritoneal macrophages and allow us to consider sigma-1 receptors as a new regulatory component of the signaling complex of the store-dependent Ca2+ entry in macrophages. Sigma-1 receptors may affect store-dependent Ca2+ entry by modulating the binding between the main components of the protein complex of the store-dependent Ca2+ entry - STIM1 proteins in the endoplasmic reticulum membrane and Orai1 channels in the plasmalemma (Srivats et al., 2016).
The results may also contribute to a more detailed understanding of the molecular mechanisms of the pharmacological action of phenothiazine neuroleptics. In addition, the data obtained may be of importance for the treatment of diseases mediated by impaired functioning of sigma-1 receptors. Thus, changes in the subcellular localization, expression, and signaling functions of sigma-1 receptors are known to lead to the development of a wide range of human diseases (Su et al., 2010, 2016; Rousseaux and Greene, 2016; Schmidt and Kruse, 2019; Aishwarya et al., 2021). The involvement of these receptors in the pathophysiology of neuropsychiatric disorders (schizophrenia, anxiety disorders, depressive states, and dementia) (Hayashi and Su, 2004; Tsai et al., 2009, 2014; Ishikawa and Hashimoto, 2010; Hayashi, 2015; Voronin et al., 2020), neurodegenerative (Alzheimer’s, Huntington’s, and Parkinson’s diseases; amyotrophic lateral sclerosis) (Ryskamp et al., 2017, 2019; Penke et al., 2018; Hayashi, 2019; Schmidt, Kruse, 2019; Yang et al., 2019; Herrando-Grabulosa et al., 2020; Zhemkov et al., 2021), oncological (Kim and Maher, 2017; Soriani and Rapetti-Mauss, 2017; Pontisso and Combettes, 2021) and cardiovascular (Stracina and Novakova, 2018; Aishwarya et al., 2021) diseases; pain syndromes (Merlos et al., 2017a, 2017b) and retinopathy (Wang et al., 2017; Smith et al., 2018) has been revealed. This made it possible to consider sigma-1 receptors as promising pharmacological targets for the treatment of these diseases.
Recently, the possible role of sigma-1 receptors in the pathophysiology of coronavirus infection (COVID-19) has also been studied. Evidence is emerging that sigma-1 receptors may be a promising therapeutic target in the treatment of patients with COVID-19. It is believed that sigma-1 receptors regulate the key mechanisms of the adaptive stress response of host cells and are involved in the early stages of viral replication (Vela, 2020; Hashimoto, 2021).
Many repurposed drugs included in complex the-rapy regimens for patients with COVID-19 are often identified as sigma-1 receptor ligands. These include the neuroleptics haloperidol, CP, and TFP (Plaze et al., 2020; Vela, 2020). It is believed that CP is the most promising drug (Muric et al., 2020; Nobile et al., 2020; Plaze et al., 2020; Stip, 2020; Stip et al., 2020). There is evidence that cationic amphiphilic compounds, which include phenothiazine neuroleptics, have antiviral activity and inhibit the entry and replication of RNA viruses (Otręba et al., 2020; Vela, 2020; Gitahy Falcao Faria et al., 2021). Thus, CP has been shown to inhibit SARS-CoV-2 replication in monkey cells (VeroE6 line) and human alveolar epithelial cells (A549-ACE2 line) (Plaze et al., 2021). In addition, the sigma-1 receptor ligands haloperidol (Hoertel et al., 2021a) and CP (Hoertel et al., 2021b) have already passed clinical trials as drugs for the treatment of patients with COVID-19.
It is also known that viruses have evolved mechanisms to disturb host cell Ca2+ homeostasis and increase [Ca2+]i, because Ca2+ is essential for virus entry, replication, maturation and release (Zhoua et al., 2009; Chen et al., 2019). In this regard, the inhibiton of virus-induced [Ca2+]i increase via inhibiting calcium release channels in the endoplasmic reticulum membrane (inositol-1,4,5-triphosphate receptors and ryanodine receptors) or Ca2+ entry channels in the plasmalemma (voltage- and store-dependent Ca2+ channels) is one of the approaches in the treatment of viral infections (Chen et al., 2019). Thus, it was found that blockers of the voltage-dependent Ca2+ channels nifedipine and amlodipine reduce mortality and decrease the risk for mechanical ventilation in elderly patients with COVID-19 and hypertension (Solaimanzadeh, 2020; Zhang et al., 2020).
