Abstract
The increased prevalence of cognitive impairment, specifically among the aging population, has attracted attention in recent years. Trans-cinnamaldehyde (TCA), which is isolated from cinnamon, has recently drawn attention because of its potent anti-inflammatory and antioxidant properties. This study aimed to investigate the effects of TCA on learning and memory using a mouse model of cognitive impairment induced by a combination of D-galactose (D-gal) and aluminum chloride (AlCl3). TCA (10 and 30 mg/kg/day) was administered orally for 30 days after the induction of cognitive impairment. The Morris water maze (MWM) task was performed to directly evaluate the neuroprotective effects of TCA on memory and spatial learning abilities. We found that TCA treatment attenuated cognitive impairment and reduced brain damage in the D‑gal- and AlCl3-treated mice. To further investigate the mechanisms involved in the effects of TCA, we analyzed the nuclear factor erythroid 2-related factor (Nrf2) and related signaling pathways. We found that TCA upregulated AMP-activated protein kinase (AMPK), Nrf2, heme oxygenase 1 (HO-1) and NAD(P)H dehydrogenase [quinone] 1 (NQO-1); this suggests that TCA may attenuate cognitive dysfunction by reducing oxidative stress. We concluded that TCA reduced D-gal and AlCl3-induced cognitive dysfunction through activation of the AMPK-mediated Nrf2/HO-1 signaling pathway in the brain. These results suggest that TCA may be a candidate for treating age-associated cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Memory is the basic cognitive function that is most affected by aging [1], and cognitive decline has a significant social and economic impact on older adults and their families and caregivers [2]. Decreased cognitive functions include impairments in working memory, long-term memory, information processing, attention, and cognitive flexibility [3, 4]. Additionally, these conditions are accompanied by more serious aging-associated diseases such as amyloidosis and vascular dementia [5].
Reactive oxygen species (ROS) hypothetically play a vital role in the pathogenesis in several neurodegenerative diseases, including Alzheimer’s disease (AD) [6]. The central nervous system (CNS) is particularly susceptible to oxidative stress because of its high oxygen consumption and relatively low levels of ROS-scavenging enzymes and endogenous antioxidants compared with those in other organs [7–9]. The imbalance between the generation and elimination of reactive oxygen species (ROS) can cause extensive and lasting damage in the CNS. This damage may accelerate memory impairment [10–12].
Treatment with D-galactose (D-gal) may increase neurodegeneration by increasing the activity of acetylcholinesterases (AChE), ROS levels, and cognitive deficits and by inhibiting antioxidant enzymes [13]. Aluminum causes neuronal vacuolization, cerebral cortex atrophy, and cognitive/memory dysfunction [14]. Furthermore, treatment with aluminum chloride (AlCl3) and D-gal induce neurodegenerative symptoms similar to AD in rodents; these symptoms include memory impairment, high levels of amyloidogenic proteins, cholinergic dysfunction, and the formation of senile plaques and neurofibrillary tangles [15–17].
Trans-cinnamaldehyde (TCA), a compound derived from cinnamon, is used as a natural spice for cooking and food processing. It is generally recognized as safe by the United States Food and Drug Administration (FDA). Extensive studies focusing on the broad scope of medicinal applications for TCA have been carried out [18–20]. These studies reported that a cinnamon extract, which contains TCA, causes antioxidant, antidiabetic, antineoplastic, and anti-inflammatory effects in various diseases [21–23]. TCA has also been demonstrated to exhibit powerful anti-inflammatory activities by affecting the activity of toll-like receptor 4 (TLR4), nuclear factor-κB (NFκB) and mitogen-activated protein kinase (MAPK) [24]. Considering the reported beneficial effects of TCA, this study aimed to determine whether TCA treatment can prevent memory deficiencies induced by D-gal and AlCl3.
MATERIALS AND METHODS
Animals. Sixty BALB/C eight-week-old male mice (20–22 g body weight) were purchased from Daehan Biolink Co., Ltd (Eumseong, Korea). All mice were housed in plastic containers under constant temperature (23 ± 1°C), humidity (60 ± 10°C), and a 12-h light/dark cycle and given free access to food and water. After a week of adaptation, mice were randomly divided into five experimental groups (n = 12 per group). All animal studies were performed per the “Principles of Laboratory Animal Care” (National Institutes of Health publication number 80-23, revised in 1996) and approved by the Animal Care and Use Guidelines Committee of Kyung Hee University (approval number: KHUASP(SE)-17-126-1).
