Abstract
New RAGE- and CD147-mediated mechanisms of damage to the hippocampus of mice due to the accumulation of amyloid β (Aβ), the development of local inflammation, metabolic disorders and damage to the blood–brain barrier in two experimental models of Alzheimer’s disease were studied in vivo. The new effects of Aβ in the hippocampal tissue in chronic Alzheimer’s type neurodegeneration, which characterize neuroplasticity disorders, were studied, as were angiogenesis, the structural and functional integrity of the blood–brain barrier, and the development of local neuroinflammation in conjunction with the features of the expression of RAGE and CD147 proteins. Early neurodegenerative changes in the hippocampus associated with the accumulation of Aβ are associated with the intensification of neoangiogenesis and the formation of aberrant intercellular contacts in the endothelial layer of cerebral microvessels in individual hippocampal subregions and the development of local neuroinflammation. As neurodegeneration progresses, neoangiogenesis in the hippocampus is suppressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
One of the most common neurodegenerative diseases in the world is Alzheimer’s disease (AD), which is characterized by progressive loss of neurons, primarily the hippocampus, the development of cognitive impairment and other signs of dementia. Currently, the disease remains incurable and reduces the duration and quality of life of patients, which makes AD a global medical and socioeconomic problem (Graham et al., 2017).
Much attention has been paid to questions devoted to the etiology and pathogenesis of AD since the discovery of the disease. It is known that the biochemical features of the disease are deposition of amyloid plaques in the extracellular space and intracellular accumulation of hyperphosphorylated neurofibrillary tangles. However, the question of the contribution of amyloid β (Aβ) and the protein to the onset and development of AD remains open (Armstrong, 2013). In the last decade, the role of disorders of the neurovascular unit (NVU) in the pathogenesis of AD is discussed. NVU is a collection of brain cells (cerebral endotheliocytes, pericytes, perivascular astrocytes, and neurons). Forms of disruption of the development of NVU in AD include changes in the permeability of the blood–brain barrier (BBB) and microcirculation due to impaired gliovascular control of local blood flow and neuron–astroglial metabolic conjugation (van de Haar et al., 2016; Liu et al., 2018).
Pathology of cerebral microvasculature is one of the main risk factors for dementia (Arvanitakis et al., 2016). In addition to this, cerebral amyloid angiopathy, being an important cause of the violation of BBB permeability and one of the signs of AD pathology, causes the development of vascular disorders, which contributes to a decrease in cognitive function (Saito and Ihara, 2016). Thus, the question arises of a causal relationship between neurodegeneration and the vascular component in the development of AD. This becomes especially clear when you consider that vascular disorders develop quite early, including before the first signs of neurodegeneration (Iturria-Medina et al., 2016), for which reason it is appropriate to search for new molecular mechanisms of damage to the NVU and BBB.
Damage to blood vessels causes BBB dysfunction, which in turn leads to damage to neurons and the accumulation of Aβ in the brain (Salmina et al., 2016).
In general, clinical and experimental studies have shown that cerebrovascular dysfunction plays an important role in the development of AD, as well as cerebral amyloid angiopathy, with deposition of amyloid in large vessels and a decrease in clearance of amyloid from the brain parenchyma, including due to changes in amyloid transport through the endothelium and his inability to degrade (Carare et al., 2013; Steinberg et al., 2014; Keable et al., 2016).
It is assumed that excessive amyloidogenesis leads to neoangiogenesis and the formation of a network of microvessels with excessive permeability of the vascular wall and enhanced transport of amyloid from blood to the brain through the damaged BBB (Biron et al., 2011). In accordance with this hypothesis, in AD, increased expression of hippocampal endotheliocytes of proteins transporting Aβ from blood to brain tissue (RAGE proteins) is recorded (Miller et al., 2008). It is known that the receptor for protein glycation end products (RAGE) mediates many physiological functions—neuron growth, survival, and regeneration—as well playing an important role in proinflammatory reactions, inducing pro-inflammatory cytokines; being the main mediator of the innate immune response; and being able to participate in a number of pathological processes, including sugar diabetes, asthma, and systemic amyloidosis (Kierdorf and Fritz, 2013; Ramasamy et al., 2016). Recent studies have demonstrated the involvement of RAGE in the transport of neuropeptide oxytocin to brain tissue (Yamamoto et al., 2019), which that provides the effect of oxytocin in relation to social behavior.
