Abstract
Catecholamine dopamine (DA) is an important neurotransmitter and hormone involved in many physiological processes and stress reactions in vertebrates and invertebrates. The aim of the work was to clarify the presence and localization of dopamine system elements (tyrosine hydrokinase (TH), dopamine beta-hydrokinase (DAbetaHK), and dopamine type 1 receptors (DA-R1)) in the Achatina achatina gastropod atrial neuroendocrine complex (NEC) cells. The snail atrial NEC cells are generated by large granular cells (GCs) and nerve fibers that are in close contact with them. The methods of histochemistry, immunofluorescence staining, and immunoelectron microscopy were used. Glyoxylic acid-induced fluorescence demonstrated the presence of catecholamines in the nerve fibers and GC. TH-like and DAbetaH-like immunoreactive material was found both in nerve fibers and in GC granules. DA-R1-positive material was detected only in nerve fibers. In addition, it was demonstrated that exogenous DA induces an enhanced degranulation of GC in vivo. Data obtained indicate the involvement of dopamine system in the functioning of snail NEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Biogenic amine dopamine (DA) acts as a hormone and neurotransmitter (a member of catecholamine group) in the animal organism. DA is generated from L-tyrosine, which, under the action of tyrosine hydroxylase (TH), is converted into dihydroxyphenylalanine (L-DOPA), which in turn is converted to DA under the action of L-DOPA decarboxylase. DA itself is a biochemical precursor of norepinephrine and adrenaline. The conversion of DA to norepinephrine occurs with the involvement of dopamine beta hydroxylase (DA-beta-H). As a neurotransmitter, DA synthesized by neurons is involved in nerve impulse transmission through the synaptic gap and is the main CNS transmitter. Its important role in normal functioning of the brain and its damages attracts the attention of researchers in the field of neuropharmacology, neurology, and psychiatry (Marsden, 2006). DA activity is not limited to the CNS. It also performs hormonal functions and is involved in regulation of many physiological processes. Pharmacological DA properties related to the cardiovascular and renal systems are well known (Casagrande, 1991).
The hormone affects somatic cells through dopamine receptors (DA-R) belonging to the class of transmembrane G protein-coupled receptors. To date, five DA-R subtypes were identified in vertebrates. DA is an evolutionary conservative hormone and is found in sponges, which have no nervous system (Hongwei Liu et al., 2004). In invertebrates (that have nervous system starting from nematodes), DA is involved in the development of behavioral reactions (Barron et al., 2010). In insects, DA is the only physiologically significant catecholamine (Mustard et al., 2005; Verlinden, 2018). The relatively simple Caenorhabditis elegans nervous system is widely used to study the mechanism of the effect of DA (McDonald et al., 2006).
DA has been identified as the main, and presumably the only, catecholamine in gastropods (Zhuravlev, 1999). The DA content in their hemolymph changes when exposed to stress factors; at the same time, the direction of this change is controversial. Thus, different types of effects (high temperature, decreased salinity, and exposure to air) cause a decrease in DA concentration in the scallop hemolymph (Chen et al., 2008). At the same time, an increase in the level of DA in hemolymph occurs in the oyster due to similar effects (Lacoste et al., 2001). DA has been found in both the central and peripheral nervous systems of mollusks (Faller et al., 2008). Its involvement in many biological processes has been demonstrated, including nutrition (Quinlan et al., 1997; Wieland and Gelperin, 1983), respiration (Moroz and Winlow, 1992), egg laying (Werkman et al., 1990), and albumin gland secretion (Kiehn et al., 2001; Saleuddin et al., 2000). DA-beta-H enzyme, for which DA is a substrate required for synthesizing norepinephrine, has been found in the Viviparus freshwater gastropod (Ottaviani et al., 1993) and Litopenaeus vannamei white shrimp (Cheng et al., 2016), while T-H (involved in DA biosynthesis) was found in the nervous system of two Biomphalaria genus members (Vallejo et al., 2014).
