Abstract
Academician N.S. Enikolopov’s method of mechanochemical solid-phase modification of polymers under conditions of intensive shear deformation has resolved one of the most important issues in cellulose processing, its dissolution. The resulting method of solid-phase dissolution in N-methylmorpholine Nрoxide (MMO) has afforded highly concentrated solutions of cellulose and a series of synthetic polymers, as well as mixed solutions based on them in a wide range of concentrations. It has been shown for the first time that the highly concentrated phase (cellulose concentration up to 45%) formed during phase separation in the presence of a precipitant undergoes a transition into nonequilibrium columnar mesophase state. The addition of synthetic polymers, which form crystal solvates with MMO but do not interact with cellulose, to solutions of cellulose in MMO shifts the columnar mesophase formation process into equilibrium. The resulting composite fibers have exhibited high strength and deformation properties. The mechanisms of interaction of various types of polymers with MMO, as well as these between cellulose and the polymers with MMO, at different stages of the mixed compositions preparation (from solid-phase mechanical activation during shear deformation to the transition into a fluid state and spinning) have been elucidated. The performed experiments have made it possible to identify the directions for targeted tuning of cellulose structure and properties, as well as the fabrication of compositions based on it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Solid-phase mechanochemical activation in a shear field, a new direction in polymer chemistry developed by the outstanding scientist N.S. Enikolopov [1–5] has served as an impetus for the development of research on the possibility of its application to address the issues which can be hardly resolved using conventional approaches. For example, the case of cellulose, dissolution of which is a sophisticated task due to its structural features, is special among numerous issues related to transfer of polymers in a solution. Regularity of the cellulose macromolecules structure, the presence of a dense system of hydrogen bonds, and relatively high chain rigidity (the Kuhn’s segment has been estimated as of 75 Å) strongly limit the range of potential solvents of cellulose. Cellulose is decomposed prior to melting, and dissolution is the only approach to obtain the molding products (fibers, films, etc.) based on it.
Until recently, an environmentally hazardous viscose process accompanied by the release of toxic products (carbon disulfide and hydrogen sulfide, as well as heavy metals salts) was the main method of dissolving cellulose. The appearance of a new environmentally friendly direct cellulose solvent, N-methylmorpholine N-oxide (MMO), in the 70s of the 20th century was like an explosion that made it possible to develop an environmentally friendly industrial MMO process for the production of a new cellulose hydrate fiber Lyocell, a real alternative to the viscose process [6–10]. The new process has required such financial costs for its development and capital investments in the industrial implementation that the cost of this fiber is clearly overestimated today. This is largely due to the energy costs to produce spinning solutions, including the cost of electricity for evaporation of water from the pre-obtained cellulose pulp in a 50% aqueous solution of MMO. Such dehydration is required to dissolve cellulose, since only the MMO monohydrate (13.3% of water) is the solvent; the dissolution efficiency increases with lowering the water content, but the limiting factor is the melting point of the binary solvent, which should not exceed reasonable temperatures: 76°C for the monohydrate and 120–130°C for MMO containing 8–10% of water.
The classical works of N.S. Enikolopov have shown that chemical reactions can occur in solids at the moment of the shear deformation impact, their rate being 3–8 orders of magnitude higher in comparison with the liquid phase [1, 11]. The core mechanochemical idea of solid-phase reactions involves mixing, grinding, and activation of the solid phase, as well as mechanochemical transformations as such. As a result of intensive mechanical action, the composite polymer system is crushed and amorphized with the in situ formation of highly active interfaces required for the physical and chemical transformations to occur [12]. The maximum achieved degree of grinding is higher, the greater the energy intensity of the grinding unit and the lower the strength and plasticity of the particles. For the ball mills, it is higher the larger the diameter of the drum and the mass of the balls.
It is important that the ideology of solid-phase reactions has been extended not only to initiate covalent interaction between the components, but also to dissolve the so popular cellulose in MMO. Already in the 1990s, a mixture of cellulose powders and MMO was dispersed and activated in a wide range of concentrations, up to 50% of the polymer, at different processing times in different ball mills. Tuning the depth of the mechanochemical activation by the temperature of the system transition to a homogeneous fluid state at heating has made it possible to determine the modes of the highest efficiency of the solid-phase interaction of cellulose with MMO and to obtain the superconcentrated solutions for the first time [13–16].
