Abstract
The structure and function of the central nervous systems of opisthobranch gastropods have been studied extensively. However, the organisation and function of the peripheral nervous system are poorly understood. The cephalic sensory organs (CSOs) are known to be chemosensory structures in the head region of opisthobranchs. In the present study, we used immunohistochemical methods and confocal laserscanning microscopy to comparatively examine the CSOs of different opisthobranchs, namely Acteon tornatilis, Aplysia punctata, Archidoris pseudoargus and Haminoea hydatis. We wanted to characterise sensory epithelia in order to infer the function of sensory structures and the organs they constitute. Immunoreactivity against the three antigens tyrosine hydroxylase, FMRFamide and serotonin was very similar in the CSOs of all investigated species. Tyrosine hydroxylase-like immunoreactivity was detected primarily in subepidermal sensory cell bodies, which were much more abundant in the anteriorly situated CSOs. This observation indicates that these cells and the respective organs may be involved in contact chemoreception and mechanoreception. The dominant features of FMRFamide-like immunoreactivity, especially in the posterior CSOs, were tightly knotted fibres which reveal glomerulus-like structures. This suggests an olfactory role for these organs. Serotonin-like immunoreactivity was detected in an extensive network of efferent fibres, but was not found within any peripheral cell bodies. Serotonin-like immunoreactivity was found in the same glomerulus-like structures as FMRFamide-like immunoreactivity, indicating a function of serotonin in the efferent control of olfactory inputs. Besides this functional implication, this study could also add some knowledge on the doubtful homology of the CSOs in opisthobranch gastropods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Opisthobranchia are morphologically extremely diverse marine gastropods occurring almost exclusively in marine habitats across the world. While the central nervous system of these animals has been well studied (Ono and McCaman 1984; Longley and Longley 1986; Croll 1987; Elekes 1992; Sudlow et al. 1998; Croll et al. 2001; Newcomb et al. 2006), relatively little is known about the organisation and function of their peripheral nervous systems. Like other gastropods, all species of the Opisthobranchia possess sensory structures in the head region, the so-called cephalic sensory organs (CSOs). These organs have already been investigated in histological (Merton 1920; Wolter 1967; Edlinger 1980; Huber 1993), ultrastructural (Storch and Welsch 1969; Emery and Audesirk 1978; Davis and Matera 1982; Boudko et al. 1999; Göbbeler and Klussmann-Kolb 2007), behavioural (Audesirk 1975; Wolter 1967) and electrophysiological (Jahan-Parwar 1972; Jacklet 1980) studies. The CSOs can take the shape of a cephalic shield, a Hancock’s organ, a lip, a lip organ, oral tentacles, an oral veil or rhinophores. According to Jahan-Parwar (1972), Audesirk (1975), Bicker et al. (1982) and Croll (1983) these organs are primarily involved in chemoreception. Chemoreception is generally the most important modality for marine gastropods because auditory and visual information are limited (Audesirk 1975; Chase 2002; Wertz et al. 2006, 2007). Chemical senses are used in finding food, avoiding predators, homing and interacting with conspecifics (Emery 1992); however, the CSOs are also sensitive to mechanical stimuli (Jahan-Parwar 1972; Bicker et al. 1982), water currents (Wolter 1967; Storch and Welsch 1969) and light (Chase 1979; Jacklet 1980).
The distribution of neurotransmitters in the CSOs of opisthobranchs has been studied in different opisthobranch taxa (Salimova et al. 1987; Moroz et al. 1997; Croll 2001; Croll et al. 2003; Wertz et al. 2006, 2007; Hochberg 2007; Wollesen et al. 2007). These studies primarily focussed on the distribution of serotonin, whereas investigations of other neurotransmitters or neuropeptides were less extensive. Comparative studies are lacking to date. Table 1 provides an overview over the immunohistochemical studies labelling catecholamines, FMRFamide and serotonin in the nervous system of adult euthyneuran gastropods done so far.
In the present study, we compared the distribution of serotonin, tyrosine hydroxylase (TH) and FMRFamides in the cephalic sensory organs of several different opisthobranchs. TH is an enzyme which catalyses the initial step in the conversion of tyrosine to the catecholamines (S.-Rozsa 1984), and therefore indicative of catecholamines. Catecholamines have been detected in the central nervous systems of different pulmonate gastropods (Elekes et al. 1991; Hernadi et al. 1993; Hernadi and Elekes 1999), in the central and peripheral nervous systems of the opisthobranchs Aplysia depilans, Aplysia fasciata and Aplysia californica (Salimova et al. 1987; Croll 2001) and in the central nervous system and CSOs of the opisthobranch Phestilla sibogae (Croll et al. 2001, 2003). FMRFamide (Phe-Met-Arg-Phe-NH2)-related peptides comprise a family of neuropeptides which were isolated first from the ganglia of the bivalve Macrocallista nimbosa (Lightfoot, 1786) (Price and Greenberg 1977), but are also ubiquitous in other molluscs, like in species of the Polyplacophora, Cephalopoda and Gastropoda (Price et al. 1987). According to Cottrell (1989) FMRFamides are chemical messengers in both the central and peripheral nervous systems. They have been detected in the central and peripheral nervous systems of different pulmonate gastropods (Cooke and Gelperin 1988; Elekes and Nässel 1990; Elekes 1992; Suzuki et al. 1997) and in the central nervous system and CSOs of the opisthobranchs Phestilla sibogae (Croll et al. 2001, 2003) and Aplysia californica (Elekes 1992; Wollesen et al. 2007). Serotonin (5-HT) is a biogenic monoamine which is synthesized in the nervous system from the amino acid tryptophan (S.-Rozsa 1984). It has been investigated in numerous studies of the central nervous system of gastropods (Ono and McCaman 1984; Longley and Longley 1986; Hernadi et al. 1989; Elekes 1992; Sudlow et al. 1998; Hernadi and Elekes 1999; Croll et al. 2001; Shirahata et al. 2004; Newcomb et al. 2006; Hochberg 2007) and the CSOs of a variety of opisthobranchs (Moroz et al. 1997; Croll et al. 2003; Wertz et al. 2006, 2007; Hochberg 2007; Wollesen et al. 2007).