Previously, we demonstrated the presence of DA in hemolymph of intact achatinas and a sharp increase in its concentration under stress caused by a short-term gravitational load; among catecholamines in the achatina hemolymph, DA is present in the highest concentration, which is 1200 pg/mL normally and increases by more than four times under stress (Martynova et al., 2015).
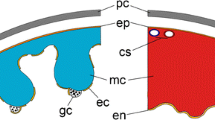
Neuroendocrine complexes (NECs), which are located in the atrium between endothelial cells and muscle fibers and consist of large granular cells (GCs) and nerve fibers in close contact with them (accompanied by interstitial cells), are involved in hormonal regulation of physiological processes in gastropods. All these cell elements contain granules, in which a number of bioactive substances were identified, such as atrial natriuretic peptide (Martynova et al., 2004), Hsp70 heat shock protein (Martynova et al., 2007), P substance and FRMF-amide (Shabel’nikov et al., 2008), serotonin, histamine (Bystrova et al., 2014), and corticoliberin (Martynova et al., 2018). The aim of this work was to study the presence and localization of dopamine system components (TH, DA-beta-H, and DA-R1) in atrial NEC in the achatina. In addition, the effect of exogenous DA on GC degranulation was tested in vivo.
MATERIALS AND METHODS
Animals
The atria of the Achatina achatina giant African snail were used as experimental material. The snail population was held in the laboratory for several years at a constant temperature (about 25°С) and artificial light mode (12 h light) on a vegetarian diet. Adult achatinas weighing 15–20 g were taken in the experiment.
Histofluorescent Tissue Staining
Histochemical detection of monoamines by means of glyoxylic acid was performed according to the method of (De la Torre and Surgeon, 1976) modified by Zaitseva et al. (2015). Isolated mollusk atrium was incubated 1.5 h at 4°С in 1 M glyoxylic acid (Sigma G10601, United States) freshly prepared on a solution containing 1 M sodium bicarbonate, 0.3 M saccharose, and 0.1 M HEPES, pH 7.4. After the incubation, the tissue was straightened by dissecting needles on the cover glass, dried initially for 30 min at room temperature and then 40 min at 60°С, and then embedded in paraffin oil. The fluorescence was registered using a Leica DFC420c camera (Leica Microsystem, Germany) on an AxioVert 200V microscope (Carl Zeiss, Germany) equipped with a Filter Set02 filter (Carl Zeiss, Germany). The excitation wavelength and emission were 365 and 420 nm, respectively.
Immunofluorescent Tissue Staining
The analysis was conducted following a standard immunocytochemical protocol. Isolated atria were stretched with dissecting needles on the slide and fixed with 4% PFA for 1 s at 4°С. After threefold washing in PBS, the tissue was treated with DMSO with carbinol (1 : 4) for 1 h and placed in pure carbinol for 1 day at a temperature of −25°C. The tissue was again washed in PBS three times for 5 min and treated with 10% fetal serum to block nonspecific antibody binding. After this, the tissue was incubated with the first antibodies for 1 day at 4°C and then with the second antibodies. Images were obtained using an Axiovert 200 M microscope (Carl Zeiss, Germany) equipped with a Leica DFC 420C digital camera (Leica Microsystems, Germany) with a lens magnification of 40×.
Immunoelectron Microscopy
Isolated atria were fixed in 2.5% glutaraldehyde on 0.1 M cacodylate buffer, pH 7.2, with a content of saccharose of 2% for 1.5 h at 4°С. Postfixation was performed in 1% OsO4 on 0.1 M cacodylate buffer, pH 7.4, with a content of saccharose of 5% for 1 h at 4°С. The tissue samples were then dehydrated in a series of ethanol solutions with increasing concentration and embedded in Araldite–Epon mixture. Ultrathin sections were prepared with a diamond knife on an LKB-Ultratome. To conduct immunochemical tests, ultrathin sections were collected on nickel grids, treated with 3% hydrogen peroxide for 20 min to loosen the resin, incubated 1 day with the first antibodies at 4°С in a humid chamber and after washing in PBS incubated with the second antibodies for 1 h at room temperature, stained with uranyl acetate and plumbum citrate, and viewed on a Zeiss Libra 120 electron microscope (Carl Zeiss, Germany) at an accelerating voltage of 80 kV.