As a result of the research, the mechanism of solid-phase mechanochemical dissolution of cellulose in MMO was established and a technological scheme of the original solid-phase MMO process for obtaining new fibers was created, which were assigned the Orcel trademark [10]. The solid-phase method of producing cellulose spin dopes has clear advantages over the traditional MMO process, primarily due to the smaller number of stages and a higher concentration of the resulting spinning solution. The transition of the system to a fluid state is carried out by heating the activated solid-phase pre-solution to the melting temperature of the solvent. Schematic diagrams of the traditional-MMO and solid-phase MMO processes are shown below.

High efficiency of the MMO interaction with the OH groups of cellulose ensures the presence of a semipolar N→О donor bond in the MMO molecule, with two lone-electron pairs at the oxygen atom, capable of interacting with other proton-containing groups. MMO can exist in three thermodynamically equilibrium crystal hydrate forms [17, 18]. Figure 1 displays the two-component diagram of the MMO–Н2О system with three thermodynamically equilibrium crystal hydrate forms of MMO: the 2.5-hydrate form (I) containing 28% of water, with Тm = 38°C, inducing only swelling of cellulose, the monohydrate one (II) with water content 13.3% and Тm = 76°C (MH MMO), and the anhydrous one with Тm ~182°C.
The most widely used form of MMO is the monohydrate, combining sufficiently high dissolving ability and relatively low melting temperature. Solutions containing up to 15% of cellulose can be obtained using MMO monohydrate. Further decrease in water content in MMO leads to an enhancement of the dissolving ability and an increase in the melting temperature, up to the anhydrous MMO (Тm of the MMO anhydride is 182°C). However, it is impossible to exploit the high reactivity of anhydrous MMO, since its melting temperature is close to this of vigorous thermal decomposition.
Thermodynamic activity of MMO, a highly polar donor solvent, is determined by its composition. More complete realization of high dissolving ability of MMO requires that the sterically bulk solvent molecules penetrate the ordered regions of cellulose. In the conventional MMO process this issue is addressed by introducing additional stages of pre-treatment of cellulose with an aqueous dilute solution of the solvent, followed by the energy-intensive stage of water elimination using special equipment and requiring continuous monitoring of the system composition (see the scheme above), whereas in the solid-phase MMO process the same is achieved via a single-stage mechanochemical activation.
The high efficiency of the solid-phase interaction of MMO with cellulose has given impetus to the expansion of the research in this direction. Taking advantage of the methodology of the solid-phase mechanical activation allowed dissolution in MMO of such hydrophobic synthetic polymers as rigid-chain thermotropic liquid crystalline alkylene-arene copolyesters and industrial polymers: aromatic and aliphatic polyamides, segmented polyurethanes, and various acrylonitrile-based copolymers. The mechanisms of interaction of the considered polymers with MMO during the activation as well as properties and structure of the solutions and the obtained fibers have been investigated [20–23].
At present, mixtures of polymers rather than individual ones are widely used to obtain new materials with predefined properties. In this regard, the creation of blended systems based on cellulose and PAN copolymers seems very promising, in particular, due to the fact that both polymers are not only sources of wool- and cotton-like materials, which is important in the textile industry, but also precursors of diverse carbon fibers. The PAN carbon fibers exhibit high carbon residue content (up to 50%) and high mechanical characteristics that allow the use as reinforcing components of composite materials. At the same time, the cellulose-based carbon fibers, with lower carbon residue content (about 15–20%) and moderate mechanical properties, are absolutely indispensable as heat-shielding materials owing to low heat conductance. It can be assumed that the combination of cellulose and PAN in a single precursor fiber may have a synergistic effect in the carbon fibers, i.e., increased strength compared to cellulose carbon fibers and reduced thermal conductivity compared to PAN carbon fibers. With the obvious importance and urgency of the issue, its solution has not been developed for a long time, due to the lack of a common solvent and the energy-intensive stage of mixing, and has become possible thanks to the development of the methodology for solid-phase mechanochemical co-dissolution of cellulose and PAN in MMO. The mechanisms of interaction between various PAN copolymers and MMO, as well as between cellulose, PAN copolymers, and MMO at different stages of the blended compositions production: from solid-phase to transition into a fluid state and spinning, have been elucidated.