The purpose of the current study was to investigate the distribution of the cell bodies and fibres containing the three types of antigens mentioned above within different types of CSOs and to compare these distributions among different opisthobranch taxa in order to reveal insights into the function and evolution of the different types of CSOs.
Materials and methods
Individuals of Acteon tornatilis (Linné, 1758) (Acteonoidea), Aplysia punctata Cuvier, 1803 (Anaspidea) and Archidoris pseudoargus (Rapp, 1827) (Nudibranchia) were collected in the intertidal near Roscoff (France). The specimens of Haminoea hydatis (Linné, 1758) (Cephalaspidea s.str.) were obtained from a laboratory culture at the Goethe-University, Frankfurt. All animals were anesthetized by injection with 7% MgCl2. The neuroanatomy of nerves was determined by macropreparation of 5–6 animals. For paraffin histology, the CSOs of 1–2 animals per species were examined and for whole mounts the CSOs of a minimum of 4 and a maximum of 20 animals were examined for each primary antibody. The CSOs were cut off and fixed for 4–24 h at room temperature in 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS, pH 7.3). For detection of tyrosine hydroxylase, the CSOs were fixed for 30 min in 99% methanol and 1% acetic acid at −18°C and subsequently transferred for 10 min each in 70, 50 and 30% methanol. After fixation the CSOs were washed several times in PBS. For the neuroanatomy of nerves the CSOs were additionally bathed overnight in 100% ethanol.
Paraffin histology
The CSOs were dehydrated in a graded isopropyl alcohol series and transferred and embedded in paraffin. Ten-micrometre-thick sections were cut with a Leica RM 2165 microtome and placed on glass microscope slides. Paraffin was removed with rotihistol. The sections were washed three times in PBS and were preincubated for 1 h with 0.2% Triton and 1% normal goat serum (NGS) in PBS. Following incubation, the sections were given another three washes in PBS and were next incubated for 24 h with either anti-FMRFamide (ImmunoStar Incorporated, Hudson, WI, USA) or anti-serotonin (Acris, Hiddenhausen,Germany) at room temperature. Both antibodies were raised in rabbit and were used at 1:100 dilutions in PBS. After incubation with primary antibody the sections were washed again three times in PBS and were then incubated for 24 h in goat anti-rabbit antibodies labelled with either FITC or TRITC (Jackson ImmunoResearch Laboratories, West Groove, PA, USA). The secondary antibodies were also used at 1:100 dilutions. After another several washes in PBS the sections were covered with glass coverslips in a solution of three parts glycerol to one part 0.1 M TRIS buffer (pH 8.0) with the addition of 2% n-propyl gallate. Additional control experiments without primary antibody incubation were done and no staining was observed in these preparations. As positive controls the central ganglia of individuals of Aplysia punctata were incubated with the same primary and secondary antibodies at the same dilutions. These ganglia showed an intense fluorescence in specific cells, as shown by Croll et al. (2004) for Aplysia californica.
Whole mounts
The CSOs were bathed overnight in blocking solution, either of 4% Triton and 1% NGS in PBS for detection of FMRFamide and serotonin or of 1% Triton and 1% normal sheep serum (NSS) in PBS for the detection of TH. After three washes in PBS the CSOs were incubated in one of the primary antibodies for 48 h at 4°C. Anti-FMRFamide and anti-serotonin were used in 1:500 dilutions. The monoclonal anti-TH antibody (ImmunoStar Incorporated, Hudson, WI, USA) was developed in mouse and was diluted 1:250. Following three washes in PBS the CSOs were incubated either for 24 h in goat anti-rabbit antibodies labelled with FITC (for FMRFamide) or TRITC (for serotonin) or for 36 h in sheep anti-mouse antibodies labelled with TRITC (for TH) at 4°C. The secondary antibodies were used at the same dilution as the primary antibodies. After another several washes in PBS the CSOs were mounted on object slides in a solution of three parts glycerol to one part TRIS buffer with the addition of 2% n-propyl gallate and covered with glass coverslips. Positive and negative control experiments were performed as described for paraffin histology.
Confocal microscopy and image processing
Preparations were viewed and photographed on a confocal laser scanning microscope (Leica TCS SP5). The distance between the optical sections was generally 1 μm and the resulting image stack was photographed as maximal projections. Cell diameters were determined by average over a minimum of ten cells. Image processing was performed with Adobe Photoshop 6.0 (Adobe Systems Incorporated, San Jose, CA, USA) and the schematic diagrams were prepared with CorelDRAW 11 (Corel Corporation, Ottawa, ON, Canada).