The role of the CD147 molecule in the pathogenesis of AD is interesting, since there is evidence that an important role is played by CD147 in the functioning of the cardiovascular, nervous, and immune systems (Agrawal et al., 2013; Kanyenda et al., 2014; Seizer et al., 2014). CD147 is functionally coupled to lactate transporters (MCT1, MCT4) and a γ secretase involved in the proteolysis of the amyloid precursor protein, as well as regulating the expression and activity of matrix metalloproteinases (Uspenskaya et al., 2018). CD147 is expressed by endothelial cells and NVU/BBB astroglia and can functionally integrate several signaling pathways activated by the implementation of angiogenesis and barrier genesis mechanisms.
It is noteworthy that the “classic” RAGE ligands—the end products of glycation—can induce the expression of CD147 in some inflammatory effector cells (Bao et al., 2010), whereas the ligand CD147—s100A9—effectively interacts with RAGE, for example, in areas of thrombosis (von Ungern-Sternberg et al., 2018). Thus, it is logical to suggest that the ligands of both receptors can act as a so-called “alarmin” that is released from damaged cells and initiates local inflammation and microcirculation disorders. The category of alarms also includes proteins of the HMGB1 group, which initiate many of the mechanisms underlying the so-called “secondary alteration” in inflammation in acute cerebrovascular accidents (Kim et al., 2018) and head injury and epilepsy (Paudel et al., 2018). HMGB1 are known to act as RAGE ligands, including in the brain in Alzheimer’s disease (Deane, 2012; Meneghini et al., 2013), are responsible for some mechanisms of damage to the BBB (Festoff et al., 2016).
In connection with the foregoing, and also taking into account the role of local neuroinflammation in the progression of Alzheimer’s type neurodegeneration, CD147 and RAGE can be considered as potential target molecules, the change in the expression of which can change the nature of the course of cerebrovascular pathology in AD.
The aim of this study was to study the molecular mechanisms of impaired cerebral neoangiogenesis and the structural and functional integrity of the BBB in experimental modeling of asthma in vivo assessment of the role of Aβ in the regulation of endothelial cell metabolism of cerebral microvessels, processes of cerebral angiogenesis and maintaining the structural and functional integrity of the BBB in normal conditions and in the development of cerebral amyloid angiopathy.
MATERIALS AND METHODS
Neurodegeneration Modeling
Two AD models were used—a transgenic model (formed neurodegeneration) and a model with intrahippocampal administration of amyloid (early stages of the disease and realization of the neurotoxic effect of Aβ). Animals were kept in cages with free access to water and feed at a constant temperature of 20 ± 2°C and a fixed lighting mode (12 h of light and 12 h of darkness).
Three groups of animals were used in the experiment. The control group was C57BL/6 mice, males aged 4 months (n = 5), these being false-operated animals after the introduction of the solvent Aβ—phosphate-buffered saline (PBS). Genetic model of asthma was provided by mice of the B6SLJ-Tg line (APPSwFlLon,PSEN1*M146L*L286V)6799Vas, males aged 9 months (n = 5), were obtained from Jackson Laboratory (United States). The experimental AD group consisted of C57BL/6 mice, males aged 4 months (n = 5). AD was simulated by intragippocampal injection of 1 μL of a preparation of aggregated Aβ 1-42 (Sigma-Aldrich, United States) along stereotactic brain coordinates in the CA1 zone of the hippocampus. Aβ was dissolved in PBS to a concentration of 50 μM, followed by aggregation in an incubator at 37°C for 7 days (Epelbaum et al., 2015). Evaluation of AD symptoms beginning 7 days after surgery was performed using “Recognition of a New Object” neurobehavioral testing (Sipos et al., 2007, Miedel et al., 2017). The experimental model of AD was verified by staining with a 0.015% solution of Thioflavin S (Sigma-Aldrich, United States). After the introduction of amyloid into the brain tissue, fluorescent amyloid plaques with a characteristic emission spectrum were observed.