Thus, our data on the suppression of both phases of Ca2+ responses, induced by disulfide-containing immunomodulators and inhibitors of endoplasmic Ca2+–ATPases in rat peritoneal macrophages, by sigma-1 receptor ligands (CP and TFP), further confirm the versatility of the effects of phenothiazine derivatives and suggest that phenothiazine neuroleptics have therapeutic potential as sigma-1 receptor ligands.
REFERENCES
Aishwarya, R., Abdullah, C.S., Morshed, M., Remex, N.S., and Bhuiyan, M.S., Sigmar1’s molecular, cellular, and biological functions in regulating cellular pathophysiology, Front. Physiol., 2021, vol. 12, Art. ID 705575. https://doi.org/10.3389/fphys.2021.705575
Alexander, S.P.H., Benson, H.E., Faccenda, E., Pawson, A.J., Sharman, J.L., McGrath, J.C., Catterall, W.A., Spedding, M., Peters, J.A., and Harmar, A.J., The concise guide to pharmacology 2013/14: overview, Br. J. Pharmacol., 2013, vol. 170, p. 1449 https://doi.org/10.1111/bph.12444
Alon, A., Schmidt, H., Zheng, S., and Kruse, A.C., Structural perspectives on sigma-1 receptor function, Adv. Exp. Med. Biol., 2017, vol. 964, p. 5.
Amer, M.S., McKeown, L., Tumova, S., Liu, R., AL Seymour, V., Wilson L.A., Naylor, J., Greenhalgh, K., Hou, B., Majeed, Y., Turner, P., Sedo, A., O’Regan, D.J., Li, J., Bon, R.S., Porter, K.E., and Beech, D.J., Inhibition of endothelial cell Ca2+ entry and transient receptor potential channels by sigma-1 receptor ligands, Br. J. Pharmacol., 2013, vol. 168, p. 1445.
Antushevich, A.A., Antonov, V.G., Grebenyuk, A.N., Antushevich, A.E., Ladanova, T.V., and Burova, E.B., Pathophysiologic rationale of effectiveness of glutoxim supportive therapy add-on to radiotherapy management of oropharyngeal cancer, Vestn. Ross. Voenno-Med. Akad., 2013, vol. 3, no. 43, p. 32.
Ayano, G., First generation antipsychotics: pharmacokinetics, pharmacodynamics, therapeutic effects and side effects: a review, Res. Rev. J. Chem., 2016, vol. 5, p. 53.
Berlansky, S., Humer, C., Sallinger, M., and Frischauf, I., More than just simple interaction between STIM and Orai proteins: CRAC channel function enabled by a network of interactions with regulatory proteins, Int. J. Mol. Sci., 2021, vol. 22, p. 471. https://doi.org/10.3390/ijms22010471
Berridge, M.J., Bootman, M.D., and Lipp, P., Calcium, a life and death signal, Nature, 1998, vol. 395, p. 645.
Berridge, M.J., Lipp, P., and Bootman, M.D., The versatility and universality of calcium signaling, Nat. Rev. Mol. Cell Biol., 2000, vol. 1, p. 11.
Berridge, M.J., Bootman, M.D., and Roderick, H.L., Calcium signalling: dynamics, homeostasis and remodeling, Nat. Rev. Mol. Cell Biol., 2003, vol. 4, p. 517.
Borisov, A.E., Kozhemyakin, L.A., Antushevich, A.E., Ketliskaya, O.S., Kashchenko, V.A., Chepur, S.V., Katsalucha, V.V., Vasyukova, E.L., Novichenkov, A.O., and Motushchuk, I.E., Clinical and experimental grounds of the regional and systemic administration of the thiopoetin group medicines for cirrhosis of the liver. First communication, Vestn. Khir. im. I.I. Grekova, 2001, vol. 4, no. 2, p. 32.