Results
Structure and innervation of the CSOs
The four investigated opisthobranch taxa possess different types of cephalic sensory organs. Acteon tornatilis possesses a cephalic shield which consists of a pair of antero-lateral lobes and a pair of postero-lateral lobes (Fig. 1a). A groove extends along the lateral ventral margins of the anterior-lateral lobes. The cephalic shield is innervated by three cerebral nerves. The anterior part is innervated by the Nervus labialis (N2), the posterior part is innervated by the Nervus rhinophoralis (N3) and the Nervus clypei captitis (Nclc). The investigated CSOs of Aplysia punctata are completely different from those of A. tornatilis. There are two pairs of CSOs, the posterior rhinophores and the anterior oral tentacles (Fig. 1b). Both pairs are rolled and have inner grooves. Each organ possesses a peripheral ganglion at the base of this groove. The Nervus labialis (N2) innervates the oral tentacles and the Nervus rhinophoralis (N3) serves the rhinophores. Archidoris pseudoargus also possesses a posterior pair of rhinophores and an anterior pair of oral tentacles (Fig. 1c). The rhinophores consist of shafts that have about 20 apical lamellae. The oral tentacles are short bulbs which are covered by the mantle. The oral tentacles are innervated by the Nervus labialis (N2) and the rhinophores by the Nervus rhinophoralis (N3). A large ganglion extends through the longitudinal axis of the rhinophores. In Haminoea hydatis we investigated three types of CSOs: the Hancock’s organ, the lip organ and the cephalic shield (Fig. 1d). The Hancock’s organ is situated posteriorly and the lip organ anteriorly underneath the cephalic shield. These CSOs are innervated by four cerebral nerves. The cephalic shield is innervated by the Nervus clypei captitis (Nclc) and the Nervus oralis (N1). The Nervus labialis (N2) innervates the lip organ and the Nervus rhinophoralis (N3) the Hancock’s organ.
Schematic diagrams of frontal views of Acteon tornatilis (a), Aplysia punctata (b), Archidoris pseudoargus (c) and Haminoea hydatis (d), showing innervation of the investigated cephalic sensory organs. The left sides of the diagrams show the innervation patterns of the cerebral nerves. The right sides of the diagrams show the location of the cephalic sensory organs. acs anterior cephalic shield; cg cerebral ganglion; cs cephalic shield; e eye; ga ganglion; gr, groove; ho Hancock’s organ; lo lip organ; N1 nervus oralis; N2 nervus labialis; N3 nervus rhinophoralis; Nclc nervus clypei captitis; ot oral tentacle; pcs posterior cephalic shield; rh rhinophore
Tyrosine hydroxylase (TH)-like immunoreactivity
TH-like immunoreactivity (lir) was found in all investigated CSOs of all investigated species. The predominant TH-like immunoreactive structures were bipolar cell somata which had diameters of 6.5 μm, were located subepidermally and bore dendrites that penetrated the epidermis (Fig. 5). The distributions of these somata varied within the different CSOs of one species as well as among species. In Acteon tornatilis these somata were found scattered across the entire cephalic shield, with increasing concentrations in the anterior region (Figs. 2a, 5a), especially in the lateral groove (~4,200 somata/mm²). In Aplysia punctata the number of TH-like immunoreactive somata was less than in A. tornatilis (Figs. 2b, 5b). The somata occurred along the complete lengths of the grooves in the oral tentacle as well as the rhinophores, with the highest concentrations near their tips. However, the somata were more common in the oral tentacles (~1,000 somata/mm²) than in the rhinophores (~300 somata/mm²). This also applied to Archidoris pseudoargus. In this species the TH-like immunoreactive somata were located in the tissue constituting the lamellae of the rhinophores as well as across the entire surface of the oral tentacles (Fig. 5c). Haminoea hydatis showed the highest density of TH-like immunoreactive somata in the lip organ (Fig. 2c, 5d). Here up to 6,400 somata/mm² were observed. Within the Hancock’s organ the catecholaminergic somata occurred in smaller numbers (~1,200 somata/mm²) at the posterior end and the lateral margin (Figs. 2d, 5d). In the cephalic shield of H. hydatis we found TH-like immunoreactive somata as well, but exclusively in the posterior region (~3,200 somata/mm²).
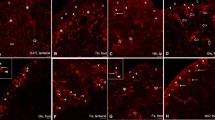
Confocal micrograph of tyrosine hydroxylase (TH)-like immunoreactivity in the cephalic sensory organs. Bipolar cell somata (arrow heads) with dendrites (arrows) beneath the epidermis (epi) in the groove of the anterior cephalic shield of Acteon tornatilis (a), the groove of the oral tentacle of Aplysia punctata (b), the lip organ of Haminoea hydatis (c) and Hancock’s organ of Haminoea hydatis (d). Bipolar cell somata (arrow head) with dendrites (arrows) beneath the epidermis (epi) and secondary nerve cells (snc) with dendrites (arrows) and axons (thick arrow) in the groove of the anterior cephalic shield of Acteon tornatilis (e). Immunoreactive fibres in the Nervus labialis (n) innervating the lip organ of Haminoea hydatis (f). epi epidermis; n nerve; snc secondary nerve cell
In addition to the subepidermal cell type described above, we found a second type of TH-like immunoreactive somata in A. tornatilis (Fig. 2e). These somata had diameters of 7.5 μm and were located exclusively in the lateral groove of the anterior cephalic shield. We observed one striking characteristic of these somata; in contrast to the somata described above their dendrites did not penetrate the epidermis. Instead, the dendrites seemed to extend to the subepidermal somata of the first cell type.
TH-like immunoreactivity was also observed within subepidermal networks of nerve fibres. These were especially abundant in the cephalic shield of A. tornatilis and H. hydatis as well as in the oral tentacles of A. punctata and A. pseudoargus. TH-lir could also be detected in cerebral nerves innervating the CSOs, e.g. the Nervus rhinophoralis of A. pseudoargus and the Nervus labialis of H. hydatis (Fig. 2f).
FMRFamide-like immunoreactivity
FMRFamide-like immunoreactivity (lir) was detected in diverse structures including nerves, peripheral ganglia, somata and fibres (Fig. 5). The predominant structures that showed FMRFamide-lir immunoreactivity were patches of tightly knotted fibres located along the major nerve branches (Fig. 3a). The distribution and density of these patches varied within the CSOs of each species as well as among species. In Acteon tornatilis such patches could only be found in low densities along the major nerve branches of the anterior cephalic shield (Figs. 3a, 5a). In Aplysia punctata both pairs of CSOs contained many such patches, which were more numerous in the rhinophores (Fig. 3b). In contrast, Archidoris pseudoargus possessed tightly knotted fibres in the tentacles, whereas no patches could be observed in the rhinophores (Fig. 5c). Haminoea hydatis contained tightly knotted fibres in all investigated CSOs. They were most densely concentrated at the lateral margin of the Hancock’s organ (Figs. 3c, 5d), whereas the lip organ and the cephalic shield possessed only a few such fibres. In addition to the patches of tightly knotted fibres, all investigated taxa contained FMRFamide-lir in their cerebral nerves (Fig. 3b, d) and in the neuropil and somata of their peripheral ganglia, when present (Fig. 3d, e, f).