Drug treatment. The TCA (C80687), D-gal (G0750) and AlCl3 (294713) were purchased from Sigma Aldrich (St. Louis, MO, USA). To induce cognitive deficiencies, mice were injected intraperitoneally with D-gal (120 mg/kg/day) and AlCl3 (20 mg/kg/day) dissolved in saline (0.9% NaCl) for 60 days, referring to the previous studies [25–27]. Control mice were injected with the same volume of saline during this period. TCA dissolved in saline was injected intraperitoneally for 1 month (Fig. 1). Mice were divided into four groups according to treatment: control group: saline + saline (WT-CON), mild cognitive impairment group: D-gal + AlCl3 + saline (MCI-CON), TCA at 10 mg/kg/day group: D-gal + AlCl3 + TCA at 10 mg/kg/day (TCA 10), and TCA at 30 mg/kg/day group: D-gal + AlCl3 + TCA at 30 mg/kg/day (TCA 30).
Graphical abstract and Schedule of the experiment. Schedule of the experiment. Eight-week-old BALB/C mice were injected intraperitoneally with D-gal (120 mg/kg/day) and AlCl3 (20 mg/kg/day) for 60 days. TCA (30 mg/kg/day) was injected intraperitoneally for 30 days. After behavioral analyses, mice were euthanized, and brain samples were collected for further analysis.
Morris water maze. We performed the Morris water maze (MWM) task to evaluate the spatial memory performance. The water maze was a white tank (1.0 m diameter, 30 cm height) filled with 20 cm of water (22–24°C). We added white opaque nontoxic paint to the water to hinder visibility. Also, a submerged Plexiglas platform (10 cm diameter; 6–8 mm below the surface of the water) was located at a fixed position throughout the training session. The position of the platform varied from mouse to mouse while being counterbalanced across experiment groups. In this study, all mice were habituated to the maze 1 day before training, and all animals were subjected to three trials per day. A training session consisted of a series of three trials per day over 8 consecutive days (24 trials in total). During each of the three trials, animals were randomly placed at different starting positions equally spaced around the perimeter of the pool. Mice were given 60 s to find the submerged platform. If the mouse did not mount the platform within 60 s, it was guided to the platform. The time to mount the platform was recorded as the latency for each trial. All mice were allowed to remain on the platform for 10 s before being returned to their home cage. A single-probe trial, in which the platform was removed, was performed after the hidden platform task had been completed (day 9). Each mouse was placed into one quadrant of the pool and allowed to swim for 60 s. All trials were recorded using a charge-coupled device camera connected to a video monitor and a computer.
RNA isolation and quantitative real-time PCR. The mice were anesthetized with intraperitoneal injection of 2.5% Avertin (2,2,2-tribromoethanol) and immediately perfused through the heart with PBS. Mouse hippocampi were isolated after behavioral testing and euthanization. The transcription of cytokines was analyzed by quantitative real-time PCR (qRT-PCR). Using the Hybrid-R (GeneAll, Seoul, Republic of Korea), we extracted the total RNA from mouse hippocampi and measured the concentration using a NanoDrop ND-1000 spectrophotometer (Thermo-Fisher, Waltham, MA, USA). Next, RNA samples (3 μg) were converted to cDNA using TOPscript RT DryMIX (Enzynomics, Daejeon, Republic of Korea). The cDNA was amplified through qRT-PCR using TOPreal qPCR 2× PreMIX (SYBR Green; Enzynomics) and the CFX Connect Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). Table 1 describes primers, which were synthesized at COSMO Genetech (Seoul, Republic of Korea).
