Abstract
Loranthus micranthus (LM) is used in the treatment of diabetes and mental disorders in Nigerian folklore medicine. We studied the short-term memory behavior as well as the activity of acetylcholinesterase, antioxidant status and glycolytic flux in cerebrum and cerebellum of diabetic rats treated with LM extract. Histopathological evaluation was used to assess the level of damage on brain tissue due to diabetes complications. Treatment with LM improved short-term memory behavior, significantly reduced the levels of lipid peroxidation (LPO), increased glutathione (GSH) levels and enhanced the activities of antioxidant enzymes. Activities of glucose metabolism enzymes were augmented while acetylcholinesterase (AChE) activity was reduced by LM treatment compared with the untreated diabetic group. Histopathology evaluation revealed the possible influence of LM in reversing diabetes-induced damage in brain tissue. Further studies are required to accentuate the potentials of LM extract in assuaging diabetes-induced neurological deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Earlier studies from human subjects and animal models revealed that free radical-induced oxidative stress plays a prominent role in cognitive and neurobehavioral dysfunction in diabetes condition [1]. Mechanisms that could implicate oxidative stress in diabetes are glucose autoxidation, protein glycation, among others in the affected organs [2], including the brain. Whenever the system encounters a process that favors production of reactive oxygen species as in the case of diabetes condition, it is often associated with attendant compromise in natural antioxidant defense systems in affected tissues. Subsequent to induction of oxidative stress, antioxidant enzymes are known to be enhanced to protect cellular functions which maintain homeostasis in the system [3]. The extreme vulnerability of the brain function to oxidative stress-mediated neuronal damage is well known and this has been substantially implied to play a vital role in neurodegeneration and neurobehavioral decline in diabetes, ageing and Alzheimer’s disease [4].
Pharmacologically, bioactive ingredients like flavonoids, polyphenols, glycosides of triterpenes, steroids and alkaloids have been identified in LM plant which could be the reason behind its promising hypoglycemic activity, as previously demonstrated in diabetic animal models [5]. This has been inferred to be due to their antioxidant, anti-inflammatory, immuno-modulatory activities and propensity to regulate hormonal levels [6]. There is an emerging increase in the substitution of ethnomedicinal plants over synthetic drugs in recent times due to their numerous healing functions and fewer side effects in the management of diabetes complications [7]. As the support for the use of plant extracts in treatment and management of metabolic disorders gain wide acceptance, it has become a need of the moment to decipher the anti-diabetic activities of LM and its attendant neurological function augmentation. LM has been reported to decrease blood glucose levels and control the loss of body weight in diabetes mellitus [5, 8, 9].
In view of the previous studies on anti-diabetic activities of LM, the present study aims at the biochemical investigation of the influence of LM on neurological deficits in animal models of STZ-induced diabetes; to the best of our knowledge, this study is the first to evaluate the possible attenuating role of LM on neurological deficits in diabetes condition.
MATERIALS AND METHODS
Drug and Chemicals
Streptozotocin (STZ), and all biochemical consumables and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA) and the British Drug Houses (Poole, Dorset, UK).
Collection and Extraction of LM Leaves
LM leaves were harvested from a forest around AE-FUNA, Nigeria. Authentication of LM leaves was done at the Department of Botany, University of Ibadan, where a voucher specimen already exist in the herbarium. Afterwards, fresh LM leaves were air dried and blended into powder using an electric grinder. 50 g of the powder was suspended in 100 mL water and 200 mL methanol (1 : 2) and kept in an incubator at 25°C for 48 h. The extraction was done by maceration. The mixture was then filtered and the filtrate was dried by low pressure. 9 g of extract was collected. The extract was suspended in water in a fixed dose and used for treatment. A stock solution of LM leaves (100 mg/mL) was prepared fresh every other day with distilled water for the period of this study.