Confocal micrograph of FMRFamide-like immunoreactivity in the cephalic sensory organs. Patches of tightly knotted fibres (g) and nerves (n) in the anterior cephalic shield of Acteon tornatilis (a), the rhinophore of Aplysia punctata (arrow heads indicate subepidermal somata) (b) and the Hancock’s organ of Haminoea hydatis (c). Nervus rhinophoralis (n) and rhinophoral ganglia (ga) of Aplysia punctata (d). Higher magnification showing isolated somata (arrow heads) within the rhinophoral ganglion (ga) of Aplysia punctata (e). Rhinophoral ganglion (ga) and nerve (n) in the rhinophore of Archidoris pseudoargus (f). Multipolar cell (arrow head) with dendrites (arrow) in the rhinophore of Archidoris pseudoargus (g). Nerve fibres (nf) beneath the epidermis (epi) in the anterior cephalic shield of Acteon tornatilis (h) and in the lip organ of Haminoea hydatis (i). cns central nervous system; epi epidermis; g glomerulus; ga ganglion; n nerve; nf nerve fibre
Aplysia punctata and Haminoea hydatis possessed FMRFamide-lir in peripheral somata (Fig. 5). These somata were generally bipolar, located subepidermally and their dendrites penetrated the epidermis. The somata in A. punctata had diameters of 7.5 μm and were distributed in low densities over the length of the rhinophore (Fig. 3b) and the grooves of the oral tentacles. The somata in H. hydatis were restricted to the anterior part of the Hancock’s organ and to the posterior part of the cephalic shield.
In addition to the bipolar somata we found another type of FMRFamide-like immunoreactive soma in the rhinophores of A. pseudoargus. These somata were multipolar with more than one dendrite (Figs. 3g, 5c). They had diameters of 11 μm and were located subepidermally basally to the lamellae. In contrast to the bipolar somata, the dendrites of the multipolar cells were not observed to penetrate the epidermis.
All investigated CSOs possessed FMRFamide-lir in a network of subepidermal fibres that did not penetrate the epidermis. In A. tornatilis, the subepidermal network of fibres was located on the ventral side of the entire cephalic shield (Fig. 3h). Both A. punctata and A. pseudoargus possessed isolated subepidermal fibres throughout their rhinophores and oral tentacles. In H. hydatis the subepidermal fibres were located primarily in the anterior region of the Hancock’s organ, throughout the entire lip organ (Fig. 3i) and in low densities within the cephalic shield.
Serotonin-like immunoreactivity
Serotonin-like immunoreactivity (lir) was found within the same patches of tightly knotted fibres (Fig. 4a, b), peripheral ganglia (Fig. 4c, d) and nerves (Fig. 4b, c) as FMRFamide-lir in all investigated taxa except for Haminoea hydatis. Therefore, the distribution of serotonin is not shown in the diagrams of the whole CSOs in Fig. 5. Serotonin-lir was also detected in a network of subepidermal nerve fibres in all investigated CSOs. These fibres did not penetrate the epidermis. In Acteon tornatilis a dense subepidermal network of fibres was located on the dorsal side of the entire cephalic shield (Fig. 4e). In contrast, Aplysia punctata possessed only a small number of subepidermal fibres in the rhinophores and tentacles. This distribution was also observed in Archidoris pseudoargus, where serotonin-like immunoreactivity was found within a small number of subepidermal fibres in the rhinophores and on the ventral surfaces of the tentacles. In H. hydatis the fibres were most densely concentrated in the anterior part of all investigated CSOs (Fig. 4f). No serotonin-like immunoreactive somata were found within any of the investigated CSOs.
Confocal micrograph of serotonin-like immunoreactivity in the cephalic sensory organs. Patches of tightly knotted fibres (g) and nerves (n) in the rhinophore (a) and oral tentacle (b) of Aplysia punctata. Immunoreactive fibres within the rhinophoral ganglion (ga) and nerves (n) in the rhinophore of Aplysia punctata (c) and Archidoris pseudoargus (d). Nerve fibres (nf) beneath the epidermis (epi) within the anterior cephalic shield of Acteon tornaltilis (e) and the Hancock’s organ of Haminoea hydatis (f). epi epidermis; g glomerulus; ga ganglion; n nerve; nf nerve fibre
Summary of the distributions of immunoreactivity in the cephalic sensory organs of Acteon tornatilis (a), Aplysia punctata (b), Archidoris pseudoargus (c) and Haminoea hydatis (d). The diagrams of the whole CSOs show the distribution of FMRFamide-like immunoreactivity on the right side and the distributions of TH-like immunoreactivity on the left side. The distributions of serotonin are very similar to those of FMRFamide and therefore are not shown in the diagrams of the whole CSOs. The small diagrams represent higher magnification views of the distributions of TH, FMRFamide and serotonin (5-HT) beneath the epidermis of the CSOs. The higher magnification view of Archidoris pseudoargus (c) only represents the distributions in the rhinophores. acs anterior cephalic shield; ax axon; bl basal lamina; cg cerebral ganglion; cs cephalic shield; d dendrite; epi epidermis; e eye; g glomerulus; ga ganglion; gr groove; ho hancock’s organ; lo lip organ; mc multipolar cell; nf nerve fibre; ot oral tentacle; pcs posterior cephalic shield; rh rhinophore; sc subepidermal cell; snc secondary nerve cell
Discussion
Our studies revealed that immunohistochemistry against the three types of neurotransmitters tyrosine hydroxylase, FMRFamide and serotonin revealed distinct structures within the CSOs. The distribution patterns of TH-, FMRFamide- and serotonin-like immunoreactivity in the four investigated Opisthobranchia species are summarised in Fig. 5.