Antibodies
Polyclonal antibodies to TH (Abcam, ab112, United States) at a dilution 1 : 100, polyclonal antibodies to DA-beta-H (Enzo, BML-DZ1020) at a dilution 1 : 50, and monoclonal antibodies to DA-R1 (Sigma, D2944, United States) at a dilution 1 : 50 were used for immunofluorescent staining. ALEXA 488 (Abcam, ab150115, United States) at a dilution 1 : 600 (in the case of the first monoclonal antibodies) and rabbit antibodies against rat immunoglobins conjugated with FITC (Sigma, F9887, United States) at a dilution 1 : 200 (in the case of the first polyclonal antibodies) were used as the second antibodies. For EM-immunocytochemical reactions, polyclonal antibodies against DAbetaH (Enzo, BML-DZ1020) at a dilution 1 : 100 and monoclonal antibodies against DA-R1 (Sigma, D2944, United States) at a dilution 1 : 1000 were used. Antibodies against rabbit immunoglobulins (for polyclonal antibodies) or against mouse immunoglobulins (for monoclonal antibodies) conjugated with a colloidal gold (Sigma, G7402, United States) at a dilution 1 : 10 were used as secondary antibodies.
Effect of DA on Snail Atrial GC in vivo
An amount of 0.06 mL physiological solution (61 mM, 3.3 mM KCl, 10.7 mM CaCl2, 13 mM MgSO4, 10 mM Hepes, pH 7.4) containing DA hydrochloride (Sigma) at a concentration of 4000 pg/mL was injected into the snail body cavity. A pure physiological solution at the same volume was introduced into the control animals. Intact animals served as another control. The atria were isolated in 1.5 h, fixed, and poured into epoxy resins according to the material preparation protocol for immunoelectron microscopy (see above). The analysis was carried out on semithin sections stained with alcian blue.
Statistical Processing
The calculation of the portion of degranulated cells was conducted in several random fields of view in preparations obtained in three independent experiments with three snails in each. Data are presented as a mean and its error; the difference was estimated by means of Student’s t-criterion and considered significant at P < 0.05.
RESULTS
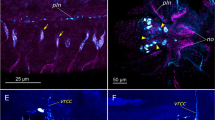
The presence of catecholamines in the snail atrium was detected histochemically by means of glyoxylic acid. A positive reaction was observed in nerve fibers and GC granules (Fig. 1a).
Dopamine system components in Achatina achatina atrium: histochemical detection of (a) catecholamines, (b, c, d, f) immunofluorescent, and (e, g) EM-immunocytochemical staining. (a) Glyoxylate-induced fluorescence is present in GC (asterisks) and nerve fibers (arrows); (b, c) T-H-like immunoreactivity in (b) GCs and (c) nerve fibers, arrows indicate nerve fibers, GCs are marked with asterisks; (d) expression of DA-beta-H-like material in GC (asterisks) and nerve fibers (arrows); (e) presence of DA-beta-H-like material in neurosecretory granules of new fibers (N) and GC granules (GC); (f) presence of DA-R1-like material in nerve fibers (arrows); (g) presence of DA-R1 in neurosecretory granules of nerve fibers, adjacent nerve fibers of one nerve bundle contain more (two asterisks) and less (one asterisk) intensely stained neurosecretory granules. (a–d, f): lens magnification: 40×. Scale segment: 1 µm.
TH expression was studied by the fluorescent immunohistochemistry method. TH-immunoreactive material was found in GC and nerve fibers (Figs. 1b and 1c).
D-beta-H expression in the snail atrium tissues was studied by the fluorescent immunohistochemistry and EM-immunocytochemistry methods. Immunofluorescent analysis demonstrated the presence of D-beta-H-immunopositive material in atrial GC and nerve fibers coming to them (Fig. 1d). When conducting EM-immunocytochemical staining, the label was found over neurosecretory granules of nerve fibers and granules of gliointerstitial cells, as well as over GC granules (Fig. 1e).