Western blot. The mice were anesthetized with intraperitoneal injection of 2.5% Avertin (2,2,2-tribromoethanol) and immediately perfused through the heart with PBS. Mouse hippocampi were isolated after behavioral testing and euthanization. The hippocampi were weighed and lysed in a volume 10× that of RIPA lysis buffer plus protease and phosphatase inhibitors. Equal amounts of protein samples (20 μg) in the sample buffer were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 120 min at 110 V. And it transferred electrophoretically to immunoblotting PVDF membranes (0.45 μm) for 80 min at 90 V by PowerPac™ Basic Power Supply (Bio-Rad Laboratories). The membranes were then pretreated with a blocking solution (5% skim milk, 0.1% Tween 20 in TBS) for 1 h at room temperature and reacted with primary antibodies in the blocking solution overnight at 4°C. The following primary antibodies were used (Table 2): Nrf2 (rabbit, 1 : 500, Cell Signaling Technology, #12721), phosphor-AMPKalpha (rabbit, 1 : 500, Cell Signaling Technology, #2535), AMPKalpha (rabbit, 1 : 500, Cell Signaling Technology, #2532), HO-1 (rabbit, 1 : 1000, Cell Signaling Technologies, #5853) and NQO1 (rabbit, 1 : 1000, Cell Signaling Technologies, #62262). Next, membranes were washed with the washing solution (0.1% Tween 20 in TBS) for 5 × 10 min and reacted with HRP-conjugated secondary antibodies against mouse or rabbit IgG in the blocking solution for 1 h at room temperature. Membranes were rewashed with the washing solution for 5 × 10 min. Protein signals were detected by an enhanced chemiluminescence reagent (Bio-Rad Laboratories) and visualized by ChemiDoc (Bio-Rad Laboratories). After that, we detected β-actin on the same blot as an internal control for the normalization of protein loading. The intensity of bands was quantified using ImageJ software (Bethesda, MD, USA).
Statistical analysis. All data were analyzed by using SPSS version 25 (IBM corporation, NY, USA) with statistical significance defined as a P value less than 0.05. The results are presented as the mean ± Standard Deviation(SD). Parametric tests such as ANOVA were used when the data satisfied the null hypothesis of the Levene’s test. Tukey’s post hoc test was performed if the P value was < 0.05 in one-way ANOVA. In case of Levene’s test results do not satisfy null hyperphothesis, the Kruskal–Wallis test followed by Dunn’s post hoc multiple comparisons was used to compare among the experimental groups. All graphs were constructed using GraphPad Prism 5.0 software (GraphPad software Inc., CA, USA).
RESULTS
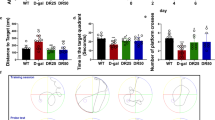
TCA improves cognitive function in mice with a D-gal- and AlCl3-induced cognitive deficiencies in the Morris water maze task. To examine whether TCA improves cognitive performance in the D-gal- and AlCl3-induced model of cognitive deficiencies, we assessed memory formation using the MWM task, which is widely used to measure hippocampus-dependent learning and memory of mice [28]. We assessed spatial memory by determining the escape time in a hidden platform using three trials per day. In this study, the MCI-CON group exhibited markedly impaired learning and memory compared with the WT-CON group (Fig. 2a). Although there was no significant difference in the escape latency times between the MCI-CON and TCA 10 groups, the TCA 30 group (p < 0.01) exhibited a significant decrease in escape latency time and a decreased tendency similar to the WT-CON (p < 0.01) group (Fig. 2a). Additionally, representative navigation paths on day 8 of training provided evidence that spatial learning acquisition was impaired in the MCI-CON group compared with the TCA 30 group (p < 0.05), which exhibited a navigation pattern similar to that of WT-CON mice (Fig. 2b). The platform was removed after the last training session, and the mice were given 60 s to find the missing platform during the probe trial. As illustrated in Fig. 2, the time spent in the target quadrant for the MCI-CON group was significantly less than that for the TCA 30 group (p < 0.01).
TCA improves cognitive function in D-gal- and AlCl3-induced cognitive deficiencies in mice. (a) 60 days of D-gal and AlCl3 induced cognitive impairment, and 30 days of TCA treatment showed no significant difference in weight between groups. (b) Spatial learning and memory were analyzed by estimating the time to reach a hidden platform for 8 consecutive days. To analyze cognitive function, we measured the time required to reach the platform. (c) After 7 days of training, a probe task was performed. The amount of time that the mouse spent in the target quadrant was measured during a 60 s probe test. Time spent in the target quadrant. All results are expressed as the mean ± Standard Deviation (SD) (n = 12–16 per group). **p < 0.01 compared with the WT CON group; ##p < 0.01 compared with the MCI CON group.