Tyrosine hydroxylase (TH)-like immunoreactivity
The distribution of TH-lir was very similar within the CSOs of the four investigated taxa. They all exhibited subepidermal bipolar TH-like immunoreactive somata. These somata possessed dendrites that penetrated the epidermis and were much more abundant in the anterior CSOs (e.g. the oral tentacles) than in the posterior CSOs (e.g. the rhinophores). These findings are consistent with those of Croll (2001) in Aplysia californica and Croll et al. (2003) in Phestilla sibogae who therefore suggested that these cells function in contact chemoreception or mechanoreception. Acteon tornatilis possessed an additional type of TH-like immunoreactive somata that could not be detected in any of the other investigated taxa. The dendrites of these bipolar cells extended to the subepidermal sensory cells and did not penetrate the epidermis. These cells may be secondary nerve cells that obtain and relay information from the subepidermal primary sensory cells. Croll (2001) also described a second type of TH-like immunoreactive somata in A. californica that lie more deeply within the tissue and occasionally appear to be multipolar. He suggested they play a role in the lateral spread of sensory information within the peripheral neural plexus. These somata may correspond to the second type of somata found in this study. However, to clearly identify the role of these TH-like immunoreactive somata, further studies, especially electrophysiological investigations, are needed. In addition to the TH-like immunoreactive somata, TH-lir was detected in fibres of the nerves innervating the different CSOs. These fibres are presumably the centrally projecting axons of the sensory somata.
FMRFamide-like immunoreactivity
The distribution of FMRFamide in the peripheral nervous system of adult opisthobranchs has only been investigated by Croll et al. (2003) in the CSOs of Phestilla sibogae. In this study, as well as in our study, the dominant features of FMRFamide-lir were patches of tightly knotted fibres. These patches possibly correspond to glomerulus-like structures (Boudko et al. 1999; Croll et al. 2003). Glomeruli have recently been reported in the rhinophores of Aplysia punctata (Wertz et al. 2006) and in sensory areas of A.californica (Moroz 2006) and are also well-known in the tentacles of the pulmonate Achatina fulica (Chase and Tolloczko 1986). In generall glomeruli are considered to be involved in processing of olfactory stimuli. The glomerulus-like structures observed in the present study were concentrated in the posterior cephalic sensory organs of the investigated taxa, especially in the rhinophores of A. punctata and the Hancock’s organ of Haminoea hydatis. This suggests an olfactory role for these organs. Both the rhinophores of A. punctata and the Hancock’s organ of H. hydatis have already been proposed to be involved in chemoreception by Audesirk (1975) and Edlinger (1980). While the rhinophores of A. punctata contained numerous glomeruli, the rhinophores of Archidoris pseudoargus were lacking glomeruli. These findings are consistent with the new work of Wertz et al. (2007). It is questionable whether the rhinophores of A. pseudoargus are involved in olfaction or whether there are other structures that serve this function. Instead of glomerulus-like structures, the rhinophores of A. pseudoargus possessed FMRFamide-like immunoreactive neuropil in a large ganglion that extended over the entire longitudinal axis of the rhinophore. According to Bicker et al. (1982) the rhinophore and tentacular ganglion of Pleurobranchaea californica serve mainly as peripheral integrating and relay stations for sensory inputs. Wertz et al. (2006) demonstrated by means of calcium imaging experiments that olfactory stimuli are relayed and processed in the rhinophoral ganglion of A. punctata. It is likely that the rhinophoral ganglion also serves as a relay station of olfactory stimuli in A. pseudoargus and therefore glomeruli are not required. In contrast, the rhinophores of A. punctata possessed an FMRFamide-like immunoreactive ganglion and additional glomerulus-like structures. This contrast between A. punctata and A. pseudoargus could be due to the fact that the rhinophoral ganglion of A. pseudoargus extends over the entire length of the rhinophore, whereas the peripheral ganglion of A. punctata is restricted to the base of the rhinophoral groove. The rhinophores of A. pseudoargus and A. punctata have completely different structures and hence may have different functions or origins, as already described by Gosliner (1994). The rhinophores of A. pseudoargus consist of a shaft with lamellae, whereas those of A. punctata are rolled. Thus the rhinophores of A. pseudoargus, in contrast to those of A. punctata, are not primarily olfactory organs but rather sense other modalities, e.g. detection of water currents. The involvement of the rhinophores of A. pseudoargus in rheotaxis has been described by Wolter (1967).
In addition to the FMRFamide-like immunoreactive glomeruli-like structures and peripheral ganglia, FMRFamide-lir was detected within a small number of subepidermal bipolar somata within the investigated CSOs of A. punctata and H. hydatis but not of A. tornatilis and A. pseudoargus. Such bipolar FMRFamide-like immunoreactive somata were also described for the tentacle tip of the pulmonate Limax marginatus (Suzuki et al. 1997). The dendrites of the cells penetrated the epidermis and therefore Suzuki et al. (1997) suggested that these cells are primary sensory neurons and contribute to chemical or mechanical reception, as suggested for the TH-like immunoreactive cells. Only the somata in the rhinophore of A. pseudoargus look different from those found in the other investigated CSOs because these cells are multipolar. Multipolar cells that show FMRFamide-lir have not yet been described in the opisthobranchs but as already mentioned above, Croll (2001) described the existence of TH-like immunoreactive multipolar cells in peripheral tissues of A. californica and suggested a role in the lateral spread of sensory information within the peripheral neural plexus. Based on our data, it is not clear whether the FMRFamide-lir multipolar cells of A. pseudoargus correspond to the multipolar cells described by Croll (2001) and hence may also play a role in this modality.