D-R1 expression in the snail atrium tissue was also studied by the fluorescent immunohistochemistry and EM-immunocytochemistry methods. Both methods identified DA-R1 positive material only in the snail atrium nerve fibers (Figs. 1f, 1g). The intensity of labeling neurosecretory granules of adjacent nerve fibers of one bundle was significantly different (Fig. 1g).
DA effect on GC degranulation in vivo was analyzed on semithin sections embedded in the snail atrium epoxy resins. Intact and granulated GCs are well distinguishable on the preparations stained with alcian blue (Figs. 2a, 2b). A spontaneous degranulation process takes place in snail atrial GCs under normal physiological conditions, and visibly empty granule chambers are present in some the cells. The number of such GCs increased dramatically after the introduction of exogenous DA. The portion of degranulated GCs in intact snail atria was 14.9 ± 3.1%. After the introduction of physiological solution without DA, the portion of degranulated GC slightly increased and was 23.8 ± 4.1%, which was not statistically different from the norm. After 1.5 h had passed after the injection of DA, an almost threefold increase in the portion of degranulated GC occurred; it was 65.4 ± 0.85%, which differs both from the norm and from the control with the introduction of physiological solution (P < 0.01). In addition to partially degranulated GCs, EM allowed to fully degranulated GCs to be detected in the experimental variant (Fig. 2c). Such small-sized cells devoid of granules cannot be identified at the light-optical level and could not be counted when calculating the portion of degranulated GCs on histological preparations. Consequently, dopamine caused more significant degranulation of GCs than was demonstrated by the analysis at the light-optical level.
Effect of dopamine on degranulation of achatina atrial GC. (a, b) Semi-thin sections of atrium tissues stained with alcian blue. In intact snails, the atrium mainly contains GCs filled with granules (a), while degranulated GCs are for the most part found in the atrium after dopamine introduction (b). GC are marked with arrows. Lens magnification: 40×. (c) Electronogram demonstrating completely degranulated GCs. Cell identification as a GC is made possible by its location between outgrowth of endothelial cell (E) and muscle fiber (M) and close contact with the nerve ending (N), as well as by clusters of small vacuoles and granules typical for GCs under the basal GC plate (arrows). Scale segment: 1 µm.
DISCUSSION
Histochemical reaction detected the presence of catecholamines in GCs and nerves of the atrium. The complete composition of catecholamines in NEC elements has not been established, but DA is one of them (as has been shown by our study). The main enzymes of the chain of tryptophan biochemical conversion to DA and then to norepinephrine (TH and DA-beta-H) were found here, with the presence of the latter entailing that norepinephrine is also present in NEC. Thus, nerve endings that are in contacting with GCs as a part of NEC are dopaminergic. GCs themselves synthesize and secrete DA, which can function in this case as a hormone. With hemolymph current, DA enters the ventricle from the atrium and then spreads rapidly throughout the organism, driven by the beating of the heart. Regulation of the heart’s functioning itself is one of the possible functions of DA secreted by GCs (just as happens in vertebrates) (Polakowski et al., 2004). In addition, it is known that this hormone in mollusks is involved in many physiological functions. Thus, there is evidence of the effect of DA on sensitive epithelium of the olfactory organs and the movement of tentacles (Rosshchin and Balaban, 2012), on ciliated cells of the Mytilus edulis larva velum, in which it inhibits the nutrition rate (Beiras and Widdows, 1995), on the spawning retention in the Dreissena polymorpha zebra mussel (Fong et al., 1993), and on a decrease in the activity of sole muscle contraction waves in the Helix sp. snail (Pavlova, 2001). The regulation of all these processes can be mediated by DA secreted by GC as a part of NEC.
The results of the work allow one to add DA to the already available extensive list of bioactive substances present in NEC cells.