Brailoiu, G.C., Deliu, E., Console-Bram, L.M., Soboloff, J., Abood, M.E., Unterwald, E.M., and Brailoiu, E., Cocaine inhibits store-operated Ca2+ entry in brain microvascular endothelial cells: critical role for sigma-1 receptors, Biochem. J., 2016, vol. 473, p. 1.
Bruce, J.I.E. and Elliott, A.C., Pharmacological evaluation of the role of cytochrome P450 in intracellular calcium signaling in rat pancreatic acinar cells, Brit. J. Physiol., 2000, vol. 131, p. 761.
Carafoli, E. and Krebs, J., Why calcium? How calcium became the best communicator, J. Biol. Chem., 2016, vol. 291, art ID 20849.
Chen, X., Cao, R., and Zhong, W., Host calcium channels and pumps in viral infections, Cells, 2019, vol. 9, p. 94. https://doi.org/10.3390/cells9010094
Choi, S.-Y., Kim, Y.-H., Lee, Y.-K., and Kim, K.-T. 2001. Chlorpromazine inhibits store-operated calcium entry and subsequent noradrenaline secretion in PC12 cells, Br. J. Pharmacol., 2019, vol. 132, p. 411.
Chu, U.B. and Ruoho, A.E., Biochemical pharmacology of the sigma-1 receptor, Mol. Pharmacol., 2016, vol. 89, p. 142.
Cobos, E.J., Entrena, J.M., Nieto, F.R., Cendán, C.M., and Del Pozo, E., Pharmacology and therapeutic potential of sigma (1) receptor ligands, Curr. Neuropharmacol., 2008, vol. 6, p. 344.
Conrad, R.E., Induction and collection of peritoneal exudate macrophages, in Manual of Macrophages Methodology, New York: Marcell Dekker, 1981, p. 5.
Cruzblanca, H., Gamiño, S.M., Bernal, J., and Alvarez-Leefmans, F.J., Trifluoperazine enhancement of Ca2+-dependent inactivation of L-type Ca2+ currents in Helix aspersa neurons, Invert. Neurosci., 1998, vol. 3, p. 269.
Delprat, B., Crouzier, L., Su, T.-P., and Maurice, T., At the crossing of ER stress and MAMs: a key role of sigma-1 receptor?, Adv. Exp. Med. Biol., 2020, vol. 1131, p. 699.
Dilsaver, S.C., Antipsychotic agents: a review, Am. Fam. Phys., 1993, vol. 47, p. 199.
Gasparre, G., Abate, C., Carlucci, R., Berardi, F., and Cassano, G., The σ1 receptor agonist (+)-pentazocine increases store-operated Ca2+ entry in MCF7σ1 and SK-N-SH cell lines, Pharmacol. Rep., 2017, vol. 69, p. 542.
Gitahy Falcao Faria, C., Weiner, L., Petrignet, J., Hingray, C., Ruiz De Pellon Santamaria, A., Villoutreix, B.O., Beaune, P., Leboyer, M., and Javelot, H., Antihistamine and cationic amphiphilic drugs, old molecules as new tools against the COVID-19?, Med. Hypotheses, 2021, vol. 148, art. ID 110508. https://doi.org/10.1016/j.mehy.2021.110508
Goeger, D. E., Riley, R. T., Dorner, J. W., and Cole, R.J., Cyclopiazonic acid inhibition of the Ca2+ transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles, Biochem. Pharmacol., 1988, vol. 37, p. 978.
Grynkiewicz, G., Poenie, M., and Tsien, R.Y., A new generation of Ca2+ indicators with greatly improved fluorescence properties, J. Biol. Chem., 1985, vol. 260, p. 3440.
Hanner, M., Moebius, F.F., Flandorfer, A., Knaus, H.G., Striessnig, J., Kempner, E., and Glossman, H., Purification, molecular cloning, and expression of the mammalian sigma1-binding site, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, art. ID 8072. https://doi.org/10.1073/pnas.93.15.8072
Harper, J.L. and Daly, J.W., Inhibitors of store-operated calcium channels: imidazoles, phenothiazines, and other tricyclics, Drug Dev. Res., 1999, vol. 47, p. 107.