Effects of TCA on the Nrf2 signaling pathway in the brain of D-gal- and AlCl3-treated mice. Previous studies indicated that the antioxidant effects are a representative benefit of TCA [29, 30]. Nrf2 regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation. In addition, some reports have demonstrated that cognitive function is associated with the Nrf2 signaling pathway [31, 32]. Therefore, we assessed whether TCA could regulate Nrf2 levels in D-gal- and AlCl3-treated mice. As illustrated in Fig 3a, the MCI-CON group exhibited decreased Nrf2 levels compared to those in the WT-CON group. However, Nrf2 levels were markedly increased in the TCA 30 group, thus indicating that TCA might restore cognition through the Nrf2 signaling pathway. To clarify the antioxidant effects of TCA induced by Nrf2 signaling, the hippocampal protein levels of HO-1 and NQO1 were evaluated using immunoblots. HO-1 levels were slightly decreased in the MCI-CON group, compared to those in the WT‑CON group. TCA treatment represented an increased tendency of HO-1 compared to the MCI-CON group, but there were no significant differences between the groups (Fig. 3b). In the case of NQO1, the MCI-CON group exhibited significantly lower expression than that in the WT-CONgroup (p < 0.05). However, NQO1 levels were markedly increased in a concentration-dependent manner in the TCA 10 group and TCA 30 treatment group (P < 0.01) (Fig. 3c).
Effects of TCA on the Nrf2 signaling pathway in the brains of D-gal- and AlCl3-treated mice. (a–c) Representative immunoblot images and quantifications for proteins relating to the Nrf2 signaling pathway. Immunoblot was carried out using antibodies against Nrf2 (a), HO-1 (b) and NQO1 (c) using total protein lysates from the brain. All results are expressed as the mean mean ± Standard Deviation(SD) (n = 3–10 per group). * p < 0.05 compared with the WT CON group; ### p < 0.001, ## p < 0.01 compared with the MCI CON group.
Effects of TCA on the LKB1/AMPK expression of D-gal- and AlCl3-treated mice. AMP-activated protein kinase (AMPK), a major regulator of cellular energy homeostasis and metabolism, also regulates Nrf2 expression [33]. We used westernblot analyses to measure changes in cerebral AMPK activity between groups and found that AMPK activation was increased by TCA treatment (p < 0.05, Fig. 4a). The serine-threonine liver kinase B1 (LKB1), also known as serine/threonine kinase 11, is one of the main kinases that mediate AMPK phosphorylation [34]. LKB1 expression was significantly increased in the TCA 30 group compared to that in the MCI-CON group (p < 0.01, Fig. 4b). Collectively, our results indicate that TCA-mediated cognitive improvement is associated with regulation of the LKB1/AMPK/Nrf2 signaling pathway.
Effects of TCA on the LKB1/AMPK expression of D-gal- and AlCl3-treated mice. (a) Representative immunoblot images and quantifications for AMPK proteins. (b) mRNA expression levels of LKB1. All results are expressed as the mean ± Standard Deviation (SD) (n = 4–7 per group). *** p < 0.001 compared with the WT CON group; # p < 0.05, ## p < 0.01 compared with the MCI CON group.
DISCUSSION
The present study demonstrates that TCA-dependent activation of the Nrf2 pathway may ameliorate behavioral dysfunction and neurological deficiencies in a D-gal- and AlCl3-induced mouse model of cognitive impairment. We found that TCA protected against cognitive dysfunction and activate the AMPK/Nrf2 pathway to exert antioxidant effects. Therefore, TCA might have potential as an effective natural product for counteracting aging and aging-associated diseases including cognitive impairment. Notably, this is the first time the effect of TCA is demonstrated on the learning and memory impairment in the D-gal and AlCl3-induced mouse model.
Cinnamon has been widely used in food as a flavoring agent and as a component of traditional medicine in Asia [35]. TCA, which is a major component of cinnamon exhibits a wide range of biological activities, including antitumoral, antibacterial, antidiabetic, anti-inflammatory, and antioxidant activities [21–23]. It also exerts neuroprotective effects on neurodegenerative models including AD and Parkinson’s disease [36, 37]. Because of these positive effects, we hypothesized that TCA might protect against D-gal- and AlCl3-induced cognitive impairment in mice.