Serotonin-like immunoreactivity
Unlike the distribution of tyrosine hydroxylase and FMRFamides, the distribution of serotonin has already been studied in detail in the peripheral nervous systems of various opisthobranchs (Moroz et al. 1997; Croll et al. 2003; Wertz et al. 2006, 2007; Hochberg 2007) as well as in the pulmonate Helix pomatia (Hernadi and Elekes 1999). No peripheral serotonin-like immunoreactive somata were found in any of the investigated CSOs and serotonin was found primarily in subepidermal nerve fibres that did not penetrate the epidermis; therefore, these fibres appear to be efferent. These findings are consistent with the observation of only efferent fibres in the CSOs of A. punctata (Wertz et al. 2006), A. pseudoargus (Wertz et al. 2007), Phestilla sibogae (Croll et al. 2003), Pleurobranchaea californica and Tritonia diomedea (Moroz et al. 1997) and the pulmonate H. pomatia (Hernadi and Elekes 1999). Serotonin-lir was found in the same patches of entangled fibres and peripheral ganglia as FMRFamide. These results agree with Moroz et al. (1997), who suggested that serotonin may play a role in the peripheral modulation of sensory inputs to the central nervous system. If these entangled fibres indeed correspond to glomerulus-like structures, serotonin may play a role in the efferent control of olfactory inputs.
Conclusions
Here, we demonstrate that the distribution of sensory structures shows characteristic patterns for different CSOs. The distribution of these structures within the CSOs leads us to the conclusion that the different types of CSOs have different functions. The posterior CSOs, i.e. the rhinophores of Aplysia punctata and the Hancock’s organ of Haminoea hydatis, generally contain many glomerulus-like structures and therefore probably primarily fulfil an olfactory function, which is also supported by their location. The anterior CSOs, i.e. the oral tentacles, the lip organ and the anterior cephalic shield, comprise numerous bipolar sensory neurons which are probably involved in contact chemoreception and mechanoreception. Thus, the anterior CSOs may play a role in these modalities. Another point which argues for a function in contact chemoreception and mechanoreception is that the anterior CSOs are situated close to the substrate and thus may serve to discriminate between different types of substrate.
Aside from the conclusions about the function of the different CSOs, this study additionally provides insight into their evolution. Since the distribution of cell bodies and glomeruli containing FMRFamide is very similar especially in the posterior CSOs of A. punctata (Anaspidea) and H. hydatis (Cephalaspidea) and since innervation patterns for these organs are very similar in the two species (data of second author, not shown here), we propose that these CSOs in Anaspidea and Cephalaspidea are homologous structures indicating that the two taxa may be more closely related to each other than to A. tornatilis (Acteonoidea) and A. pseudoargus (Nudibranchia), where these FMRFamide-like immunoreactive structures are missing. This assumption is in agreement with molecular systematic studies by Vonnemann et al. (2005) and Klussmann-Kolb et al. (2008) who revealed a sister-group relationship between the Anaspidea and the Cephalaspidea as well as between the Nudipleura (Nudibranchia plus Pleurobranchoidea) and the Acteonoidea, respectively.
These sister-group relationships would either imply that the rhinophores of the Anaspidea and the Nudibranchia have evolved independently from each other as already suggested by Gosliner (1994) or that the rhinophores evolved only once within the Euthyneura. The first hypothesis is supported by Edlinger (1980) and Huber (1993), who proposed that the rhinophores of the Anaspidea derive from the Hancock’s organ of the Cephalaspidea. However, this would imply that the Cephalaspidea take a more basal position within the Opisthobranchia than the Anaspidea, a hypothesis that is not supported by current phylogenetic analyses (Vonnemann et al. 2005; Klussmann-Kolb et al. 2008).
In addition to these implications it seems that the distribution of some immunoreactive structures in the posterior CSOs of the investigated opisthobranch taxa is comparable to that of pulmonate gastropods (Suzuki et al. 1997; Hernadi and Elekes 1999) which supports a close relationship of these two groups of Gastropoda. This is in agreement with former phylogenetic studies (e.g. Salvini-Plawen and Steiner 1996; Ponder and Lindberg 1997; Dayrat et al. 2001; Dayrat and Tillier 2002) and affirm the second assumption that the posterior CSOs evolved only once within all Euthyneura.
Nevertheless, further immunohistochemical investigations on more taxa of opisthobranchs as well as pulmonate gastropods are nescessary to obtain insights in the evolution of the CSOs within Euthyneura.