Harper, J.L., Shin, Y., and Daly, J.W., Loperamide: a positive modulator for store-operated calcium channels?, Proc. Natl. Acad. Sci. U. S. A., 1997, vol. 94, art. ID 14912. Hashimoto, K., Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor, Eur. Arch. Psychiatry Clin. Neurosci., vol. 271, p. 249. https://doi.org/10.1007/s00406-020-01231-x
Hayashi, T., Sigma-1 receptor: the novel intracellular target of neuropsychotherapeutic drugs, J. Pharmacol. Sci., 2015, vol. 127, p. 2.
Hayashi, T., The sigma-1 receptor in cellular stress signaling, Front. Neurosci., 2019, vol. 13, p. 733. https://doi.org/10.3389/fnins.2019.00733
Hayashi, T. and Su, T.-P., Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders, CNS Drugs, 2004, vol. 18, p. 269.
Hayashi, T. and Su, T.-P., Sigma-1 receptor chaperones at the ER–mitochondrion interface regulate Ca(2+) signaling and cell survival, Cell, 2007, vol. 131, p. 596.
Hayashi, T., Maurice, T., and Su, T.-P., Ca2+ signalling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration, J. Pharmacol. Exp. Ther., 2000, vol. 293, p. 788.
Herrando-Grabulosa, M., Gaja-Capdevila, N., Vela, J.M., and Navarro, X., Sigma 1 receptor as a therapeutic target for amyotrophic lateral sclerosis, Br. J. Pharmacol., 2020, vol. 178, p. 1336.
Hoertel, N., Sanchez-Rico, M., Vernet, R., Jannot, A.-S., Neuraz, A., Blanco, C., Lemogne, C., Airagnes, G., Paris, N., Daniel, Ch., Gramfort, A., Lemaitre, G., Bernaux, M., Bellamine, A., Beeker, N., and Limosin, F., Observational study of haloperidol in hospitalized patients with COVID-19, PLoS One, 2021a, vol. 16, art. ID e0247122. https://doi.org/10.1371/journal.pone.0247122
Hoertel, N., Sanchez-Rico, M., Vernet, R., Jannot, A.-S., Neuraz, A., Blanco, C., Lemogne, C., Airagnes, G., Paris, N., Daniel, Ch., Gramfort, A., Lemaitre, G., Bernaux, M., Bellamine, A., and Beeker, N., Observational study of chlorpromazine in hospitalized patients with COVID-19, Clin. Drug Invest., 2021b, vol. 41, p. 221. https://doi.org/10.1007/s40261-021-01001-0
Ishikawa, M. and Hashimoto, K., The role of sigma-1 receptors in the pathophysiology of neuropsychiatric diseases, J. Receptor, Ligand Channel Res., 2010, vol. 3, p. 25.
Ito, K., Nakazawa, K., Koizumi, S., Liu, M., Takeuchi, K., Hashimoto, T., Ohno, Y., and Inoue, K., Inhibition by antipsychotic drugs of L-type Ca2+ channel current in PC12 cells, Eur. J. Pharmacol., 1996, vol. 314, p. 143.
Itzhak, Y., Ruhland, M., and Krahling, H., Binding of umespirone to the sigma receptor: evidence for multiple affinity states, Neuropharmacology, 1990, vol. 29, p. 181.
Kekuda, R., Prasad, P.D., Fei, Y.J., Leibach, F.H., and Ganapathy, V., Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1), Biochem. Biophys. Res. Commun., 1996, vol. 229, p. 553. https://doi.org/10.1006/bbrc.1996.1842
Kim, F.J. and Maher, C.M., Sigma1 pharmacology in the context of cancer, Handb. Exp. Pharmacol., 2017, vol. 244, p. 237.
Kruse, A., Structural insights into sigma1 function, Handb. Exp. Pharm., 2017, vol. 244, p. 13.