In view of this, it seems timely to conduct a systematic review and analysis of the research to identify the general patterns of physico-chemical interactions during solid-phase dissolution of polymers in MMO under shear deformation, which determine the properties and structure of the fluid solutions and fibers obtained on their basis, and to overview the approaches to the targeted creation of new polymer compositions with the required set of properties.
FEATURES OF SOLID-PHASE INTERACTION OF CELLULOSE WITH MMO UNDER THE ACTION OF SHEAR DEFORMATION
According to basic principles of mechanochemistry, high efficiency of the solid-phase activation of cellulose under the action of MMO can be caused by the influence of the macrokinetic factors of the dissolution process: availability of any of its parts to the interaction with the solvent which, at so intensive dispersion, penetrates the least accessible interfibrillar space, as well as the interaction at the molecular level.
Studies of the effect of the degree of cellulose dispersion on the efficiency of the solid-phase reactions with MMO have established that the particle size of the cellulose powder should not exceed 250 μm, and the degree of amorphization should not exceed 10%. To establish the nature of the observed high reactivity of the cellulose–MMO mixtures subject to solid-phase activation, a set of comparative physico-chemical studies of the initial reagents, cellulose–MMO mixtures upon impact of shear deformation and pressure, and mechanical mixtures of the same composition, processed separately in the same modes and then homogenized, have been carried out.
The results of investigation of the interaction with water of various mixtures containing from 3 to 25% cellulose, obtained in an adiabatic calorimeter (Table 1), have made it possible to identify a common trend for both systems, an increase in the thermal effect of the considered mixtures interaction with water with a decrease in the cellulose concentration in the mixtures, probably due to the difference in the heats of interaction of MMO and cellulose with water [24]. However, despite the fact that the values of enthalpy of interaction with water for mechanical mixtures are much higher than in the case of the activated mixtures with water, the difference in the enthalpies is nearly constant and equals 222 J/g, almost identical to the enthalpy of cellulose dissolution in the MMO melt (224 J/g), and in direct contact of solid cellulose with the MMO melt. Of course, the obtained values have been determined with certain inaccuracy, but they provide quite convincing evidence of the similar nature of the interactions occurring between the cellulose and the MMO in the solid phase and in direct contact of the solid cellulose with the MMO melt, but at a lower temperature (about 300 K), almost equal to the melting point of the activated solid system. In mechanochemical processes, the solid phase is considered as the medium in which physical and chemical processes take place. During the deformation mixing of a mixture of cellulose powder and MMO, an interfacial boundary is formed, where atoms come into contact with each other and penetrate the interfacial boundary with subsequent dissolution in the bulk of the second component at a rate 3–4 times higher than the rate of high-temperature dissolution in the liquid phase.
To elucidate the molecular mechanism of the interaction between MMO molecules and cellulose macromolecules, various approaches have been used. For example, investigation of the activated cellulose–MMO system by dielectric method by T.I. Borisova et al. [25] has revealed that the first stage consists in the embedding of the MMO molecules between the (101) crystallographic planes of cellulose, the N → O8 bond of the MMO molecules being at a distance closer that 3 Å from the primary С6–ОН hydroxyl groups of cellulose, available for hydrogen bonds formation. In the molecules of MMO monohydrate, the semipolar N → O bond is strongly electron-donor, which leads to the rupture of the ОН6–ОН3' intermolecular bonds in cellulose and the bonding of between the OН6O8 and ОНЗ'О8 fragments in the cellulose–MMO system. The rupture of the intermolecular hydrogen bonds increases the probability of the interaction of the MMO molecules with the hydroxyl groups involved in the intramolecular hydrogen bonding, ОН2'–ОН6 and O5–OН3'. High degree of solvation of the inter- and intramolecular hydrogen bonds in cellulose inevitably results in the transfer of cellulose macromolecules into the solution.
Although the interaction of the cellulose hydroxyl groups with the donor solvent MMO is of the electron-donor-acceptor nature rather than chemical one [5, 6], the attempts have been made to isolate the formed Н-complex via sublimation of the unbound MMO under vacuum and investigation of the sample having reached constant mass by means of X-ray diffraction analysis and IR spectroscopy. Comparative analysis of the X-ray diffraction patterns and the spectral regions of the isolated H-complex and individual components of the system have revealed the absence of their additivity, which can be considered a direct evidence for the solid-phase complex formation reaction occurring between cellulose and MMO under conditions of simultaneous all-round pressure, shear, and forced plastic flow, and the formation of solid pre-solutions.