Animals Ethics statement and Experimental Design
Adult male Wistar rats (140 ± 6 g) obtained from the Animal house, Alex-Ekwueme Federal University Ndufu-Alike, Nigeria, were acclimatized for 7 days prior to this study. Animals were kept in plastic cages placed in a well-ventilated animal house, fed with rat chow and water ad libitum and subjected to natural photoperiod of 12 h light/dark. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science (NAS) and published by the National Institute of Health. The experiment was performed in accordance with the guidelines and approval of institutional animal ethics committee AE-FUNA. Diabetes was induced with single intraperitoneal administration of STZ (60 mg/kg), freshly prepared in citrate buffer (0.1 M, pH 4.5) to the rats after an overnight fast. After 72 h of STZ injection, blood samples were collected through the tail vein and blood glucose levels were determined with an Accu-Chek glucometer (Roche Diagnostics GmbH, Mannheim, Germany). The rats with fasting blood glucose levels above 230 mg/dL were considered as diabetic and selected for the study. Five groups of 6 rats each were maintained throughout the investigation period. Group I: control rats received distilled water orally at 2 mL/kg for 14 days. Group II: diabetic untreated rats. Group III: diabetic rats treated with glibenclamide (GB) orally at 5 mg/kg for 14 days. Group IV: diabetic rats treated with LM leave orally at 100 mg/kg for 14 days. Group V: diabetic rats treated with LM leave orally at 200 mg/kg for 14 days. Levels of fasting blood glucose and body weight were monitored throughout the treatment period. The dose of LM leave was selected from earlier data published elsewhere [10]. Twenty-four hours following the last treatment, the overnight-fasted rats were sacrificed by cervical dislocation, and brain samples were collected, weighed and cerebrum was separated from the cerebellum. Subsequently, part of brain samples was stored frozen at –20°C until the determination of biochemical estimations, while part of the samples was fixed in 10% neutral buffered formalin solution for subsequent histopathological analysis.
Evaluation of Neurobehavioral Function
Tests were conducted after last administration using the Y-maze, open-field test (OFT) and tail suspension test (TST). All behavioral tests were carried out in a quiet dimly lit room between the hours of 9 am and 3 pm. All behavioral tests were videotaped and later scored by two independent observers who were unaware of the experimental protocol. All apparatus used for the tests were cleaned with 10% ethanol to remove possible bias due to smell left by the previous animal.
Y-Maze
The Y-maze was performed as previously described [11] using protocols adapted from Mori et al. [12]. This test evaluates short-term spatial memory using spontaneous alternating behaviors. Here, a three-armed Y-shaped maze is used. Rats are placed on a predetermined start arm and allowed to roam freely for 8 min. Arm entry (hind limbs completely in the arm) is scored. Entering all 3 arms in the overlapping triplet sets is defined as spontaneous alternation. The percentage of spontaneous alternation is calculated as: (spontaneous alternation/(total number of arm entries – 2)) × 100.
OFT
This assesses locomotor and exploratory activities in rats using protocols previously described [13]. Here, an apparatus consisting of a box (72 × 72 × 36 cm) with the floor divided into 18 × 18 square units was used. Rats are placed in the centre of the box and allowed to roam freely for 5 min. Locomotion frequency (number of crossings from one square to the other), rearing frequency (number of times the animals stood on their hind paws), rearing against the wall (no of times the animals stood on their hind paws against the wall), hinding (calculated by adding the rearing frequency to rearing against the wall), are amongst the parameters scored.
TST
This test assesses depressive or despair-like behavior in rats. The idea is based on the fact that rats will develop an immobile posture when exposed to unavoidable stress of being suspended by their tail [14]. Here, rats were suspended individually by their tail from a retort stand with an adhesive tape for 6 min, and the amount of time the rats spend immobile is recorded.
Brain Antioxidant Function and Acetylcholinesterase Activity
Homogenization of brain samples (cerebrum and cerebellum) of rats was carried out in Tris buffer (50 mM, pH 7.4) containing potassium chloride (1.15%), the homogenate was then centrifuged at 10 000 g for 15 min at 4°C. The supernatant was thereafter separated for protein concentration evaluation following the method of Lowry et al. [15]. The evaluation of various antioxidant enzymes and oxidative stress parameters were analyzed according to existing protocols; superoxide dismutase (SOD) activity, as described by Misra and Fridovich [16]. Catalase (CAT) activity was done by the method of Clairborne [17]. The level of glutathione (GSH) was evaluated following the method described by Jollow et al. [18]. GSH-Px activity was assessed by the method of Rotruck et al. [19]. Level of Lipid peroxidation (LPO) was carried out following the method described by Farombi et al. [20] and acetylcholinesterase activity was estimated by the method of Ellman et al. [21].