The concept of DA as a regulator of immune reactions is developed during the last 20 years; its effect on the activity of lymphocytes, who are immune effector cells of vertebrates, was demonstrated (Basu and Dasqupta, 2000). Ottaviani et al. in their numerous works demonstrated the similarity of a set of bioactive molecules involved in the development of the immune response, stress reaction, and neuroendocrine regulation in invertebrates (particularly, mollusks) and vertebrates; at the same time, these authors consider hemolymph cells (hemocytes) as the main immunocompetent cells of mollusks (Blom and Ottaviani, 2017; Malagoli and Ottaviani, 2017). The existence of a single neuroendocrine–immune regulatory network, in which DA and the enzymes of its biochemical transformations occupy a key position, has been hypothesized in mollusks (Cheng et al., 2016, 2017). In particular, it has been demonstrated that DA modulates the immune response to the introduction of bacteria in scallops (Zhou et al., 2011). The detection of DA system components in snail atrium NEC cells indicates their possible involvement (along with hemocytes) in the functioning of neuroendocrine–immune regulatory network, namely, in the immune protection, neuroendocrine regulation of physiological processes, and stress reactions. DA is an important element of them.
We detected DA-R1-like immunoreactivity in neurosecretory granules of nerve fibers, but not in GCs. We used antibodies to the first type of DA-R, guided by the available data on their presence in the CNS of the Lymnaea stagnalis great pond snail (Hernádi et al., 2012), albumin gland of the Helisoma duryi snail (Mukai et al., 2004), and ovarian tissues of the Crassostrea angulate oyster (Yang et al., 2013). However, as demonstrated, there are at least three subtypes of DA receptors in invertebrates (Mustard et al., 2005). It is possible that the achatina atrial GCs sense the effect of DA through another subtype of DA-R. The different intensities of neurosecretory granule labeling in neighboring axons of the same nerve bundle by antibodies against DA-R1 is notable. We observed a similar picture when working with antibodies against the heat shock protein (Martynova et al., 2007) and P substance and FMRF-amide (Shabel’nikov et al., 2008), as well as against corticoliberin and its receptors (Martynova et al., 2018). This corresponds to physiological data on the mixed character of gastropod nerves (Zhuravlev, 1999). The presence of DA in secretory granules of GCs and DA-R1 in nerve fibers indicates the possibility of hormonal signal transmission from GCs to the CNS. DA acts as a sensor neurotransmitter in this case.
There are data on the involvement of dopaminergic nerves in chemo- and mechanoreception in gastropods (Croll et al., 2003) and signal transmission from peripheral nervous system to CNS, where they affect the behavioral reactions (Bédécarrats et al., 2013). Thus, NEC can determine physiological and psychological state of the snail. The process of transgranulation of granular material from the mast cells to the nerve endings in contact with them has been described for vertebrates and has been interpreted as a form of communication of the immune and nervous systems (Wilhelm et al., 2005). Gastropod atrial GCs exhibit many similarities with mast cells—common bioactive substances contained in the granules, close contacts with nerve fibers, and active involvement in stress response. In the context of this work, it is important to note the detection of DA and TH in the mast cell granules (Rönnberg et al., 2012; Rönnberg and Pejler, 2012).
The introduction of exogenous DA caused a significant increase in secretory process in atrial GCs. Previously, we demonstrated using the method of high performance liquid chromatography that the dopamine concentration in the achatina hemolymph increases under stress up to 5300 pg/mL (Martynova et al., 2015). Thus, the DA dose that we selected for the injection (4000 pg/mL) is comparable with hormone concentration in the snail hemolymph under stress and is not damaging. Previously, we also demonstrated that the stress effects cause GC degranulation. Based on data obtained in this work, the involvement of DA in the activation of this process can be suggested. We found no DA-R1 in GCs, and it is not clear through what receptors DA modulates the secretory process.
Thus, dopamine system elements (TH, DbetaGC, and DA-R1) are involved in the snail atrium NEC elements, and its activations lead to an increase in degranulation of secretory GCs as a part of the NEC (which is the stress response component). The data that have been obtained enrich our ideas about the mechanisms of NEC functioning.