Particular attention has been drawn to a decrease in the melting point and, accordingly, this of the dissolution of mixtures subject to solid-phase activation in comparison with the melting point of the MMO used, which is enhanced with an increase in the cellulose content in the mixture. The revealed dependences have been in coincidence with the data in [18, 26, 27], yet the observed temperature depression has been more prominent. For example, whereas in the case of MMO monohydrate the increase in the cellulose content from 3 to 15% has led to the decrease in the melting point of the solution by 2–6°C [2] or by 6–13°C [26], the activated mixtures based on MMO with Тm = 170 and 150°C, containing 30% of cellulose, melt at 130°C, i.e., the temperature depression has been of 40 and 20°C, respectively.
The revealed depression in the melting temperature with an increase in the cellulose content is caused by the interaction of the system components at the molecular level. Using the Flory–Huggins equation to assess this interaction, the Flory–Huggins interaction parameter χ, a quantitative measure of the polymer–solvent interaction has been determined [18], its negative value being abnormally low, –3. As a rule, negative χ values for various polymer systems are only slightly below –1. Further research aiming to refine this parameter, revealed even more prominent anomaly of the χ values, exceeding the earlier observed negative values by almost an order of magnitude [26, 27]. The obtained abnormally low values of χ are likely caused by the fact that it is not quite correct to use the classical Flory–Huggins theory for the cellulose–MMO system, since the special features of the considered system (high polarity of both cellulose and MMO, as well as the solid-phase complex formation) require the account for additional factors.
The possibility of formation of the solid H-complexes (solvates) with Тm 30–50°C lower than the melting point of initial MMO during the mechanophysical activation of cellulose allows the use of the most reactive high-melting hydrate forms of MMO to obtain the solutions containing up to 50% of cellulose. The preparation of the superconcentrated solutions has afforded to come close to an issue which cannot be considered solved, namely, the existence of the LC ordering in the solutions of cellulose in MMO.
LIQUID-CRYSTALLINE STATE OF SOLUTIONS OF CELLULOSE IN MMO
The cellobiose residue containing of two glucopyranose cycles is an elementary unit of cellulose. The presence of an asymmetrical carbon atom imparts chirality (non-equivalence to the mirror structure) to the glucopyranose cycle. This fact leads to optical activity of the unit and the entire macromolecule, ultimately determining the LC structure of cellulose in a solution as well as cellulose derivatives on a solution or a melt. The features of the formation of the LC state in solutions and melts of cellulose derivatives have been considered in sufficient detail in [28‒31]. Strange as it may seem, cellulose derivatives (in particular, ethers, such as hydroxypropylcellulose) can form thermotropic as well as lyotropic LC systems, even in water [29]. Evidently, this fact is due to global rupture of the H-bonds in cellulose and an increase in the degree of anisotropy of individual macromolecules (i.e., their rigidity).
As far as cellulose itself is considered, despite potential possibility of its transition into the LC state predicted back in the 1960s‒1970s [28], realization of the LC ordering in its solutions has remained undetermined. The major issue consists in the complexity of preparation of highly concentrated cellulose solutions, even in such thermodynamically good solvent as MMO, and their high viscosity.
The values of the Kuhn’s segment of cellulose are of 70‒100 Å depending on the solvent, whereas the critical concentration \(V_{2}^{*}\) of the LC transition, according to the classical Flory equation, can be calculated as
with Х being the degree of the macromolecules asymmetry, which should be of 40‒50 wt %.
The first, and still actively cited, report on the LC state of solutions f cellulose in MMO containing up to 25% of the polymer belongs to French researchers [32, 33]. However, further numerous research have shown that the optical anisotropy and the features of rheological behavior observed by the authors are due to phase inhomogeneity of the highly concentrated solutions and the photoelasticity effect. Moreover, the cellulose concentration in the solution, of 20‒25 wt %, is definitely too low in comparison with the above theoretical estimation of the critical concentration of the transition of the cellulose–MMO system in the LC state.
Preparation of the solutions of cellulose in MMO with concentration of 50% has made it possible to investigate the possibility of realization of the LC ordering in them. For example, rheological properties as well as structure and phase transformations in solutions in MMO containing 25‒55% of cellulose with degree of polymerization 300 and 600 and temperature of 20‒180°C have been studied by means of polarization microscopy, small-angle polarized light scattering, and X-ray diffraction analysis [34‒39].