Krutetskaya, Z.I., Milenina, L.S., Naumova, A.A., Butov, S.N., Antonov, V.G., and Nozdrachev, A.D., Sigma-1 receptor antagonist haloperidol attenuates Ca2+ responses induced by glutoxim and molixan in macrophages, Dokl. Biochem. Biophys., 2017, vol. 472, no. 1, p. 74.
Krutetskaya, Z.I., Milenina, L.S., Naumova, A.A., Butov, S.N., Antonov, V.G., and Nozdrachev, A.D., Trifluoperazine attenuates store-dependent Ca2+ entry in macrophages, Dokl. Biochem. Biophys., 2018a, vol. 478, no. 1, p. 44.
Krutetskaya, Z.I., Milenina, L.S., Naumova, A.A., Butov, S.N., Antonov, V.G., and Nozdrachev, A.D., Sigma-1 receptor antagonist haloperidol attenuates store-dependent Ca2+ entry in macrophages, Dokl. Biochem. Biophys., 2018b, vol. 480, no. 1, p. 162.
Krutetskaya, Z.I., Milenina, L.S., Naumova, A.A., Butov, S.N., Antonov, V.G., and Nozdrachev, A.D., Amitriptyline attenuates Ca2+ responses induced by glutoxim and molixan in macrophages, Dokl. Biochem. Biophys., 2018c, vol. 481, no. 1, p. 222.
Maurice, T. and Su, T.-P., The pharmacology of sigma-1 receptors, Pharmacol. Ther., 2009, vol. 124, p. 195.
McNaughton, N.C.L., Green, P.J., and Randall, A.D., Inhibition of human α1E subunit-mediated Ca2+ channels by the antipsychotic agent chlorpromazine, Acta Physiol. Scand., 2001, vol. 173, p. 401. https://pubmed.ncbi.nlm.nih.gov/28315267
Merlos, M., Burgueño, J., Portillo-Salido, E., Plata-Salamán, C.R., and Vela, J.M., Pharmacological modulation of the sigma 1 receptor and the treatment of pain, Adv. Exp. Med. Biol., 2017a, vol. 964, p. 85. https://doi.org/10.1007/978-3-319-50174-1_8
Merlos, M., Romero, L., Zamanillo, D., Plata-Salamán, C., and Vela, J.M., Sigma-1 receptor and pain, Handb. Exp. Pharmacol., 2017b, vol. 244, p. 131.
Monahan, R.A., Dvorak, H.F., and Dvorak, A.M., Ultrastructural localization of nonspecific esterase activity in guinea pig and human monocytes, macrophages and lymphocytes, Blood, 1981, vol. 58, p. 1089.
Monnet, F.P., Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance, Biol. Cell, 2005, vol. 97, p. 878.
Muric, N.N., Arsenijevic, N.N., Milica, M., and Borovcanin, M.M., Chlorpromazine as a potential antipsychotic choice in COVID-19 treatment, Front. Psychiatry, 2020, vol. 11, art. ID 612347. https://doi.org/10.3389/fpsyt.2020.612347
Nakazawa, K., Higo, K., Abe, K., Tanaka, Y., Saito, H., and Matsuki, N., Blockade by calmodulin inhibitors of Ca2+ channels in smooth muscle from rat vas deferens, Br. J. Pharmacol., 1993, vol. 109, p. 137.
Nobile, B., Durand, M., Courtet, P., Van de Perre, P., Nagot, N., Molès, J.P., and Olié, E., Could the antipsychotic chlorpromazine be a potential treatment for SARS-CoV-2?, Schizophrenia Res., 2020, vol. 223, p. 373.
Ogata, N. and Narahashi, T., Potent blocking action of chlorpromazine on two types of calcium channels in cultured neuroblastoma cells, J. Pharmacol. Exp. Ther., 1990, vol. 252, p. 1142.
Ogata, N., Yoshii, M., and Narahashi, T., Differential block of sodium and calcium channels by chlorpromazine in mouse neuroblastoma cells, J. Physiol., 1990, vol. 420, p. 165.