Immunohistochemistry
Two days after surgery, transcardial perfusion was performed with 4% paraformaldehyde followed by brain sampling. The brain was fixed in 10% neutral buffered formalin, and then immersed in a 20% sucrose solution. Sections were made with a thickness of 50 μm using a Microtome Thermo Scientific Microm HM 650 (Thermo Fisher Scientific, United States). The method of indirect immunohistochemistry for free-floating sections was carried out registration of target markers (Encinas and Enikolopov, 2008).
After washing in PBS, sections were blocked with 3% goat serum albumin in PBS and 1% Triton X-100 for 1 h at room temperature, followed by overnight incubation with primary antibodies to RAGE (ab3611; Abcam, UK), HMGB1 (ab77302; Abcam, UK), to CD147 (ab108317; Abcam, United Kingdom), JAM1 (ab52647; Abcam, United Kingdom), and CD31 (PECAM-1)-FITC (F8402; Sigma-Aldrich, United States). All antibodies were used in a dilution of 1 : 1000. After incubation with primary antibodies, the sections were washed and incubated with secondary antibodies conjugated with Alexa (Abcam, United Kingdom) at a dilution of 1 : 1000 for 2 h at room temperature. Cell nuclei were stained with DAPI (Thermo Fisher Scientific, United States).
Images were obtained using an Olympus FV 10i confocal microscope (Olympus, Japan). In the sections of the brain, the number of cells expressing target markers were counted in at least five fields of view.
The fraction of cells expressing the target molecules (of the total number of cells in the vascular region in the field of view calculated from the nuclei of DAPI-positive cells localized in the vascular region) was counted in at least five fields of view. Target label colocalization was evaluated using the Pearson correlation coefficient using specialized software for the Olympus FV 10i microscope (Zinchuk et al., 2007).
Statistical processing of the results was carried out using nonparametric statistics. To compare the indices in independent samples, the Mann–Whitney test was used, and the comparison of dependent samples was carried out using the Wilcoxon test. The differences were considered significant when p < 0.05. The results are presented as mean values and their errors.
RESULTS
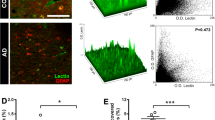
Intrahippocampal administration of Aβ caused the development of Alzheimer’s type neurodegeneration in experimental animals, which was accompanied by an increase in the number of HMGB1-immunopositive cells in the dentate gyrus of the hippocampus—4.6 ± 0.6% compared with the control group (1.8 ± 0.4%) (p = 0.0014) (Fig. 1a).
Relative amount of immunopositive cells on group proteins HMGB1 (a), RAGE (b), CD147 (c), and CD31 (d) in control mice and mice with a model of Alzheimer’s disease. Immunohistochemistry using appropriate antibodies. Control—false-operated animals with the introduction of PBS; AD—Alzheimer’s disease is modeled by the intragippocampal administration of an amyloid β drug; B6SLJ—mice with a genetic model of Alzheimer’s disease. CA1, CA2, CA3, DG—zones of the hippocampus.
Unidirectional changes were found during the registration of RAGE-immunopositive cells—an increase in the number of hippocampal cells expressing RAGE in the experimental group to 13.2 ± 2.6%, compared with mock-operated animals (5.9 ± 0.7%) (p < 0.05) (Fig. 1b).
Considering that Aβ and HMGB1 are endogenous ligands of RAGE proteins, an increase in RAGE expression in endothelial cells of animal hippocampus with a simultaneous increase in HMGB1 expression and Aβ accumulation in experimental AD indicates the realization of RAGE-mediated mechanisms of local inflammation and endothelial dysfunction.