References
Audesirk TE (1975) Chemoreception in Aplysia californica. I. Behavioral localization of distance chemoreceptors used in food-finding. Behav Biol 15:45–55. doi:10.1016/S0091-6773(75)92066-0
Bicker G, Davis WJ, Matera EM (1982) Chemoreception and mechanoreception in the gastropod mollusc Pleurobranchaea californica. II. Neuroanatomical and intracellular analysis of afferent pathways. J Comp Physiol 149:235–250. doi:10.1007/BF00619217
Boudko DY, Switzer-Dunlap M, Hadfield MG (1999) Cellular and subcellular structure of anterior sensory pathways in Phestilla sibogae (Gastropoda, Nudibranchia). J Comp Neurol 403:39–52. doi: 10.1002/(SICI)1096-9861(19990105)403:1≤39::AID-CNE4≥3.0.CO;2-B
Cardot J, Fellman D (1983) Immunofluorescent evidence of an FMRFamide-like peptide in the peripheral nervous system of the gastropod mollusc Helix aspersa. Neurosci Lett 43:167–172. doi:10.1016/0304-3940(83)90182-9
Chase R (1979) Photic sensitivity of the rhinophore in Aplysia. Can J Zool 57:698–701
Chase R (2002) Behavior and its neural control in gastropod molluscs. Oxford University Press, New York
Chase R, Tolloczko B (1986) Synaptic glomeruli in the olfactory system of a snail, Achatina fulica. Cell Tissue Res 246:567–573. doi:10.1007/BF00215198
Chiasson BJ, Baker MW, Croll RP (1994) Morphological changes and functional recovery following axotomy of a serotonergic cerebrobuccal neurone in the land snail Achatina fulica. J Exp Biol 192:147–167
Cooke IRC, Gelperin A (1988) Distribution of FMRFamide-like immunoreactivity in the nervous system of the slug Limax maximus. Cell Tissue Res 253:69–76
Cottrell GA (1989) The biology of the FMRFamide-series of peptides in molluscs with special reference to Helix. Comp Biochem Physiol 93A(1):41–45. doi:10.1016/0300-9629(89)90189-8
Croll RP (1983) Gastropod chemoreception. Biol Rev Camb Philos Soc 58:293–319. doi:10.1111/j.1469-185X.1983.tb00391.x
Croll RP (1987) Distribution of monoamines in the central nervous system of the nudibranch gastropod, Hermissenda crassicornis. Brain Res 405:337–347. doi:10.1016/0006-8993(87)90303-9
Croll RP (2001) Catecholamine-containing cells in the central nervous system and periphery of Aplysia californica. J Comp Neurol 441:91–105. doi:10.1002/cne.1399
Croll RP, Boudko DY, Hadfield MG (2001) Histochemical survey of transmitters in the central ganglia of the gastropod mollusc Phestilla sibogae. Cell Tissue Res 305:417–432. doi:10.1007/s004410100394
Croll RP, Boudko DY, Pires A, Hadfield MG (2003) Transmitter contents of cells and fibres in the cephalic sensory organs of the gastropod mollusc Phestilla sibogae. Cell Tissue Res 314:437–448. doi:10.1007/s00441-003-0778-1
Croll RP, Staubauch S, Klussmann-Kolb A (2004) FMRFamide-like immunoreactivity in the central nervous systems and periphery of Aplysia californica. World Congress of Malacology, Perth, Australia
Davis WJ, Matera EM (1982) Chemoreception in gastropod molluscs: electron microscopy of putative receptor cells. J Neurobiol 13(1):79–84. doi:10.1002/neu.480130109
Dayrat B, Tillier S (2002) Evolutionary relationships of euthyneuran gastropods (Mollusca): a cladistic re-evaluation of morphological characters. Zool J Linn Soc 135:403–470. doi:10.1046/j.1096-3642.2002.00018.x
Dayrat B, Tillier A, Lecointre G, Tillier S (2001) New clades of euthyneuran gastropods (Mollusca) from 28S rRNA sequences. Mol Phyl Evol 19(2):225–235. doi:10.1006/mpev.2001.0926
Edlinger K (1980) Zur Phylogenie der chemischen Sinnesorgane einiger Cephalaspidea (Mollusca-Opisthobranchia). Z Zool Syst Evol 18:241–256
Elekes K (1992) Neurotransmitters in the gastropod CNS: comparative immunocytochemistry. Acta Biol Hung 43(1–4):213–220
Elekes K, Nässel DR (1990) Distribution of FMRFamide-like immunoreactive neurons in the central nervous system of the snail Helix pomatia. Cell Tissue Res 262:177–190. doi:10.1007/BF00327760
Elekes K, Kemenes G, Hiripi L, Geffard M, Benjamin PR (1991) Dopamine-immunoreactive neurones in the central nervous system of the pond snail Lymnaea stagnalis. J Comp Neurol 307:214–224. doi:10.1002/cne.903070205
Emery DG (1992) Fine structure of olfactory epithelia of gastropod molluscs. Microsc Res Tech 22:307–324. doi:10.1002/jemt.1070220402
Emery DG, Audesirk TE (1978) Sensory cells in Aplysia. J Neurobiol 9:173–179. doi:10.1002/neu.480090207
Göbbeler K, Klussmann-Kolb A (2007) A comparative ultrastructural investigation of the cephalic sensory organs in Opisthobranchia (Mollusca, Gastropoda). Tissue Cell 39(6):399–414. doi:10.1016/j.tice.2007.07.002
Gosliner TM (1994) Gastropoda: Opisthobranchia. Microscopic anatomy of invertebrates Volume 5. Mollusca I:253–355
Hernadi L, Elekes K (1999) Topographic organization of serotonergic and dopaminergic neurons in the cerebral ganglia and their peripheral projection patterns in the head areas of the snail Helix pomatia. J Comp Neurol 411:274–287. doi: 10.1002/(SICI)1096-9861(19990823)411:2≤274::AID-CNE8≥3.0.CO;2-9
Hernadi L, Elekes K, S-Rozsa K (1989) Distribution of serotonin-containing neurons in the central nervous system of the snail Helix pomatia. Cell Tissue Res 257:313–323. doi:10.1007/BF00261835
Hernadi L, Juhos S, Elekes K (1993) Distribution of tyrosine-hydroxylase-immunoreactive and dopamine-immunoreactive neurons in the central nervous system of the snail Helix pomatia. Cell Tissue Res 274:503–513. doi:10.1007/BF00314547