The considered solutions have been optically anisotropic, yet the stability of the anisotropy has been affected by the concentration of cellulose in the solution. During heating of the solutions containing up to 30% of cellulose, the anisotropy has disappeared at 80‒90°C. During repeated heating–cooling treatment, these solutions have been completely isotropic and have not revealed the light transmission in crossed polaroids, irrespectively of temperature. Birefringence of the solutions containing 30 to 40% of cellulose have also vanished upon heating, yet at a higher temperature (130‒140°C), the anisotropy being partially recovered on cooling. The highly viscous rubber-like solutions containing up more than 45% of cellulose have not lost the optical anisotropy upon repeated and prolonged keeping at a high temperature (up to thermal decomposition), however, this fact is likely due to photoelasticity of the concentrated solutions and inhomogeneity of their phase composition (even the presence of the swollen cellulose regions). However, even whereas the overall pattern of birefringence is maintained, pronounced changes in texture have been observed in individual areas of the considered sample: the birefringence weakening on cooling and its strengthening on heating.
The X-ray diffraction analysis of the obtained solutions has been performed. The solutions containing up to 40% of cellulose have been found amorphous. Their diffractograms contained only a diffuse maximum at 2θ = 16°‒20°, corresponding to the short-range order in the system. The wide- and small-angle investigation of the 50% solutions of cellulose in MMO has revealed a new reflection at about 9о at 80°C (Fig. 2), evidencing the appearance of a long-range ordering with the layered interchain periodicity in the system. The increase in temperature to 125°C led to strengthening of the reflection.
The obtained experimental results evidenced the appearance of optical anisotropy in concentrated solutions of cellulose in MMO, in the non-oriented stare as well as under deformation. However, it is impossible to unambiguously conclude on the nature or transformation of the birefringent texture of concentrated solutions of cellulose in MMO. Assignment of the observed optical anisotropy is complicated by the phase nonuniformity of the considered highly concentrated solutions of cellulose and their noticeable photoelasticity.
Summing up the entire set of the conducted research has made it possible to conclude that the anisotropy of the highly concentrated solutions containing up to 45% of cellulose is due to the kinetic factors, namely, slow relaxation of the forced orientation appearing during the formation of the highly concentrated viscous solution. Optical anisotropy of the solutions containing 50% of cellulose, as per the X-ray diffraction analysis, is caused by the presence of the layered long-range periodicity, but the system cannot be considered a thermodynamically equilibrium state, since complete phase homogeneity has not been achieved due to high viscosity and elasticity of the solution. It is logical to conclude that at cellulose concentration in the solution in MMO above 45% the system approaches the limit of cellulose solubility in MMO.
EVOLUTION OF CELLULOSE STRUCTURE BY STAGES OF SPINNING, FROM A SOLUTION TO CELLULOSE AND COMPOSITE ORIENTED FIBER
During the dry-wet spinning of a solution of cellulose in MMO, liquid oriented jet of the solutions is passed into an aqueous spin bath, where the mass transfer processes over a certain time result in the phase separation of the solution, which allows monitoring of the evolution of the cellulose structure at different stages of the process.
The curves of distribution of intensity in the meridional and equatorial diffractograms of the obtained gel fiber practically coincide, the diffractograms being diffuse (Fig. 3).
Spinning of cellulose fiber under conditions of uniaxial tension deformation leads to drastic changes in the cellulose structure. The freshly spun gel fiber with incompletely removed solvent and, hence, higher concentration of cellulose in comparison with the initial solution contains a metastable modification of cellulose. The photo X-ray diffractogram of the sample (Fig. 4) has revealed distinct equatorial reflections.
The presence of only a single strong equatorial reflection in the diffractogram and, respectively, sharp drop in the intensity with an increase in the diffraction angle 2θ is usually related to an increase in disordering due to conformation disturbance along the chain. Such character of the diffractograms is generally typical of the mesophase structures such as 2D columnar mesophase [40], i.e., molecules of MMO hinder the formation of intramolecular (no ordering along the cellulose chains) as well as intermolecular H-bonds. The 2D ordering in the positions of the centers of gravity of the cellulose–MMO associates is schematically shown in Fig. 5.
Upon complete phase separation, up to the formation of cellulose fiber, the diffraction scattering pattern is more significantly changed.