At the next stage, we evaluated some features of angiogenesis realized through the CD147 molecule in experimental AD. In animals with a genetic model of AD in the CA3 hippocampus, the number of endotheliocytes expressing CD147 (23 ± 1.6%) was less (p = 0.025) than in the control group (39.1 ± 3.1%) (Fig. 1c). At the same time, in the dentate gyrus of the hippocampus, an increased (p = 0.004) number of CD147-immunopositive cells (41.4 ± 4.7%) was found compared with the false-operated animals (15.3 ± 1.4%) (Fig. 1c).
In the CA1 and CA2 hippocampal subregions, no significant differences in CD147 expression were observed.
Taking into account the revealed disturbances in angiogenesis in animals with an experimental model of AD, we evaluated the features of the expression of the marker of endothelial dysfunction (CD31) and adhesive contact protein (JAM1) in the hippocampus. In the experimental group, the proportion of CD31-immunopositive endotheliocytes was two times higher than in the control group of animals, and amounted to 5.5 ± 0.9% versus 2.4 ± 0.4%, respectively (p = 0.0053).
However, when modeling AD in endothelial cells, there was a decrease in the CD31 colocalization index with the adhesive contact molecule—JAM1—which amounted to 21 ± 7%. In the control group, the localization index reached 92.7 ± 5.2% (p < 0.0001).
The detected increase in the number of CD31-immunopositive endotheliocytes in animals with an early disturbance model aroused interest in assessing the nature of CD31 expression in individual hippocampal subregions in animals with already formed neurodegenerative changes (genetic model of AD).
In animals with a genetic model of AD, we identified a tendency (p = 0.223) toward a reduction in the number of endotheliocytes expressing CD31 in the dentate gyrus of the hippocampus (21.5 ± 1%) compared with control (24.3 ± 1.4%). A similar situation is observed in the CA2 and CA3 zones of the hippocampus (Fig. 1d) However, in the CA1 hippocampus subregion, the proportion of CD31-immunopositive cells was higher in the experimental group (28.9 ± 1.7%) than in the control (21.4 ± 1.8%) (p = 0.028).
Thus, it can be assumed that the initial stages of Alzheimer’s type neurodegeneration are accompanied by intensification of the processes of neoangiogenesis in the hippocampus, however, as the disease progresses, neoangiogenesis is suppressed. The exception is the CA1 hippocampus subregion, in which a high level of expression of CD31 immunopositive cells is maintained.
DISCUSSION
We have obtained new data on the fundamental mechanisms of conjugation of the processes of neuroplasticity in normal and chronic neurodegeneration, as well as the mechanisms of development of amyloid angiopathy. In the course of this work, an experimental approach using two experimental AD models in vivo to confirm the possible participation in the pathogenesis of CD147- and RAGE-mediated mechanisms in the development of endothelial dysfunction, remodeling of the vascular network and impaired structural integrity of the blood–brain barrier. The new effects of Aβ in the hippocampal tissue (in the early stages and with the formed Alzheimer’s type neurodegeneration) were studied, which characterize the disorders angiogenesis (expression CD31 in various areas of the hippocampus), the structural and functional integrity of the BBB (expression of JAM1 protein), the development of local neuroinflammation (expression of HMGB1) in conjunction with the expression features of CD147 and RAGE proteins.
Early neurodegenerative changes in the hippocampus associated with Aβ accumulation are accompanied by intensification of neoangiogenesis, impaired formation of endothelial contacts, an increase in endothelial dysfunction in the hippocampus, and the development of local neuroinflammation, followed by inhibition of neoangiogenesis as the disease progresses.
The detected increase in the expression of CD31 against the background of the reduction of the expression of JAM1 in the endothelial cells of the hippocampal microvessels is maximal in the CA1 subregion. Most likely, the accumulation of Aβ in the vascular wall causes intensification of angiogenesis and the formation of new vessels with aberrant expression of JAM1, which is simultaneously associated with an increase in the expression of CD147 as a molecule that regulates the processes of angiogenesis and extracellular metabolism of amyloid. It is believed that a decrease in the number of CD31-immunopositive cells is characteristic of neuroinflammation and neurodegenerative diseases. An overall decrease in the expression of CD31 in brain tissue is characteristic of endothelial dysfunction and/or death of endothelial cells. In particular, a decrease in the level of expression of CD31 was observed in patients with AD, which suggests that there is extensive endothelial degeneration during the progression of the disease (Grammas, 2011; Magaki et al., 2018).