REFERENCES
Barron, A.B., Søvik, E., and Cornish, J.L., The roles of dopamine and related compounds in reward-seeking behavior across animal phyla, Front. Behav. Neurosci., 2010, vol. 4, p. 163. doi https://doi.org/10.3389/fnbeh.2010.00163
Basu, S. and Dasgupta, P.S., Dopamine, a neurotransmitter, influences the immune system, J. Neuroimmunol., 2000, vol. 102, pp. 113–124.
Bédécarrats, A., Cornet, C., Simmers, J., and Nargeot, R., Implication of dopaminergic modulation in operant reward learning and the induction of compulsive-like feeding behavior in Aplysia, Learn. Mem., 2013, vol. 20, pp. 318–327.
Beiras, R. and Widdows, J., Effect of the neurotransmitters dopamine, serotonin and norepinephrine on the ciliary activity of mussel (Mytilus edulis) larvae, Mar. Biol., 1995, vol. 122, pp. 597–603.
Blom, J.M.C. and Ottaviani, E., Immune–neuroendocrine interactions: evolution, ecology, and susceptibility to illness, Med. Sci. Monit. Basic Res., 2017, vol. 23, pp. 362–367.
Bystrova, O.A., Shabelnikov, S.V., and Martynova, M.G., The process of granule exocytosis in non-stimulated atrial granular cells of the snail, Achatina achatina: an ultrastructural, histochemical and immunocytochemical study, Acta Histochem., 2014, vol. 116, pp. 14–19.
Casagrande, C., Dopamine and the kidney in heart failure, Herz, 1991, vol. 16, pp. 102–115.
Chen, M., Yang, H., Xu, B., Wang, F., and Liu, B., Catecholaminergic responses to environmental stress in the hemolymph of Zhikong scallop Chlamys farreri, J. Exp. Zool. Ecol. Genet. Physiol., 2008, vol. 309, pp. 289–296.
Cheng, W., Ka, Y.W., and Chang, C.C., Dopamine beta-hydroxylase participate in the immunoendocrine responses of hypothermal stressed white shrimp, Litopenaeus vannamei, Fish Shellfish Immunol., 2016, vol. 59, pp. 166–178.
Cheng, W., Ka, Y.W., and Chang, C.C., Involvement of dopamine beta-hydroxylase in the neuroendocrine-immune regulatory network of white shrimp, Litopenaeus vannamei, Fish Shellfish Immunol., 2017, vol. 68, pp. 92–101.
Croll, R.P., Boudko, D.Y., Pires, A., and Hadfield, M.G., Transmitter contents of cells and fibres in the cephalic sensory organs of the gastropod mollusc Phestilla sibogae, Cell Tiss. Res., 2003, vol. 314, pp. 437–448.
De la Torre, J.C. and Surgeon, J.W., A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method, Histochemistry, 1976, vol. 49, pp. 81–93.
Faller, S., Stauback, S., and Klussman-Kolb, A., Comparative immunohistochemistry of the cephalic sensory organs in Opisthobranchia (Mollusca, Gastropoda), Zoomorphology, 2008, vol. 127, pp. 227–239.
Fong, P.P., Noordhuis, R., and Ram, J.L., Dopamine reduces intensity of serotonin-induced sprowning in the zebra mussel Dreissena polymorpha (Pallas), J. Exp. Zool., 1993, vol. 266, pp. 79–83.
Hernádi, L., Vehovszky, Á., and Serfőző, Z., Immunological and pharmacological identification of the dopamine D1 receptor in the CNS of the pond snail, Lymnaea stagnalis, Acta Biol. Hung., 2012, vol. 63, suppl. 2, pp. 151–159.
Kiehn, L., Saleuddin, S., and Lange, A., Dopaminergic neurons in the brain and dopaminergic innervation of the albumen gland in mated and virgin Helisoma duryi (Mollusca: Pulmonata), BMC Physiol., 2001, vol. 1, p. 9.
Lacoste, A., Malham, S.K., Cueff, A., and Poulet, S.A., Stress-induced catecholamine changes in the hemolymph of the oyster Crassostrea gigas, Gen. Comp. Endocrinol., 2001, vol. 122, pp. 181–188.