According to the X-ray diffraction analysis data, the photo X-rat diffraction pattern (Fig. 6a) and the diffractograms (Figs. 6b, 6c) of the cellulose fiber have coincided with the reference data on cellulose II, with somewhat increased identity parameter along the fiber axis: а = 8.14 Å, b = 10.34 Å, с = 9.14 Å, β = 62°. The equator (Fig. 6, curve 1) contains four reflections with large half-width (1.8°‒2.0°). The indexes of the reflections are shown in the figure; the near-equatorial reflections have not been observed (Fig. 6, curves 2, 3).
The meridian (Fig. 6c) contained four orders of the reflection of the meridional reflex (1m, 2m, 3m, 4m) corresponding to the period of 10.34 Å. Several characteristic features of the scattering patterns of the ordered cellulose are noteworthy. For example, half-width of the purely meridional reflections is significantly less than this of the equatorial ones. Therefore, the crystallites are larger in the longitudinal direction than in the transverse one (ratio l : d ∼ 10 : 1). In other words, the fiber spinning from a solution of cellulose in MMO yields a crystalline phase, in which the large crystallites are extended in the longitudinal direction. This feature, on one hand, leads to high ordering and density of the formed structures and, on the other hand, reduces the probability of the formation of the transverse hydrogen bonds with the neighbor crystalline clusters, which increases the tendency towards the system fibrillization.
Comparison of equatorial diffractograms obtained for the crystalline fiber (Fig. 6) and the gel fiber (Fig. 5) of cellulose has led to a suggestion that the ordering of the macromolecules in the plane of the 2D mesophase is similar to this of the ас plane of the crystal. This conclusion is supported by the closeness of the angular positions of the equatorial reflections of the crystal and the mesophase. The set of the results obtained for different phase states of the system showed that the solutions of cellulose in MMO are transformed into the columnar mesophase state at the polymer content of 45%, yet the type of the lyotropic LC phase can hardly be identified due to nonuniformity of the phase composition of the highly concentrated solutions.
Despite the high demand for various cellulose molding products (fibers, films, nonwovens, etc.), the researchers face a fairly important issue of expanding the areas of application of the cellulose materials by creating composite materials with the required deformation, strength, functional, and operational properties.
SOLUTIONS OF MIXTURES OF CELLULOSE AND SYNTHETIC POLYMERS IN MMO
The highly polar donor MMO is a thermodynamically good solvent with respect to hydrophilic cellulose, but can also dissolve (via the solid-phase mechanochemical dissolution) synthetic polar hydrophobic polymers such as linear thermotropic alkylene-aromatic polyesters containing mesogenic triads based on fumaric and oxybenzoic acids with hexa- (GP-6) and decamethylene (GP-10) spacers, copolyesters with different content of the elementary units of the parent homopolymers, and copolyesters with mesogenic triads of terephthalic and hydroxybenzoic acids with oxypropylene and decamethylene spacers, poly-m-phenyleneisophthalimide (PMPIA), polyurethanes, and different PAN copolymers [41‒45]. Investigation of the process of solid-phase dissolution of copolyesters and PMPIA in MMO has revealed the general features of the polymers, namely, formation of the crystal solvates during their dissolution in MMO, separating into a crystal solvate phase upon cooling. Comparative analysis of the X-ray diffraction patterns has evidenced complete absence of identity of the reflections of the crystal lattices of the initial polymers, the solvent, and the crystal solvate formed in MMO [43]. The dissolved macromolecules of copolyesters form new intermolecular species with the MH MMO molecules, the solvate adducts which give the crystal solvate phase during subsequent cooling of the solution.
Whereas a single type of the crystal solvates are formed upon dissolution of the copolyesters in MMO, dissolution of PMPIA can yield two crystal solvates depending on the crystal hydrate form of MMO. It should be specifically noticed that the bond between the polymer and the solvent in the crystal solvate is sufficiently strong, being preserved upon introduction of up to 80% of water to the system, i.e., the concentration at which MMO exists in the liquid state.
According to the X-ray diffraction analysis data, the considered composite solutions with the copolyesters, PMPIA, or PAN are amorphous, but the character of the structure changes in the course of the polymer phase separation is basically different. Since the evolution of the structure is the most striking in the cellulose–PMPIA system, let us consider the gradual formation of the cellulose composites exemplified by this system.