The earliest changes in the hippocampus, which are characterized by an increase in the number of CD31-immunopositive endotheliocytes and a fourfold decrease in the coefficient of JAM1 and CD31 colocalization in these cells, suggest that Aβ accumulation causes intensification of angiogenesis and the formation of new blood vessels, but with aberrant expression of JAM1 adhesive contact protein. In turn, impaired expression of adhesive contact proteins that ensure the integrity of the endothelial monolayer during intensive neoangiogenesis is accompanied by the formation of a functionally incompetent BBB in the early stages of Alzheimer’s type neurodegeneration.
These changes are recorded against the background of the development of local inflammation, including due to the HMGB1 protein released from damaged cells. An increase in the expression of RAGE proteins on cerebral endothelial cells provides the effect of HMGB1 on these cells, which may cause endothelial damage and dysfunction. It is known that HMGB1 is a marker of neuroinflammation and plays a significant role in the pathogenesis of AD—inducing cell degeneration, modifying the processes of Aβ aggregation, and acting as an independent mediator of the action of Aβ (Fujita et al., 2016). On the other hand, HMGB1 is a RAGE ligand in various tissues, and their interaction induces inflammation (Weber et al., 2015). In addition, the interaction of Aβ with RAGE-positive endotheliocytes, neurons and microglia initiates oxidative stress, the formation of lipid peroxides (Chen et al., 2018).
Together with the accumulation of Aβ in the vascular wall, which stimulates the activation of proinflammatory cytokines and reactive oxygen species, all these processes cause damage to neurons, smooth muscle cells and pericytes, and endothelial dysfunction, contributing to damage to the BBB (Erickson and Banks, 2013). Among other things, RAGE is considered to serve as a transporter of Aβ to the brain from peripheral blood, while RAGE receptors mediate the translocation of Aβ from the extracellular space to the intracellular (Andrade et al., 2018; Reich et al., 2018).
Similarly, in the hippocampus, the expression of CD147 increases (mainly in the dentate gyrus), which may be in the nature of a “response” reaction to the accumulation of Aβ and the intensification of remodeling of microvessels.
CD147 is a molecule the expression of which in NVU cells regulates the processes of proteolysis of the amyloid precursor protein, secretion of matrix metalloproteinases, lactate transport, and angiogenesis (Vetrivel et al., 2008; Muramatsu, 2016). It is important to note that, with brain damage, the expression of CD147 increases (Wei et al., 2014).
Interestingly, in the animals of the control group, the dentate gyrus of the hippocampus shows a minimal (in comparison with other hippocampal subregions) level of expression of CD147.
It is known that the level of expression of CD147 is inversely proportional to local production of Aβ due to the fact that CD147 negatively controls the activity of gamma-secretase associated with it in the multiprotein complex and stimulates extracellular degradation of Aβ. It is logical to assume that the high level of expression of CD147 in the dentate gyrus of the hippocampus, as well as the high levels of expression of HMGB1 and RAGE that we have registered in this region, collectively mark the intensity of inflammation, remodeling of microvessels, and amyloid metabolism.
REFERENCES
Agrawal, S.M., Williamson, J., Sharma, R., Kebir, H., Patel, K., Prat, A., and Yong, V.W., Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis, Brain, 2013, vol. 6, p. 1760.
Andrade, S., Ramalho, M.J., Pereira, M.D.C., and Loureiro, J.A., Resveratrol brain delivery for neurological disorders prevention and treatment, Front. Pharmacol., 2018, vol. 9, p. 1261.