Liu, H., Mishima, Y., Fujiwara, T., Nagai, H., Kitazawa, A., Mine, Y., Kobayashi, H., Yao, X., Yamada, J., Oda, T., and Namikoshi, M., Isolation of araguspongine M, a new stereoisomer of an araguspongine/xestospongin alkaloid, and dopamine from the marine sponge Neopetrosia exigua collected in Palau, Mar. Drugs, 2004, vol. 2, pp. 154–163.
Malagoli, D. and Ottaviani, E., Cross-talk among immune and neuroendocrine systems in molluscs and other invertebrate models, Horm. Behav., 2017, vol. 88, pp. 41–44.
Marsden, C.A., Dopamine: the rewarding years, Br. J. Pharmacol., 2006, vol. 147, suppl. 1, pp. 136–144.
Martynova, M.G., Krylova, M.I., and Bystrova, O.A., Immunocytochemical localization of atrial natriuretic peptide in endothelial and granular cells of the heart of Lophotrochozoa, Tsitologiia, 2004, vol. 46, no. 5, pp. 448–455.
Martynova, M.G., Bystrova, O.A., Shabelnikov, S.V., Margulis, B.A., and Prokofjeva, D.S., Hsp70 in the atrial neuroendocrine units of the snail, Achatina fulica, Cell Biol. Int., 2007, vol. 31, pp. 413–419.
Martynova, M.G., Shabelnikov, S.V., and Bystrova, O.A., Long-term consequences of a short-term hypergravity load in a snail model, Int. J. Astrobiol., 2015, vol. 14, pp. 489–495.
Martynova, M.G., Petukhova, O.A., Sharlaimova, N.S., Shabelnikov, S.V., and Bystrova, O.A., Components of corticotropin-releasing factor (CRF) signaling system in snail atria, Cell Tissue Biol., 2018, vol. 12, no. 4, pp. 342–349.
McDonald, P.W., Jessen, T., Field, J.R., and Blakely, R.D., Dopamine signaling architecture in Caenorhabditis elegans, Cell Mol. Neurobiol., 2006, vol. 26, pp. 593–618.
Moroz, L.I. and Winlow, W., Respiratory behaviour in Lymnaea stagnalis: pharmacological and cellular analyses, Acta Biol. Hung., 1992, vol. 43, pp. 421–429.
Mukai, S.T., Kiehn, L., and Saleuddin, A.S., Dopamine stimulates snail albumen gland glycoprotein secretion through the activation of a D1-like receptor, J. Exp. Biol., 2004, vol. 207, pp. 2507–2518.
Mustard, J.A., Beggs, K.T., and Mercer, A.R., Molecular biology of the invertebrate dopamine receptors, Arch. Insect Biochem. Physiol., 2005, vol. 59, pp. 103–117.
Ottaviani, E., Caselgrandi, E., Franchini, A., and Franceschi, C., CRF provokes the release of norepinephrine by hemocytes of Viviparus ater (Gastropoda, Prosobranchia): further evidence in favour of the evolutionary hypothesis of the mobile immune-brain, Biochem. Biophys. Res. Commun., 1993, vol. 193, pp. 446–452.
Pavlova, G.A., Effect of serotonin, dopamine and ergometrine on locomotion in the pulmonate mollusk Helix lucorum, J. Exp. Biol., 2001, vol. 204, pp. 1625–1633.
Polakowski, J.S., Segreti, J.A., Cox, B.F., Hsieh, G.C., Kolasa, T., Moreland, R.B., and Brioni, J.D., Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats, Clin. Exp. Pharmacol. Physiol., 2004, vol. 31, pp. 837–841.
Quinlan, E.M., Arnett, B.C., and Murphy, A.D., Feeding stimulants activate an identified dopaminergic interneuron that induces the feeding motor program in Helisoma, J. Neurophysiol., 1997, vol. 78, pp. 812–824.
Rönnberg, E. and Pejler, G., Serglycin: the master of the mast cell, Methods Mol. Biol., 2012, vol. 836, pp. 201–217.