Brain Glucose Metabolizing Enzymes Activity
Hexokinase activity (cytoplasmic fraction) of brain samples (cerebrum and cerebellum) was determined following the method described by Branstrup et al. [22]. Glucose-6-phosphate dehydrogenase activity was estimated by the method of Dawson et al. [23].
Brain Histopathological Analyses
Brain tissues fixed in 10% neutral buffered formalin were processed for routine histological preparations. Sections were obtained from paraffin blocks and stained with Haematoxylin and Eosin (H&E). Sections were observed under a digital research bright-field microscope (OMAX 3MP Digital Microscope, US), and digital photomicrographs were taken by a pathologist who was blinded on the groups of animals.
Statistical Analysis
Data were expressed as mean ± SEM. Data comparisons were performed using One-way ANOVA, followed by Turkey’s test for multiple comparisons. GraphPad Prism (Version 5.03, GraphPad Software, USA) was used for charts and analysis. Statistical significance was set at p < 0.05.
RESULTS
Time Course Effects of LM on Blood Glucose Level during the Study Period
Blood glucose level of the control and experimental rats at day 1th, 7th, and 14th day of the study are presented in Table 1. The STZ-treated diabetic rats showed hyperglycemia as confirmed by a significant (p < 0.001) increase in the blood glucose level when compared with the control rats. The increase in the blood glucose level of STZ-induced diabetic rats was 200 to 260% above the control rats from day 1st to 14th day. However, oral administration of GB reduced the blood sugar levels from 180 to 94% compared to the control group whereas LM extract at 100 and 200 mg/kg resulted in a blood glucose lowering activity from 199 to 100% and 180 to 54% compared with control group on the 7th and 14th days respectively, which were significant when compared with the diabetic untreated rats (Day 1 [F4,25 = 2328; p < 0.0001], Day 7 [F4,25 = 1967; p < 0.0001], Day 14 [F4,25 = 1093; p < 0.0001]).
Effects of LM on Neurobehavioral Studies of Diabetic Rats
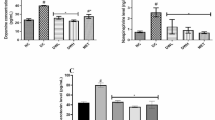
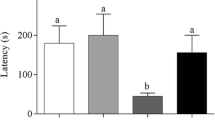
As shown in Fig. 1, Y-maze tests revealed there was a significant decrease (p < 0.05) of percentage spontaneous alternation in STZ-induced diabetes untreated group compared to control. Additionally, percentage spontaneous alternation of STZ diabetes untreated group was significantly reduced (p < 0.05) compared to groups treated with glibenclamide and 200 mg/kg extract after induction of diabetes [F4,20 = 4.95; p = 0.0061]. No significant difference is observed between STZ diabetes untreated groups and 100 mg/kg extract treated diabetic group. Activities on the OFT showed no significant difference in neither locomotion frequency [F4,20 = 2.56; p = 0.0699] nor hinding [F4,20 = 0.94; p = 0.4618] when treated groups were compared to control. Additionally, no significant difference was observed in any of the two parameters when the diabetic only group is compared to other treated groups (Fig. 1). Results of TST showed a significant increase (p < 0.05) in immobility in STZ induced diabetes only group compared to control. Additionally, immobility time of STZ diabetes only group was significantly increased (p < 0.05) compared to groups treated with glibenclamide and 200 mg/kg extract following diabetic induction [F4,20 = 6.38; p = 0.0018]. No significant difference is observed between STZ diabetes untreated groups and 100 mg/kg extract treated diabetic group (Fig. 1).
Influence of LM on Antioxidant System, and Altered AChE Activity in Cerebrum and Cerebellum of Diabetic Rats
Figures 2 and 3 represent the effects of LM on the antioxidant enzymes analyzed in cerebrum and cerebellum of experimental rats. When compared with the control, diabetic untreated group showed a significant decrease (p < 0.001) in the activities of SOD, CAT, GSH-Px and GST in a range of 60–75% in the cerebrum (SOD [F4,25 = 135.5; p < 0.0001], CAT [F4,25 = 17.51; p < 0.0001], GSH-Px [F4,25 = 11.44; p < 0.0001], GST [F4,25 = 33.72; p < 0.0001]) and cerebellum (SOD [F4,25 = 183.8; p < 0.0001], CAT [F4,25 = 19.09; p < 0.0001], GSH-Px [F4,25 = 16.30; p < 0.0001], GST [F4,25 = 33.20; p < 0.0001]). However, treatment with GB or 100 and 200 mg/kg LM significantly increased (p < 0.001) the activities of the antioxidant enzymes when compared with diabetic untreated group in a dose dependent manner.