The vsat majority of AD cases are sporadic, but the causes underying these cases remain unknown. Therefore, many transgenic mice were developed to mimic AD pathophysiology [38]. Also, non-transgenic AD mouse models induced by diverse methods including Aβ, streptozotocin, LPS, D-gal, AlCl3, high-fat diet and lesion were used for AD research [39]. Although no single mouse model recapitulates all of the aspects of the disease spectrum, each model allows for some analysis of one or two components of the disease, which is not readily possible with human patients or samples. Therefore, the choice of animal model depends on the experimental hypothesis. In this study, we used D-gal- and AlCl3-induced model. This model is commonly used because of its ability to increase free radical production, decrease antioxidant enzyme activity, attenuate the immune response, and represent age-related cognitive impairment [40]. However, the protective effects of TCA on D-gal and AlCl3-induced cognitive impairment have not been reported before. Here, we found that TCA can prevent D-gal- and AlCl3-induced cognitive impairment, although further studies are needed to exactly explain this phenomenon.
Oxidative stress plays an important role in the pathogenesis of aging-related diseases. It has been reported that abnormal oxidative stress is an early contributor to the pathogenesis and development of neurodegenerative diseases [41]. Nrf2 and its targeted antioxidants provide a defense system against oxidative stress [42]. Activated Nrf2 translocates into the nucleus and binds to the antioxidant response elements (ARE) to activate the expression of target genes, including HO-1 and NQO1. Previous reports demonstrated that TCA decreases neuroinflammation through activation of the Nrf2 signaling pathway [30]. To determine how TCA improves cognitive function in the D-gal and AlCl3 mouse model, we analyzed the Nrf2 signaling pathway. Similarly to previous studies, we found that TCA significantly increases the level of Nrf2 and NQO1 but only slightly increases HO-1 protein expression. Several signaling cascades, such as mitogen-activated protein kinases (MAPKs) and AMPK, regulate nuclear activation of Nrf2. Activated AMPK increases catabolic and decreases anabolic activities, which increases ATP generation and reduces ATP consumption [43]. Besides its role in energy homeostasis, activated AMPK has also been linked with reduced neuroinflammation and decreased redox stress. AMPK-mediated enhancement of Nrf2 signaling has been reported by some investigators [44, 45]. Similarly to previous results, we found that TCA increases AMPK activation and possibly activates the Nrf2 signaling pathway. Calcium/calmodulin-dependent protein kinase kinase 2 is a well-known enzyme to regulate AMPK phosphorylation. In our experimental conditions, however, we did not find the differences of CAMKK2 levels among experimental groups (data not shown). However, further studies are needed to exactly explain this result. One recent study demonstrated that the LKB1/AMPK and Nrf2/HO-1 axes provide an important role in prolonging the lifespan [46]. We also found that TCA increases LKB1 expression. Although subsequent studies are required regarding its exact molecular mechanisms, our results suggest that TCA may prevent age-related cognitive dysfunction through the LKB1/AMPK/Nrf2 signaling pathway (Fig. 5). Future studies will be aimed at exploring the effects of TCA and relating mechanisms on animal models relating to neurodegeneration.
REFERENCES
Albert, M. and Funkenstein, H., Diseases of the Nervous System: Clinical Neurobiology, 1992, vol. 1992, pp. 598–611.
Sharma, S., Rakoczy, S., and Brown-Borg, H., Life Sciences, 2010, vol. 87, nos. 17–18, pp. 521–536.
Moller, J., Cluitmans, P., Rasmussen, L., Houx, P., Rasmussen, H., Canet, J., Rabbitt, P., Jolles, J., Larsen, K., and Hanning, C., The Lancet, 1998, vol. 351, no. 9106, pp. 857–861.
Hovens, I.B., Schoemaker, R.G., van der Zee, E.A., Heineman, E., Izaks, G.J., and van Leeuwen, BL., Brain, Behavior, and Immunity, 2012, vol. 26, no. 7, pp. 1169–1179.
Gorelick, P.B., Scuteri, A., Black, S.E., DeCarl, I.C., Greenberg, S.M., Iadecola, C., Launer, L.J., Laurent, S., Lopez, O.L., and Nyenhuis, D., Stroke, 2011, vol. 42, no. 9, pp. 2672–2713.
Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T., Mazur, M., and Telser, J., Int. J. Biochem. Cell. Biol., 2007, vol. 39, no. 1, pp. 44–84.