Rönnberg, E., Calounova, G., and Pejler, G., Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner, Biol. Chem., 2012, vol. 393, pp. 107–112.
Roshchin, M. and Balaban, P.M., Neural control of olfaction and tentacle movements by serotonin and dopamine in terrestrial snail, J. Comp Physiol. Neuroethol. Sens. Neural. Behav. Physiol., 2012, vol. 198, pp. 145–158.
Saleuddin, A.S.M., Mukai, S.T., Almeida, K., and Hatiras, G., Membrane transduction pathway in the neuronal control of protein secretion by the albumen gland in Helisoma (Mollusca), Acta Biol. Hung., 2000, vol. 51, pp. 243–253.
Shabel’nikov, S.V., Bystrova, O.A., and Martynova, M.G., Immunolocalization of the substances P- and FMRFamide in the atrium of the snail Achatina fulica, Cell Tissue Biol., 2008, vol. 2, no. 4, pp. 451–544.
Vallejo, D., Habib, M.R., Delgado, N., Vaasjo, L.O., Croll, R.P., and Miller, M.W., Localization of tyrosine hydroxylase-like immunoreactivity in the nervous systems of Biomphalaria glabrata and Biomphalaria alexandrina, intermediate hosts for schistosomiasis, J. Comp. Neurol., 2014, vol. 522, pp. 2532–2552.
Verlinden, H., Dopamine signalling in locusts and other insects, Insect. Biochem. Mol. Biol., 2018, vol. 97, pp. 40–52.
Werkman, T.R., De, Vlieger, T.A., and Stoof, J.C., Indications for a hormonal function of dopamine in the central nervous system of the snail Lymnaea stagnalis, Neurosci. Lett., 1990, vol. 108, pp. 167–172.
Wieland, S.J. and Gelperin, A., Dopamine elicits feeding motor program in Limax maximus, J. Neurosci., 1983, vol. 3, pp. 1735–1745.
Wilhelm, M., Silver, R., and Silverman, A.J., Central nervous system neurons acquire mast cell products via transgranulation, Eur. J. Neurosci., 2005, vol. 22, pp. 2238–2248.
Yang, B., Ni, J., Zeng, Z., Shi, B., You, W., and Ke, C., Cloning and characterization of the dopamine like receptor in the oyster Crassostrea angulata: expression during the ovarian cycle, Comp. Biochem. Physiol. Biochem. Mol. Biol., 2013, vol. 164, pp. 168–175.
Zaitseva, O.V., Shumeev, A.N., Korshunova, T.A., and Martynov, A.V., Heterochronies in the formation of the nervous and digestive systems in early postlarval development of opisthobranch mollusks: organization of major organ systems of the Arctic dorid Cadlina laevis, Biol. Bull., 2015, vol. 42, pp. 186–195.
Zhou, Z., Wang, L., Shi, X., Zhang, H., Gao, Y., Wang, M., Kong, P., Qiu, L., and Song, L., The modulation of catecholamines to the immune response against bacteria Vibrio anguillarum challenge in scallop Chlamys farreri, Fish Shellfish Immunol., 2011, vol. 31, pp. 1065–1071.
Zhuravlev, V.L., Mechanisms of neurohumoral control of gastropod heart, Zh. Evol. Biochem. Physiol., 1999, vol. 35, no. 2, pp. 65–77.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research, project no. 16-04-00069.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by A. Barkhash
Abbreviations: GC—granular cell, DA—dopamine, DA-beta-H—dopamine beta hydroxylase, NEC—neuroendocrine complex, TH—tyrosine hydroxylase, DA-R1—dopamine type 1 receptors, EM—electron microscopy.
Rights and permissions
About this article
Cite this article
Bystrova, O.A., Shumeev, A.N. & Martynova, M.G. Dopamine System Components in Neuroendocrine Complexes in Snail Atrium. Cell Tiss. Biol. 13, 152–159 (2019). https://doi.org/10.1134/S1990519X19020032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X19020032