Effects of Loranthus micranthus extract in STZ induced diabetes on the activities of SOD and CAT in rat brain (cerebrum and cerebellum). Bars are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. STZ (STZ-induced diabetic); GB (Glibenclamide); LM1 (100 mg/kg Loranthus micranthus); LM2 (200 mg/kg Loranthus micranthus).
Effects of Loranthus micranthus extract in STZ induced diabetes on the activities of GSH-Px and GST in rat brain (cerebrum and cerebellum). Bars are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. STZ (STZ-induced diabetic); GB (Glibenclamide); LM1 (100 mg/kg Loranthus micranthus); LM2 (200 mg/kg Loranthus micranthus).
Figure 4 shows the influence of LM on AChE activity and levels of GSH and LPO measured in cerebrum (AChE [F4,25 = 7.39; p = 0.0005], GSH [F4,25 = 453.2; p < 0.0001], LPO [F4,25 = 32.79; p < 0.0001]) and cerebellum (AChE [F4,25 = 8.22; p = 0.0002], GSH [F4,25 = 521.6; p < 0.0001], LPO [F4,25 = 81.15; p < 0.0001]) of experimental rats. In comparison with control, we observed a significant increase (p < 0.001) in the activity of AChE and levels of LPO within the range of 35–56%, and about 68% decrease in GSH levels. However, treatment with GB or LM ameliorated the noticeable alteration in the biomarkers relative to control and diabetic group.
Effects of Loranthus micranthus extract in STZ induced diabetes on the levels of LPO, GSH and AChE activity in rat brain (cerebrum and cerebellum). Bars are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. STZ (STZ-induced diabetic); GB (Glibenclamide); LM1 (100 mg/kg Loranthus micranthus); LM2 (200 mg/kg Loranthus micranthus).
Influence of LM on the Glycolytic Flux in Cerebrum and Cerebellum of Diabetic Rats
Figure 5 depicts the effects of LM on hexokinase and G-6-PD determined in cerebrum (hexokinase [F4,25 = 8.78; p = 0.0001], G-6-PD [F4,25 = 25.03; p < 0.0001]) and cerebellum (hexokinase [F4,25 = 12.73; p < 0.0001], G-6-PD [F4,25 = 33.68; p < 0.0001]) of diabetic rats. There was a noticeable decrease in the activities of glucose metabolism enzymes in cerebrum and cerebellum of diabetic untreated rats when compared with control in a range of 50–65%. However, treatment with GB and LM (100 and 200 mg/kg body weight) significantly increased (p < 0.01) the activities of the glucose metabolism enzymes in comparison with control and diabetic untreated group.
Effects of Loranthus micranthus extract in STZ induced diabetes on the activities of hexokinase and G-6-PD in rat brain (cerebrum and cerebellum). Bars are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. STZ (STZ-induced diabetic); GB (Glibenclamide); LM1 (100 mg/kg Loranthus micranthus); LM2 (200 mg/kg Loranthus micranthus).
Effect of LM on Histopathological Alterations in Cerebrum and Cerebellum of Diabetic Rats
Figures 6 and 7 depict histopathological analysis. Mostly intact and normal appearing neurons were observed in the cerebrum and cerebellum of the brain of control rats. In the cerebrum, normal neurons were clearly observed in the cerebral cortex, as granule or pyramidal neurons with intact nuclei and obvious nucleoli. Additionally, intact large pyramidal neurons with conspicuous nucleoli were observed in the hippocampal CA fields. In the cerebellum of control rats, three layers of cerebellar neurons were clearly observed – molecular, Purkinje, and granular layer. The neurons or the cerebellum were intact, particularly the Purkinje neurons with their large appearing soma and obvious nuclei and nucleoli. Untreated STZ-induced diabetic rats presented severe neurodegenerating features particularly in the cerebrum. The cerebral cortex and hippocampal CA fields showed many neurons with nuclei disintegration and loss of nucleoli, some pyknotic nuclei, neuronal cytoplasmic vacuolations and complete loss of nuclei substance. The cerebellum of diabetic rats showed some shrunken Purkinje neurons and slight nuclei disintegration. Particularly, one untreated STZ-diabetic rat presented abnormal proliferation of Purkinje neurons and surrounding glial cells suggesting cerebellar tumour following diabetic induction. Treatment with glibenclamide and LM extract showed improved histopathological appearance in the cortex and hippocampal CA fields of the cerebrum. Particularly, in diabetic rats treated with 200 mg/kg LM extract, mostly intact neurons were seen in the cortex and hippocampus, while some degenerating neurons were obvious in diabetic rats treated with glibenclamide and 100 mg/kg LM extract. The cerebellum appears mostly intact in diabetic rats treated with glibenclamide and extracts, both 100 and 200 mg/kg.