Armstrong, R.A., What causes Alzheimer’s disease?, Folia Neuropathol., 2013, vol. 3, p. 169.
Arvanitakis, Z., Capuano, A.W., Leurgans, S.E., Bennett, D.A., and Schneider, J.A., Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study, Lancet Neurol., 2016, vol. 9, p. 934.
Bao, W., Min, D., Twigg, S.M., Shackel, N.A., Warner, F.J., Yue, D.K., and McLennan, S.V., Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications, Am. J. Physiol.—Cell Physiol., 2010, vol. 5, p. C1212.
Biron, K.E., Dickstein, D.L., Gopaul, R., and Jefferies, W.A., Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease, PLoS One, 2011, vol. 8, e23789.
Carare, R.O., Hawkes, C.A., Jeffrey, M., Kalaria, R.N., and Weller, R.O., Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy, Neuropathol. Appl. Neurobiol., 2013, vol. 6, p. 593.
Chen, F., Ghosh, A., Hu, M., Long, Y., Sun, H., Kong, L., Hong, H., and Tang, S., RAGE-NF-κB-PPARγ signaling is involved in AGEs-induced upregulation of amyloid-β influx transport in an in vitro BBB model, Neurotox. Res., 2018, vol. 2, p. 284.
Deane, R.J., Is RAGE still a therapeutic target for Alzheimer’s disease?, Future Med. Chem., 2012, vol. 7, p. 915.
Encinas, J.M. and Enikolopov, G., Identifying and quantitating neural stem and progenitor cells in the adult brain, Methods Cell Biol., 2008, vol. 85, p. 243.
Epelbaum, S., Youssef, I., Lacor, P.N., Chaurand, P., Duplus, E., Brugg, B., Duyckaerts, C., and Delatour, B., Acute amnestic encephalopathy in amyloid-β oligomer–injected mice is due to their widespread diffusion in vivo, Neurobiol. Aging, 2015, vol. 6, p. 2043.
Erickson, M.A. and Banks, W.A., Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease, J. Cereb. Blood Flow Metab., 2013, vol. 10, p. 1500.
Festoff, B.W., Sajja, R.K., van Dreden, P., and Cucullo, L., HMGB1 and thrombin mediate the blood-brain barrier dysfunction acting as biomarkers of neuroinflammation and progression to neurodegeneration in Alzheimer’s disease, J. Neuroinflam., 2016, vol. 1, p. 194.
Fujita, K., Motoki, K., Tagawa, K., Chen, X., Hama, H., Nakajima, K., Homma, H., Tamura, T., Watanabe, H., Katsuno, M., Matsumi, C., Kajikawa, M., Saito, T., Saido, T., Sobue, G., Miyawaki, A., and Okazawa, H., HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease, Sci. Rep., 2016, vol. 6, p. 31895. https://doi.org/10.1038/srep31895
Graham, W.V., Bonito-Oliva, A., and Sakmar, T.P., Update on Alzheimer’s disease therapy and prevention strategies, Ann. Rev. Med., 2017, vol. 1, p. 413.
Grammas, P., Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease, J. Neuroinflam., 2011, vol. 8, p. 26. https://doi.org/10.1186/1742-2094-8-26
van de Haar, H.J., Jansen, J.F.A., van Osch, M.J.P., van Buchem, M.A., Muller, M., Wong, S.M., Hofman, P.A.M., Burgmans, S., Verhey, F.R.J., and Backes, W.H., Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging, Neurobiol. Aging, 2016, vol. 45, p. 190.
Iturria-Medina, Y., Sotero, R.C., Toussaint, P.J., Mateos-Pérez, J.M., and Evans, A.C., Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis, Nat. Commun., 2016, vol. 21, p. 11934. https://doi.org/10.1038/ncomms11934
Kanyenda, L.J., Verdile, G., Martins, R., Meloni, B.P., Chieng, J., Mastaglia, F., Laws, S.M., Anderton, R.S., and Boulos, S., Is cholesterol and amyloid-β stress induced CD147 expression a protective response? Evidence that extracellular cyclophilin a mediated neuroprotection is reliant on CD147, J. Alzheimer’s Dis., 2014, vol. 3, p. 545.