Ossa, F., Schnell, J.R., and Ortega-Roldan, J.L., A review of the human sigma-1 receptor structure, Adv. Exp. Med. Biol., 2017, vol. 964, p. 15.
Otręba, M., Koґsmider, L., and Rzepecka-Stojko, A., Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review, Eur. J. Pharmacol., 2020, vol. 887, art. ID173553. https://doi.org/10.1016/j.ejphar.2020.173553
Penke, B., Fulop, L., Szucs, M., and Frecska, E., The role of sigma-1 receptor, and intracellular chaperone in neurodegenerative diseases, Curr. Neuropharmacol., 2018, vol. 16, p. 97.
Plaze, M., Attali, D., Petit, A.-C., Blatzer, M., Simon-Loriere, E., Vinckier, F., Cachia, A., Chretien, F., and Gaillard, R., Repurposing chlorpromazine to treat COVID-19: the reCoVery study, L’Encephale, 2020, vol. 46, p. 169.
Plaze, M., Attali, D., Prot, M., Petit, A.-C., Blatzer, M., Vinckier, F., Levillayer, L., Chiaravalli, J., Perin-Dureau, F., Cachia, A., Friedlander, G., Chretien, F., Simon-Loriere, E., and Gaillard, R., Inhibition of the replication of SARS-CoV-2 in human cells by the FDA-approved drug chlorpromazine, Int. J. Antimicrobial Agents, 2021, vol. 57, art. ID 106274. doi.org/https://doi.org/10.1016/j.ijantimicag.2020.106274
Pontisso, I. and Combettes, L., Role of sigma-1 receptor in calcium modulation: possible involvement in cancer, Genes, 2021, vol. 12, p. 139. https://doi.org/10.3390/genes12020139
Randriamampita, C. and Trautmann, A., Ionic channels in murine macrophages. Cell. Biol., 1987, vol. 105, p. 761.
Rosado, J.A., Sigma-1 receptors: a new pathway for the modulation of store-operated calcium entry, Biochem. J., 2016, vol. 473, pp. e9–e10. https://doi.org/10.1042/BJ20151144
Rousseaux, C.G. and Greene, S.F., Sigma receptors [σRs]: biology in normal and diseased states, J. Recept. Signal Transduct., 2016, vol. 36, p. 327.
Ryskamp, D., Wu, J., Geva, M., Kusko, R., Grossman, I., Hayden, M., and Bezprozvanny, I., The sigma 1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease, Neurobiol. Dis., 2017, vol. 97, p. 46.
Ryskamp, D.A., Korban, S., Zhemkov, V., Kraskovskaya, N., and Bezprozvanny, I., Neuronal sigma-1 receptors: signaling functions and protective roles in neurodegenerative diseases, Front. Neurosci., 2019, vol. 13, p. 862. https://doi.org/10.3389/fnins.2019.00862
Schmidt, H.R. and Kruse, A.C., The molecular function of σ receptors: past, present, and future, Trends Pharmacol. Sci., 2019, vol. 40, p. 636.
Schmidt, H.R., Zheng, S., Gurpinar, E., Koehl, A., Manglik, A., and Kruse, A.C., Crystal structure of the human σ1 receptor, Nature, 2016, vol. 532, p. 527.
Schmidt, H.R., Betz, R.M., Dror, R.O., and Kruse, A.C., Structural basis for σ1 receptor ligand recognition, Nat. Struct. Mol. Biol., 2018, vol. 25, p. 981.
Schuster, D.I., Arnold, F.J., and Murphy, R.B., Purification, pharmacological characterization and photoaffinity labeling of sigma receptors from rat and bovine brain, Brain Res., 1995, vol. 670, p. 14.
Smith, S.B., Wang, J., Cui, X., Mysona, B.A., Zhao, J., and Bollinger, K.E., Sigma 1 receptor: a novel therapeutic target in retinal disease, Prog. Retin. Eye Res., 2018, vol. 67, p. 130. https://doi.org/10.1016/j.preteyeres.2018.07.003
Sokolova, G.B., Sinitsyn, M.V., Kozhemiakin, L.A., and Perel’man, M.I., Glutoxim in the complex treatment of tuberculosis, Antibiot. Khimioter., 2002, vol. 47, no. 2, p. 20.