Histological analysis of cerebrum (cortex and hippocampal CA3 field) and cerebellum of control rats (a–c) and untreated STZ-diabetic rats (d–h). Note intact neurons in control with clear nuclei and obvious nucleoli in cortex and hippocampal CA field (arrows). Also note intact Purkinje neurons in the cerebellum (arrowhead) of control. Observe neurodegenerating features (dashed arrows) in untreated STZ-diabetic, include disintegrating nuclei with loss of nuclei, pyknotic nuclei, and complete loss of nuclei substances. An untreated diabetic animal showed abnormal cell proliferation (double arrows) in the cerebellum (g, h). H&E ×400 (a–f, h), ×100 (g).
Histological analysis of cerebrum (cortex and hippocampal CA3 field) and cerebellum of diabetic rats treated with glibencalmide (a–c), 100 mg/kg extract (d–f), and 200 mg/kg extract (g–i). Observe some neurodegenerating features (dashed arrows) in glibenclamide and 100 mg/kg extract treated groups. H&E ×400 (a–i).
DISCUSSION
There is an increasing existing awareness of the attenuating potentials of naturally occurring phytochemicals against pathological disorders that herald neurodegeneration. The results of the current study evidently revealed the effectiveness of LM in reversing neurological anomalies associated with diabetes complication. The chemo-protective property of LM was substantiated by the amelioration of diabetes-induced oxidative stress, improved glycolytic flux and augmentation of antioxidant enzymes, regulation of AChE activity and ultimately are versal of locomotor deficits, in rats. The attenuation of various biochemical parameters and neurological deficits in diabetic-treated rats by LM is similar to the performance of the treated rats with glibenclamide, a standard drug.
Tremendous progress in the understanding of neurodegenerative complications occasioned by diabetes and the involvement of oxidative stress as a major mechanism is noteworthy [4]. Interestingly, one of the major survival mechanisms of cells that have been implicated in diverse pathological disorders involving oxidative stress is the antioxidant defense system. In the front line of the antioxidant defense system are enzymes such as SOD and CAT which are actively implicated in the defense against oxidative cell injury owing to their capacity to wipe up free radicals [24]. Our findings revealed a significant decrease in SOD and CAT activities in the cerebrum and cerebellum of diabetic-untreated rats which suggests enzyme inhibition and inability to scavenge free radicals in the two brain sections. Moreover, elevated levels of LPO observed in the two regions of the brain during this study indicates a state of oxidative stress contingent on the reduction in the capacity of the antioxidant defense system and accumulation of ROS in diabetic rats. However, LM mediated reversal of the diabetes-induced oxidative stress was evidenced by increased SOD and CAT activities, which was followed by a reduction in LPO levels in the brain sections concomitantly. These findings therefore support the earlier report on antioxidant properties of LM [25]. Along the second line of the antioxidant defense system are GSH-dependent enzymes which protect against cell damage because they detoxify deleterious by-products generated by ROS and also help to assuage the dissemination of free radicals [26]. GSH-Px is involved in a redox homeostasis that detoxifies peroxides by reacting with GSH and converting it to GSSG, which is reduced to GSH by GSR [26]. GSH-Px, and GST-a phase two xenobiotic detoxifying enzyme are among the basic antioxidant enzymes. Hence, the alterations in the activities of these enzymes, and their substrate GSH observed in this study following the induction of diabetes in rats could portend tissue damage which may be linked with neurodegenerative diseases. Supplementation with LM improved the activity of antioxidant enzymes, which indicates protection against free radicals. The remarkable increase in AChE activity in diabetic-untreated rats may lead to a decrease in acetylcholine levels in the synaptic cleft, and reduction in this essential neurotransmitter and neuromodulator consequently diminish the effectiveness of cholinergic neurotransmission and weaken locomotor and exploratory activities in the rats. However, the ameliorating outcome on AChE activity in diabetic-treated rats with LM might boost neurotransmission and consequently improve neurobehavioral performance as observed in this study.