Keable, A., Fenna, K., Yuen, H.M., Johnston, D.A., Smyth, N.R., Smith, C., Rustam, Al-Shahi, Salman, Samarasekera, N., James, N.A.R., Attems, J., Kalaria, R.N., Wellera, R.O., and Carare, R.O., Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy, Biochim. Biophys. Acta, 2016, vol. 5, p. 1037.
Kierdorf, K. and Fritz, G., RAGE regulation and signaling in inflammation and beyond, J. Leukocyte Biol., 2013, vol. 1, p. 55.
Kim, I.D., Lee, H., Kim, S.W., Lee, H.K., Choi, J., Han, P.L., and Lee, J.K., Alarmin HMGB1 induces systemic and brain inflammatory exacerbation in post-stroke infection rat model, Cell Death Dis., 2018, vol. 4, p. 426.
Liu, X., Hou, D., Lin, F., Luo, J., Xie, J., Wang, Y., and Tian, Y., The role of neurovascular unit damage in the occurrence and development of Alzheimer’s disease, Rev. Neurosci., 2018. https://doi.org/10.1515/revneuro-2018-0056
Magaki, S., Tang, Z., Tung, S., Williams, C.K., Lo, D., Yong, W.H., Khanlou, N., and Vinters, H.V., The effects of cerebral amyloid angiopathy on integrity of the blood-brain barrier, Neurobiol. Aging, 2018, vol. 70, p. 70.
Meneghini, V., Bortolotto, V., Francese, M.T., Dellarole, A., Carraro, L., Terzieva, S., and Grilli, M., High-mobility group box-1 protein and β-amyloid oligomers promote neuronal differentiation of adult hippocampal neural progenitors via receptor for advanced glycation end products/nuclear factor-kb axis: relevance for Alzheimer’s disease, J. Neurosci., 2013, vol. 14, p. 6047.
Miedel, C.J., Patton, J.M., Miedel, A.N., Miedel, E.S., and Levenson, J.M., Assessment of spontaneous alternation, novel object recognition and limb clasping in transgenic mouse models of amyloid-β and tau neuropathology, J. Vis. Exp., 2017, vol. 123. https://doi.org/10.3791/55523
Miller, M.C., Tavares, R., Johanson, C.E., Hovanesian, V., Donahue, J.E., Gonzalez, L., Silverberg, G.D., and Stopa, E.G., Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease, Brain Res., 2008, vol. 1230, p. 273.
Muramatsu, T., Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners, J. Biochem., 2016, vol. 5, p. 481.
Paudel, Y.N., Shaikh, M.F., Chakraborti, A., Kumari, Y., Aledo-Serrano, Á., Aleksovska, K., Alvim, M.K.M., and Othman, I., HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction, Front. Neurosci., 2018, vol. 12, p. 628.
Ramasamy, R., Shekhtman, A., and Schmidt, A.M., The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic disease, Expert Opin. Ther. Targets, 2016, vol. 4, p. 431.
Reich, D., Gallucci, G., Tong, M., and de la Monte, S.M., Therapeutic advantages of dual targeting of PPAR-δ and PPAR-γ in an experimental model of sporadic Alzheimer’s disease, J. Parkinson’s Dis. Alzheimer’s Dis., 2018, vol. 1, p. 1.
Saito, S. and Ihara, M., Interaction between cerebrovascular disease and Alzheimer pathology, Curr. Opin. Psychiatry, 2016, vol. 2, p. 168.
Salmina, A.B., Pozhilenkova, E.A., Morgun, A.V., Kuvacheva, N.V., Shuvaev, A.N., Lopatina, O.L., Boitsova, E.B., and Taranushenko, T.E., Glial dysfunction and blood-brain barrier impairment in the developing brain, Adv. Neuroimmune Biol., 2016, vol. 2, p. 69.