Hochberg R (2007) Serotonin-like immunoreactivity in the central and peripheral nervous systems of the interstitial acochlidean Asperspina sp. Biol Bull 213:43–54
Huber G (1993) On the cerebral nervous system of marine Heterobranchia (Gastropoda). J Molluscan Stud 59:381–420. doi:10.1093/mollus/59.4.381
Jacklet JW (1980) Light sensitivity of the rhinophores and eyes of Aplysia. J Comp Physiol 136(3):257–262. doi:10.1007/BF00657541
Jahan-Parwar B (1972) Behavioral and electrophysiological studies on chemoreception in Aplysia. Am Zool 12:525–537
Kemenes G, Elekes K, Hiripi L, Benjamin PR (1989) A comparison of four techniques for mapping the distribution of serotonin and serotonin-containing neurons in fixed and living ganglia of the snail, Lymnaea. J Neurocytol 18(2):193–208. doi:10.1007/BF01206662
Klussmann-Kolb A, Dinapoli A, Kuhn K, Streit B, Albrecht C (2008) From sea to land and beyond—new insights into the evolution of the euthyneuran Gastropoda (Mollusca). BMC Evol Biol 8:57. doi:10.1186/1471–2148-8-57
Longley RD, Longley AJ (1986) Serotonin immunoreactivity of neurons in the gastropod Aplysia californica. J Neurobiol 17(4):339–358. doi:10.1002/neu.480170408
Merton H (1920) Untersuchungen über die Hautsinnesorgane der Mollusken. I. Opisthobranchia. Abhandl Senckenb Naturf Ges 36(4):449–473
Moroz LL (2006) Localization of putative nitrergic neurons in peripheral chemosensory areas and the central nervous sytem of Aplysia californica. J Comp Neurol 495:10–20. doi:10.1002/cne.20842
Moroz LL, Sudlow LC, Jing J, Gillette R (1997) Serotonin-immunoreactivity in peripheral tissues of the opisthobranch molluscs Pleurobranchaea californica and Tritonia diomedea. J Comp Neurol 382:176–188. doi: 10.1002/(SICI)1096-9861(19970602)382:2≤176::AID-CNE3≥3.0.CO;2-0
Newcomb JM, Fickbohm DJ, Katz PS (2006) Comparative mapping of serotonin-immunoreactive neurons in the central nervous system of nudibranch molluscs. J Comp Neurol 499:485–505. doi:10.1002/cne.21111
Ono JK, McCaman RE (1984) Immunocytochemical localization and direct assays of serotonin-containing neurons in Aplysia. Neuroscience 11(3):549–560. doi:10.1016/0306-4522(84)90044-7
Ponder WF, Lindberg D (1997) Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zool J Linn Soc 119:83–265
Price DA, Greenberg MJ (1977) Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusc. Prep Biochem 7:261–281. doi:10.1080/00327487708061643
Price DA, Davies NW, Doble KE, Greenberg MJ (1987) The variety and distribution of the FMRFamide-related peptides in molluscs. Zoolog Sci 4:395–410
Salimova NB, Sakharov DA, Milosevic I, Rakic L (1987) Catecholamine-containing neurons in the peripheral nervous system of Aplysia. Acta Biol Hung 38(2):203–212
Salvini-Plawen L, Steiner G (1996) Synapomorphies and plesiomorphies in higher classification of Mollusca. In: Taylor J (ed) Origin and evolutionary radiation of the Mollusca. Oxford University Press, The Malacological Society of London, pp 29–51
Shirahata T, Watanabe S, Kirino Y (2004) Distribution of serotonin-like immunoreactive neurons in the slug Limax valentianus. Cell Tissue Res 315:285–290. doi:10.1007/s00441-003-0820-3
S.-Rozsa K (1984) The pharmacology of molluscan neurons. Prog Neurobiol 23:79–150. doi:10.1016/0301-0082(84)90013-3
Storch V, Welsch U (1969) Über den Bau und Funktion der Nudibranchier-Rhinophoren. Z Zellforsch 97:528–536. doi:10.1007/BF00332801
Sudlow LC, Jing J, Moroz LL, Gillette R (1998) Serotonin-immunoreactivity in the central nervous system of the marine molluscs Pleurobranchaea californica and Tritonia diomedea. J Comp Neurol 395:466–480. doi: 10.1002/(SICI)1096-9861(19980615)395:4≤466::AID-CNE4≥3.0.CO;2-#
Suzuki H, Kimura T, Sekiguchi T, Mizukami A (1997) FMRFamide-like-immunoreactive primary sensory neurons in the olfactory system of the terrestrial mollusc, Limax marginatus. Cell Tissue Res 289:339–345. doi:10.1007/s004410050881
Vonnemann V, Schrödl M, Klussmann-Kolb A, Wägele H (2005) Reconstruction of the phylogeny of the Opisthobranchia (Mollusca: Gastropoda) by means of 18S and 28S rRNA gene sequences. J Molluscan Stud 71:113–125. doi:10.1093/mollus/eyi014
Wertz A, Rössler W, Obermayer M, Bickmeyer U (2006) Functional neuroanatomy of the rhinophore of Aplysia punctata. Front Zool 3:6. doi:10.1186/1742-9994-3-6
Wertz A, Rössler W, Obermayer M, Bickmeyer U (2007) Functional neuroanatomy of the rhinophore of Archidoris pseudoargus. Helgol Mar Res 61:135–142. doi:10.1007/s10152-007-0061-z
Wollesen T, Wanninger A, Klussmann-Kolb A (2007) Neurogenesis of cephalic sensory organs of Aplysia californica. Cell Tissue Res 330:361–379. doi:10.1007/s00441-007-0460-0
Wolter H (1967) Beiträge zur Biologie, Histologie und Sinnesphysiologie (insbesondere der Chemorezeption) einiger Nudibranchier (Mollusca, Opisthobranchia) der Nordsee. Z Morphol Oekol Tiere 60:275–337. doi:10.1007/BF00424637
Acknowledgments
We are grateful to Roger P. Croll for his helpful comments on an earlier version of this report. We would also like to thank the German Science Foundation (DFG) for supporting this project (KL 1303/3-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faller, S., Staubach, S. & Klussmann-Kolb, A. Comparative immunohistochemistry of the cephalic sensory organs in Opisthobranchia (Mollusca, Gastropoda). Zoomorphology 127, 227–239 (2008). https://doi.org/10.1007/s00435-008-0066-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-008-0066-4