The key enzyme catalyzing glucose phosphorylation in all organs of the body and the first step in glycolysis is hexokinase [27]. Hexokinase has been shown to be rigorously impaired during diabetes [28]. Impairment of hexokinase activity suggests impaired glucose catabolism via glycolysis ultimately leading to its accumulation resulting in hyperglycemia which could be deleterious to normal functioning of the brain due to lack of ATP. Hexokinase activity was found to decrease in diabetic rats in cerebrum and cerebellum. Treatment with LM elevated the activity of hexokinase in the two brain regions. In addition, decreased G-6-PD activity in cerebrum and cerebellum may also contribute to the decreased GSH level due to decreased production of NADPH through pentose phosphate pathway, which plays essential role in the GSH cycle to reduce the disulfide (oxidized) form of glutathione to the sulfhydryl form, in accordance with earlier report [29]. This could contribute to the brain oxidative damage experienced during diabetes condition. However, the activities of G-6-PD were significantly augmented following the administration of LM.
Assessment of short-term memory using the Y‑maze is measured by spontaneous alternating activities. Spontaneous alternation is a typical pointer to short-term spatial memory in Y-maze activities. Increase in the spontaneous alternation of treated rats relative to the control is an indicator of improved short-term memory, whereas a decrease is an indicator of opposite effects [11]. The present study indicates declining short-term memory and possibly overall cognitive abilities in STZ-induced diabetes. Memory deficit in diabetes which is observed in the present study has been supported by several previous reports. Hyperglycemia in diabetes has been implicated as majorly responsible for the observed cognitive decline [30, 31]. Additional consequences of lowered insulin and alterations include inflammatory responses which affect synaptic functions and neurodegeneration. Consequently, all these effects result in a more intense decline in cognition due to severe neuronal loss, which is observed in the present study following STZ-induced diabetes. Furthermore, the increase in cerebral neurodegeneration particularly in the cortex and hippocampus of diabetics, as shown in the present study, is also linked to cognitive decline.
The major outcome in the OFT is movement, which is greatly influenced by rodent’s locomotor output and exploratory drive. Locomotion frequency and hinding are pointers to animal’s overall locomotor and exploratory activities respectively [13]. Here we observed no changes in locomotor and exploratory activities following the induction of diabetes. Chen et al. [32] also observed no apparent effect on general locomotor activities following STZ-induced diabetes, though another study has shown impaired locomotor activity in diabetic rats [33].
The current study also evaluated the state of depression in animals using the TST, which is one of the two most common behavioral paradigms for assessing depression in rodents. Increased immobility time in the TST is predictive of depression in rodents [11, 32]. The present study showed depressive-like symptoms in STZ-induced diabetic rats. This effect has been supported by previous studies [32, 33]. Alterations in synaptic plasticity in the cortex and hippocampus, the brain regions likely associated with regulating affective behaviors may be responsible for depressive disorders, and probably diabetes associated depression. Additionally, depression may result from metabolic effects of diabetes. Dysregulated serotonergic systems have been implicated in the build-up to depressive state [34]. In the current study, treatment with glibenclamide and 200 mg/kg LM extract attenuated the cognitive decline and depressive-like behavior observed in STZ-diabetic rats. Attenuation of these behavioral deficits by glibenclamide and extract may be due to their mitigating effects on hyperglycemia, altered antioxidant status, perturbed neurotransmission and subsequent preservation of neuronal integrity.
Structural anomalies in the brain have been severally associated with diabetes mellitus and altered glycemic control [35, 36]. Neuronal degeneration and injuries in STZ-induced experimental diabetes have been consistently shown in several regions of the cerebrum including the cortex, hippocampus, striatum, and hypothalamus [31, 37] as well as in the cerebellum [38]. Our present data support these earlier studies, as we have also observed neuronal damage and degeneration following STZ-induced diabetes. Importantly, we report a possible development of tumour in the cerebellum following STZ-induced diabetes. Diabetes has been linked to increasedrisk of brain tumours and cancers, particularly glioblastoma [39–41]. Though the mechanisms for diabetes associated brain tumours and cancers is poorly understood, it has been suggested that high glucose internal environment that occurs in diabetes may promote tumour growth and development [42]. In the current study, treatment with glibenclamide and extract (particularly at 200 mg/kg) attenuated structural abnormalities seen in STZ-induced diabetic rats.