Olanow, C.W., Ann. Neurol. 1992, vol. 32, Suppl. S2-9.
Jeong, K., Shin, Y.C., Park, S., Park, J.S., Kim, N., Um, J.Y., Go, H., Sun, S., Lee, S., and Park, W., et al., J. Biomed. Sci., 2011, vol. 18, p. 14.
Zhu, J., Mu, X., Zeng, J., Xu, C., Liu, J., Zhang, M., Li, C., Chen, J., Li, T., and Wang, Y., PLoS One, 2014, vol. 9, no. 6, p. e101291.
Rosales-Corral, S., Tan, D.-X., Manchester, L., and Reiter, R.J., Oxidative Medicine and Cellular Longevity, 2015, vol. 2015.
Barone. E., Current Alzheimer Research, 2016, vol. 13, no. 2, pp. 108–111.
Luca, M., Luca, A., and Calandra, C., Oxidative Medicine and Cellular Longevity, 2015, vol. 2015, pp. Article ID 504678.
Gao, J., Zhou, R., You, X., Luo, F., He, H., Chang, X., Zhu, L., Ding, X., and Yan, T., Metabolic Brain Disease, 2016, vol. 31, no. 4, pp. 771–778.
Wang, Z., Wei, X., Yang, J., Suo, J., Chen, J., Liu, X., and Zhao, X., Neuroscience Letters, 2016, vol. 610, pp. 200–206.
Xiao, F., Li, X.-G., Zhang, X.-Y., Hou, J.-D., Lin, L.-F., Gao, Q., and Luo, H.-M., Neuroscience Bulletin, 2011, vol. 27, no. 3, pp. 143–155.
Li, Z., Zhao, G., Qian, S., Yang, Z., Chen, X., Chen, J., Cai, C., Liang, X., and Guo, J., Journal of Ethnopharmacology, 2012, vol. 144, no. 2, pp. 305–312.
Peng, X.M., Gao, L., Huo, S.X., Liu, X.M., and Yan, M., Phytotherapy Research, 2015, vol. 29, no. 8, pp. 1137–1144.
Hong, S.H., Ismail, I.A., Kang, S.M., Han, D.C., and Kwon, B.M., Phytotherapy Research, 2016, vol. 30, no. 5, pp. 754–767.
Zhao, H., Xie, Y., Yang, Q., Cao, Y., Tu, H., Cao, W., and Wang, S., Journal of Pharmaceutical and Biomedical Analysis, 2014, vol. 89, pp. 150–157.
Chen, B.-J., Fu, C.-S., Li, G.-H., Wang, X.-N., Lou, H.-X., Ren, D.-M., and Shen, T., Mini Reviews in Medicinal Chemistry, 2017, vol. 17, no. 1, pp. 33–43.
Kwon, H.-K., Hwang, J.-S., So, J.-S., Lee, C.-G., Sahoo, A., Ryu, J.-H., Jeon, W.K., Ko, B.S., Im, C.-R., and Lee, S.H., BMC Cancer, 2010, vol. 10, no. 1, p. 392.
Kim, S.H., Hyun, S.H., and Choung, S.Y., Journal of Ethnopharmacology, 2006, vol. 104, nos. 1–2, pp. 119–123.
Roussel, A.-M., Hininger, I., Benaraba, R., Ziegenfuss, T.N., and Anderson, R.A., Journal of the American College of Nutrition, 2009, vol. 28, no. 1, pp. 16–21.
Kim, D.H., Kim, C.H., Kim, M.-S., Kim, J.Y., Jung, K.J., Chung, J.H., An, W.G., Lee, J.W., Yu, B.P., and Chung, H.Y., Biogerontology, 2007, vol. 8, no. 5, pp. 545–554.
Li, Z., Chen, X., Lu, W., Zhang, S., Guan, X., Li, Z., and Wang, D., Int. J. Mol. Sci., 2017, vol. 18, no. 8.
Zhang, L., Zhang, Z., Fu, Y., Yang, P., Qin, Z., Chen, Y., and Xu, Y., Neuropharmacology, 2016, vol. 110, pp. 503–518.
Gao, L., Peng, X.M., Huo, S.X., Liu, X.M., and Yan, M., Phytotherapy Research, 2015, vol. 29, no. 8, pp. 1131–1136.