Seizer, P., Gawaz, M., and May, A.E., Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases, Cardiovasc. Res., 2014, vol. 1, p. 17.
Sipos, E., Kurunczi, A., Kasza, A., Horváth, J., Felszeghy, K., Laroche, S., Toldi, J., Párducz, A., Penke, B., and Penke, Z., Beta-amyloid pathology in the entorhinal cortex of rats induces memory deficits: implications for Alzheimer’s disease, Neuroscience, 2007, vol. 1, p. 28.
Steinberg, M., Hess, K., Corcoran, C., Mielke, M.M., Norton, M., Breitner, J., Green, R., Leoutsakos, J., Welsh-Bohmer, K., Lyketsos, C., and Tschanz, J., Vascular risk factors and neuropsychiatric symptoms in Alzheimer’s disease: the cache county study, Int. J. Geriatric Psychiatry, 2014, vol. 2, p. 153.
Ungern-Sternberg, S.N.I., von, Zernecke, A., and Seizer, P., Extracellular matrix metalloproteinase inducer EMMPRIN (CD147) in cardiovascular disease, Int. J. Mol. Sci., 2018, vol. 19. pii: E507. https://doi.org/10.3390/ijms19020507
Uspenskaya, Yu.A., Komleva, Yu.K., Gorina, Ya.V., Pozhilenkova, E.A., Belova, O.A., and Salmina, A.B., Multifunctionality of CD147 and new opportunities for diagnosis and therapy, Sib. Med. Obozr., 2018, vol. 4, p. 22.
Vetrivel, K.S., Zhang, X., Meckler, X., Cheng, H., Lee, S., Gong, P., Lopes, K.O., Chen, Y., Iwata, N., Yin, K.J., Lee, J.M., Parent, A.T., Saido, T.C., Li, Y.M., Sisodia, S.S., and Thinakaran, G., Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta, J. Biol. Chem., 2008, vol. 28, p. 19489.
Weber, D.J., Allette, Y.M., Wilkes, D.S., and White, F.A., The HMGB1-RAGE inflammatory pathway: implications for brain injury-induced pulmonary dysfunction, Antioxid. Red. Signaling, 2015, vol. 17, p. 1316.
Wei, M., Li, H., Shang, Y., Zhou, Z., and Zhang, J., Increased CD147 (EMMPRIN) expression in the rat brain following traumatic brain injury, Brain Res., 2014, vol. 1585, p. 150.
Yamamoto, Y., Liang, M., Munesue, S., Deguchi, K., Harashima, A., Furuhara, K., Yuhi, T., Zhong, J., Akther, S., Goto, H., Eguchi, Y., Kitao, Y., Hori, O., Shiraishi, Y., Ozaki, N., et al., Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice, Commun. Biol., 2019, vol. 1, p. 76.
Zinchuk, V., Zinchuk, O., and Okada, T., Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: Pushing pixels to explore biological phenomena, Acta Histochem. Cytochem., 2007, vol. 40, p. 101.
Funding
This work was carried out with state financial support of the Presidential Program for Leading Scientific Schools of the Russian Federation, project no. NSh-6240.2018.7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare they have no conflict of interest.
Statement on the welfare of animals. Animal experiments were carried out in accordance with generally accepted ethical international standards in compliance with the principles of humaneness set out in the European Community Directive (2010/63/EC) and the requirements of order of the Russian Ministry of Health no. 267 dated June 19, 2003, “On the Approval of the Guidelines of Laboratory Practice in the Russian Federation.”
Additional information
Abbreviations: Aβ—beta-amyloid β, AD—Alzheimer’s disease, BBB—blood–brain barrier, NVU—neurovascular unit, RAGE—protein glycation end product receptor.
Rights and permissions
About this article
Cite this article
Morgun, A.V., Osipova, E.D., Boitsova, E.B. et al. Vascular Component of Neuroinflammation in Experimental Alzheimer’s Disease in Mice. Cell Tiss. Biol. 14, 256–262 (2020). https://doi.org/10.1134/S1990519X20040057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X20040057