Taken together, the restorative impact of LM on neurobehavioral activity in diabetes-induced neurodegeneration is showcased as evidenced by improvement in the neurobehavioral performance which might be attributed to its ability to maintain AChE and antioxidant enzymes activities with concomitant suppression of oxidative affront. Further study into testing various fractions of the extract and its total components, to be obtained through nano-milling in order to enhance its bioavailability and ensure organic-solvent free processing is an ongoing research in our laboratory.
REFERENCES
Kaplan, M., Aviram, M., and Hayek, T., Pharm. Therap., 2012, vol. 136, pp. 175–185.
Prickaerts, J., Fahrig, T., and Blokland, A., Behav. Brain. Res., 1999, vol. 102, pp. 73–88.
Price, J., Verma, S., and Li, R.K., Heart Fail. Rev., 2003, vol. 8, pp. 213–219.
Sharifzadeh, M., Ranjbar, A., Hosseini, A., and Khanavi, M., Iran. J. Pharm. Res., 2017, vol. 16, no. 1, pp. 201–209.
Ebokaiwe, A.P., Ijomone, O.M., Edeh, O.,Oteh, I., and Ebuka, D.E., J. Basic Clin. Physiol. Pharmacol., 2018. https://doi.org/10.1515/jbcpp-2017-0092
Vinayagam, R., and Xu, B., Nutr. Metab., 2015, vol. 12, p. 60.
Channabasava, G.M., Chandrappa, C.P., and Umashankar, T., J. Diabetes Metab., 2015, vol. 6, pp. 5–14.
Osadebe, P.O., and Ukweze, S.E., J. Biol. Res. Biotech., 2004, vol. 2, pp. 18–23.
Obatomi, D.K., Bikomo, E.O., and Temple, V.J., J. Ethnopharmacol., 1994, vol. 43, pp. 13–17.
Osadebe, P.O., Omeje, E.O., Uzor, P.F., David, E.K., and Obiorah, D.C., Asian Pacif. J. Tropic. Med., 2010, vol. 3, pp. 196–199.
Ijomone, O.M., Olaibi, O.K., Biose, I.J., Mba, C., Tete, S.A., and Nwoha, P.U. Pathophysiology, 2015, vol. 22, no. 1, pp. 57–63.
Mori, Y., Cao, D., Li, X., Yin, J., Wang, Z., and Zhang, Y., Int. J. Mol. Sci., 2014, vol. 15, pp. 7667–7683.
Ijomone, O.M., Olaibi, O.K., Biose, I.J., Mba, C., Umoren, K.E., and Nwoha, P.U., Ann. Neurosci., 2014, vol. 21, no. 2, pp. 42–46.
Naqvi, F., Haider, S., Batool, Z., Perveen, T., and Haleem, D.J., Pharmcal. Rep., 2012, vol. 64, pp. 64–69.
Lowry, O.H., Rosenbrough, N.J., Farr, A.L., and Randall, R.J., J. Biol. Chem 1951, vol. 193, pp. 265–275.
Misra, H.P. and Fridovich, I., J. Biol. Chem., 1972, vol. 247, pp. 3170–3175.
Claiborne, A., Handbook of Methods for Oxygen Radical Research, Greenwald, A.R. (Ed.), Boca Raton, CRC Press, 1995, pp. 237–242.
Jollow, D.J., Mitchell, J.R., Zampaglione, N., and Gillette, J.R., Pharmacol., 1974, vol. 11, pp. 151–169.
Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., and Hoekstra, W.G., Science, 1973, vol. 179, pp. 588–590.
Farombi, E.O., Tahnteng, J.G., and Agboola, A.O., Food Chem. Toxicol., 2000, vol. 38, pp. 535–541.
Ellman, G.L., Courtney, K.D., Andres, V., and Featherstone, R.M., Biochem. Pharmacol., 1961, vol. 7, pp. 88–95.