Solaimanzadeh, I., Nifedipine and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19, Cureus, 2020, vol. 12, art. ID e8069. https://doi.org/10.7759/cureus.8069
Soriani, O. and Rapetti-Mauss, R., Sigma 1 receptor and ion channel dynamics in cancer, Adv. Exp. Med. Biol., 2017, vol. 964, p. 63. https://doi.org/10.1007/978-3-319-50174-1
Srivats, S., Balasuriya, D., Pasche, M., Vistal, G., Edwardson, J.M., Taylor, C.W., and Murrell-Lagnado, R.D., Sigma 1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1, J. Cell Biol., 2016, vol. 213, p. 65.
Stip, E., Psychiatry and COVID-19: the role of chlorpromazine, Can. J. Psychiatry, 2020, vol. 65, p. 739.
Stip, E., Rizvi, T.A., Mustafa, F., Javaid, S., Aburuz, S., Ahmed, N.N., Abdel Aziz, K., Arnone, D., Subbarayan, A., Al Mugaddam, F., and Khan, G., The large action of chlorpromazine: translational and transdisciplinary considerations in the face of COVID-19, Front. Pharmacol., 2020, vol. 11, p. 577678. https://doi.org/10.3389/fphar.2020.577678
Stracina, T. and Novakova, M., Cardiac sigma receptors—an update, Physiol. Res., 2018, vol. 67, p. S561.
Su, T.-P., Hayashi, T., Maurice, T., Buch, S., and Ruoho, A.E., The sigma-1 receptor chaperone as an inter-organelle signaling modulator, Trends Pharmacol. Sci., 2010, vol. 31, p. 557.
Su, T.-P., Su, T.-C., Nakamura, Y., and Tsai, S.-Y., The sigma-1 receptor as a pluripotent modulator in living systems, Trends Pharmacol. Sci., 2016, vol. 37, p. 262.
Tam, S.W. and Cook, L., Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H]SKF 10,047 and [3H]haloperidol binding in guinea pig brain membranes, Proc. Natl. Acad. Sci. U. S. A., 1984, vol. 81, p. 5618.
Thastrup, O., Dawson, A.P., Scharff, O., Foder, B., Cullen, P.J., Drobak, B.K., Bjerrum, P.J., Christensen, S.B., and Hanley, M.R., Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage, Agents Actions, 1989, vol. 27, p. 17.
Tolstoi, O.A., Tsygan, V.N., Klimov, A.G., Stepanov, A.V., and Antushevich, A.E., Experimental evaluation of the efficiency of the drug molixan on restoring the operation of virus-infected laboratory animals, Izv. Ross. Voenno-Med. Akad., 2019, vol. 38, no. 1, p. 271.
Trebak, M. and Kinet, J.-P., Calcium signalling in T cells, Nat. Rev. Immunol., 2019, vol. 19, p. 154.
Tsai, S.-Y., Hayashi, T., Mori, T., and Su, T.-P., Sigma-1 receptor chaperones and diseases, Cent. Nerv. Syst. Agents Med. Chem., 2009, vol. 9, p. 184.
Tsai, S.-Y., Pokrass, M.J., Klauer, N.R., De Credico, N.E., and Su, T.-P., Sigma-1 receptor chaperones in neurodegenerative and psychiatric disorders, Expert Opin. Ther. Targets, 2014, vol. 18, p. 1461. https://doi.org/10.1517/14728222.2014.972939
Vavers, E., Zvejniece, L., Maurice, T., and Dambrova, M., Allosteric modulators of sigma-1 receptor: a review, Front. Pharmacol., 2019, vol. 10, p. 223. https://doi.org/10.3389/fphar.2019.00223
Vela, J.M., Repurposing sigma-1 receptor ligands for COVID-19 therapy?, Front. Pharmacol., 2020, vol. 11, art. ID 582310. https://doi.org/10.3389/fphar.2020.582310
Vig, M. and Kinet, J.-P., Calcium signaling in immune cells, Nat. Immunol., 2009, vol. 10, p. 21.