Vorhees, C.V., and Williams, M.T., Nat. Protoc., 2006, vol. 1, no. 2, pp. 848–858.
Prabuseenivasan, S., Jayakuma, R.M., and Ignacimuthu, S., BMC Complement Altern. Med., 2006, vol. 6, p. 39.
Abou El-Ezz, D., Maher, A., Sallam, N., El-Brairy, A., and Kenawy, S., Neurochem. Res., 2018, vol. 43, no. 12, pp. 2333–2342.
Gureev, A.P., Popov, V.N., Starkov, A.A., Exp. Neurol., 2020, vol. 328, p. 113285.
Zhao, M., Tang, X., Gong, D., Xia, P., Wang, F., and Xu, S., Front. Pharmacol., 2020, vol. 11, p. 71.
Garcia, D. and Shaw, R.Jz., Mol. Cell., 2017, vol. 66, no. 6, pp. 789–800.
Majd, S., Power, J.H.T., Chataway, T.K., and Grantham, H.J.M., BMC Cell Biol., 2018, vol. 19, no. 1, p. 7.
Hajimonfarednejad, M., Ostovar, M., Raee, M.J., Hashempur, M.H., Mayer, J.G., and Heydari, M., Clin. Nutr., 2019, vol. 38, no. 2, pp. 594–602.
Peterson, D.W., George, R.C., Scaramozzino, F., LaPointe, N.E., Anderson, R.A., Graves, D.J., and Lew, J., J. Alzheimers Dis., 2009, vol. 17, no. 3, pp. 585–597.
Frydman-Marom, A., Levin, A., Farfara, D., Benromano, T., Scherzer-Attali, R., Peled, S., Vassar, R., Segal, D., Gazit, E., and Frenkel, D., et al., PLoS One., 2011, vol. 6, no. 1, p. e16564.
LaFerla, F.M., and Green, K.N., Cold Spring Harbor Perspectives in Medicine, 2012, vol. 2, no. 11, p. a006320.
Li, X., Bao, X., and Wang, R., International Journal of Molecular Medicine, 2016, vol. 37, no. 2, pp. 271–283.
Sadigh-Eteghad, S., Majdi, A., McCann, S.K., Mahmoudi, J., Vafaee, M.S., and Macleod, M.R., PLoS One, 2017, vol. 12, no. 8, p. e0184122.
Tonnies, E. and Trushina, E., J. Alzheimers Dis. 2017, vol. 57, no. 4, pp. 1105–1121.
Ni, Y.-H., Huo, L.-J., Li, T.-T., World Journal of Gastroenterology, 2017, vol. 23, no. 11, p. 2002.
Yamauchi, T., Kamon, J., Minokoshi, Ya., Ito, Y., Waki, H., Uchida, S., Yamashita, S., Noda, M., Kita, S., and Ueki, K., Nature Medicine, 2002, vol. 8, no. 11, pp. 1288–1295.
Sozen, E., Karademir, B., Ozer, N.K., Free Radic. Biol. Med., 2015, vol. 78, pp. 30–41.
Mo, C., Wang, L., Zhang, J., Numazawa, S., Tang, H., Tang, X., Han, X., Li, J., Yang, M., and Wang, Z., Antioxidants & Redox Signaling, 2014, vol. 20, no. 4, pp. 574–588.
Zimmermann, K., Baldinger, J., Mayerhofer, B., Atanasov, A.G., Dirsch, V.M., and Heiss, E.H., Free Radic. Biol. Med., 2015, vol. 88(Pt B), pp. 417–426.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07050547).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflict of interest.
Ethical approval. All animal studies were performed in accordance with the “Principles of Laboratory Animal Care” (National Institutes of Health publication number 80-23, revised in 1996) and approved by the Animal Care and Use Guidelines Committee of Kyung Hee University (approval no. KHUASP(SE)-19-041).
Rights and permissions
About this article
Cite this article
Jong-Sik Ryu, Do, J., Kang, HY. et al. The Protective Effects of Trans-Cinnamaldehyde against D-Galactose and Aluminum Chloride-Induced Cognitive Dysfunction in Mice. Neurochem. J. 15, 50–58 (2021). https://doi.org/10.1134/S1819712421010104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712421010104