Branstrup, N., Krik, J.E., and Bruni, C., J. Gerontol., 1957, vol. 12, pp. 166–171.
Dawson, R.C.M., Elliott, D.C., Elliott, W.H., and Jones K.M., Data for Biochemical Research, 2nd ed., Oxford, Clarendon Press, 1969, pp. 483–498.
Ebokaiwe, A.P. and Farombi, E.O., J. App. Life. Sci. Inter., 2016, vol. 5, pp. 1–11.
Moghadamtousi, S.Z., Hajrezaei, M., Abdul-Kadir, H., and Zandi, K., Evidence-Based Compl. Altern. Med., 2013, Article ID 273712.
Maritim, A.C., Sanders, R.A., and Watkins, J.B., J. Nutr. Biochem., 2003, vol. 14, pp. 288–294.
Vestergoard, H., Dan. Med. Bull., 1999. vol. 46, pp. 13–34.
Sato, T., Magata, K., and Koga, N., Biochem. Biophys. Res. Commun., 1998, vol. 245, pp. 378–381.
Griendling, K.K., Sorescu, D., and Ushio-Fukai, M., Circ. Res., 2000, vol. 86, pp. 494–501.
Mao, Y., Cao, D., Li, X., Yin, J., Wang, Z., and Zhang, Y., Int. J. Mol. Sci., 2014, vol. 15, pp. 7667–7683.
Wang, J., Yin, J., Song, Y., Zhang, L., Ren, Y., and Wang, D., J. Diab. Res., 2014, Article ID 796840, https://doi.org/10.1155/2014/796840
Chen, C., Wang, Y., Zhang, J., Ma, L., Gu, J., and Ho, G., Dis. Model. Mech., 2014, vol. 7, pp. 723–730.
Haider, S., Ahmed, S., Tabassum, S., Memon, Z., Ikram, M., and Haleem, D.J., Acta Neurol. Belg., 2013, vol. 113, pp. 35–41.
Meeter, M., Talamini, L., Schmitt, J.A.J., and Riedel, W.M. Neuropsychopharmacology, 2005, vol. 31, pp. 712–720.
van Elderen, S.G.C., de Roos, A., de Craen, A.J.M., Westendorp, R.G.J., Blauw, G.J., and Jukema, J.W., Neurology, 2010, vol. 75, pp. 997–1002.
van Harten, B., de Leeuw, F.E., Weinstein, H.C., Scheltens, P., and Biessels, G.J., Diabetes Care, 2006, vol. 29, pp. 2539–2548.
Huang, M., Gao, L., Yang, L., Lin, F., and Le, H., NeuroImage: Clinical, 2012, vol. 1, pp. 57–65.
Ozdemir, N.G., Akbas, F., Kotil, T., and Yilmaz, A., Turk. J. Med. Sci., 2016, vol. 46, pp. 1579–1592.
Orgel, E. and Mittelman, S.D., Curr. Diab. Rep., 2013, vol. 13, no. 2, pp. 213–222.
Chambless, L.B., Parker, S.L., Hassam-Malani, L., McGirt, M.J., and Thompson R.C., J. Neuro-Oncol., 2012, vol. 106, no. 2, pp. 383–389.
Giovannucci, E., Harlan, D.M., Archer, M.C., Bergenstal, R.M., Gapstur, S.M., and Habel, L.A., CA: Cancer J. Clin., 2010, vol. 60, no. 4, pp. 207–221.
Tieu, M.T., Lovblom, L.E., McNamara, M.G., Mason, W., Laperriere, N., Millar, B., J. Neuro-Oncol., 2015, vol. 124, pp. 119–126.
Funding
This work was supported by FUNAI Institutional based TETFUND grant with code FUNAI/FST/14/B2/022 awarded to Dr. Azubuike Ebokaiwe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that there are no conflicts of interest.
Ethical approval. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Science (NAS) and published by the National Institute of Health. The experiment was performed in accordance with the guidelines and approval of institutional animal ethics committee AE-FUNA.
Rights and permissions
About this article
Cite this article
Ebokaiwe, A.P., Ijomone, O.M., Osawe, S.O. et al. Influence of Loranthus micranthus against STZ-Induced Neurobehavioral Deficits in Diabetic Rats. Neurochem. J. 13, 283–294 (2019). https://doi.org/10.1134/S1819712419030061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712419030061