Voronin, M.V., Vakhitova, Y.V., and Seredenin, S.B., Chaperone Sigma1R and antidepressant effect, Int. J. Mol. Sci., 2020, vol. 21, art. ID. 7088. https://doi.org/10.3390/ijms21197088
Wang, L., Zhang, L., Li, S., Zheng, Y., Yan, X., Chen, M., Wang, H., Putney, J.W., and Luo, D., Retrograde regulation of STIM1-Orai1 interaction and store-operated Ca2+ entry by calsequestrin, Sci. Rep., 2015, vol. 5, p. 1.
Wang, J., Cui, X., Roon, P., Saul, A., and Smith, S.B., The role of Sigma1R in mammalian retina, Adv. Exp. Med. Biol., 2017, vol. 964, p. 267.
Wu, Z. and Bowen, W.D., Role of sigma-1 receptor c-terminal segment in inositol 1,4,5-trisphosphate receptor activation. Constitutive enhancement of calcium signaling in mcf-7 tumor cells, J. Biol. Chem., 2008, vol. 283, art. ID 28198.
Xie, Q., Zhang, Y., Zhai, C., and Bonanno, J.A., Calcium influx factor from cytochrome P-450 metabolism and secretion-like coupling mechanisms for capacitative calcium entry in corneal endothelial cells, J. Biol. Chem., 2002, vol. 277, p. 16559.
Yang, K., Wang, C., and Sun, T., The roles of intracellular chaperone proteins, sigma receptors, in Parkinson’s disease (PD) and major depressive disorder (MDD), Front. Pharmacol., 2019, vol. 10, p. 528. https://doi.org/10.3389/fphar.2019.00528
Zhang, L.-K., Sun, Y., Zeng, H., Wang, Q., Jiang, X., Shang, W.-J., Wu, Y., Li, Sh., Zhang, Y.-L., Hao, Z.-N., Chen, H., Jin, R., Liu, W., Li, H., Peng, K., and Xiao, G., Calcium channel blocker amlodipine besylate therapy is associated with reduced case fatality rate of COVID-19 patients with hypertension, Cell Discov., 2020, vol. 6, p. 96. https://doi.org/10.1038/s41421-020-00235-0
Zhemkov, V., Geva, M., Hayden, M.R., and Bezprozvanny, I., Sigma-1 receptor (S1R) interaction with cholesterol: mechanisms of S1R activation and its role in neurodegenerative diseases, Int. J. Mol. Sci., 2021, vol. 22, p. 4082. https://doi.org/10.3390/ijms22084082ps
Zhoua, Y., Freyb, T.K., and Yanga, J.J., Viral calciomics: interplays between Ca2+ and virus, Cell Calcium, 2009, vol. 46, p. 1.
Funding
This work was carried out within the framework of the research program of the Department of Biophysics of St. Petersburg State University and the Department of Clinical Biochemistry and Laboratory Diagnostics of Kirov Military Medical Academy (St. Petersburg), as well as St. Petersburg State University agreements for the performance of research works nos. 28-12-38 of March 5, 2018, and 05/03-20 of March 12, 2020.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. Experiments on animals were performed in accordance with generally accepted ethical international standards (International Guiding Principles for Biomedical Research Involving Animals, 1985) and the requirements of the Order no. 267 of June 19, 2003, of the Ministry of Health of the Russian Federation “On the Approval of the Rules of Laboratory Practice in the Russian Federation.”
Additional information
Abbreviations: [Ca2+]i, intracellular Ca2+concentration; CPA, cyclopiazonic acid; TFP, trifluoperazine; CP, chlorpromazine.
Rights and permissions
About this article
Cite this article
Milenina, L.S., Krutetskaya, Z.I., Antonov, V.G. et al. Sigma-1 Receptor Ligands Chlorpromazine and Trifluoperazine Attenuate Ca2+ Responses in Rat Peritoneal Macrophages. Cell Tiss. Biol. 16, 233–244 (2022). https://doi.org/10.1134/S1990519X22030075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X22030075