As seen from the presented diffractogram (Fig. 7), the cellulose composites containing 5% of PMPIA upon removal of the solvent have not been amorphous even without any drawing and have demonstrated a significant increase in the intensity of the scattering at 2θ = 12° in the equatorial direction, without any redistribution between the equatorial and meridional scattering at 2θ > 15° (Fig. 7).
The presence of two peaks in the diffractogram has evidenced the packing with two average intermolecular distances in the system. These distances are significantly different. Probably, the closest molecules can easily form a system of intra- and intermolecular hydrogen bonds, required for the appearance of ordered “domains”, prone to the orientation. As a result, layered 1D packing is formed at a low draw ratio (λ = 6) (Fig. 8a).
The spinning of the composite fibers under conditions of orientation drawing has resulted in further transformation of the scattering pattern. As seen in Fig. 8b, redistribution of the intensity is observed over the entire scattering area. Two reflections have been observed at the equator, as in the case of pure cellulose mesomorphic gel fiber (Fig. 4). The improvement of the packing (of the centers of gravity of the cellulose‒MMO‒PMPIA associates) of cellulose macromolecules in the basis plane (at the molecular level) has led to the transition of the layered 1D packing into the 2D columnar mesophase. Position of the equatorial reflections in the photo X-ray diffraction patterns of the oriented composite sample has completely corresponded to the position of equatorial reflections of the mesomorphic cellulose gel fiber and has coincided with the position of the principal basal (101) reflection of the cellulose crystal.
An increase in the draw ratio of the composite sample to λ = 16 has led to further improvement of its structure. The texture X-ray diffraction pattern of such sample (Fig. 8c) has revealed the appearance of the meridional reflection at 2θ = 35°. The special features of the scattering at the equatorial area, earlier assigned to the ordering in the basis plane, have been preserved. At the same time, the reflections in the quadrants of the photo X-ray diffraction pattern have not been observed. Comparison of the oriented fibers of cellulose and the composite, the draw ratio being the same, has solidly confirmed this fact. Indeed, the scanning of the composite fiber has not revealed the near-meridional and other reflections in the quadrants (Fig. 9). The absence of the quadrant reflections is the special feature of the composite fiber. At the same time, angular position and half-width of the equatorial and the meridional reflections for the cellulose and the composite (low content of the polymer additive, 4%) have been almost identical.
The marked features of the scattering pattern of the composite fiber obtained at λ = 16 have evidenced the realization of two independent ordering levels in the system: the 2D ordering in the basis plane (at the intermolecular level) and the 1D order along the fiber axis (at the molecular level), i.e., generally non-crystalline 3D ordering. In this case, the mesomorphic state is realized at the complete phase separation of the system and is equilibrium.
Hence, it has been stated for the first time that the solid-phase composite solvates of PMPIA and cellulose with MMO undergo a transition into two-phase fluid state during subsequent heating; the disperse phase of the obtained emulsions consists mainly of the PMPIA macromolecules solvated with the solvent molecules. The high affinity of the solvate shell of labile molecules of flexible-chain PMPIA to the hydroxyl groups of cellulose allows its macromolecules to integrate into the interchain space of cellulose, thus somewhat sterically hindering the intrachain ordering, which is limited to the stage of the formation of the 2D mesophase, and ruling out further formation of the crystalline phase of cellulose.
The IR investigation of solutions of PMPIA with the following formula

in MMO have shown that the amide groups of PMPIA are prone to the intermolecular association, which also involves the protons at the nitrogen atom and the oxygen atoms of the carbonyl group. In the presence of MMO containing water, the intermolecular bonds are broken and new hydrogen bonds are formed, primarily involving the protons at the nitrogen atom, the formation of these bonds is accompanied by a change in the conformation of the PMPIA chain. It can be concluded that PMPIA is prone to association with the MMO–water complex, and cellulose bound to several water molecules via the hydrogen bonds can only be linked to MMO through them. This has provided further confirmation of the validity of the above mechanism, according to which the high affinity of the introduced polymer additives to MMO is the driving force of the process of tuning the cellulose structure in a solution in MMO.
The structure features of the cellulose composite fibers determine the set of their mechanical properties over the entire range of compositions.
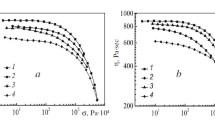
As seen from Fig. 10, the maximum values of the tension at break ε, strength σ, and the modulus Ε are observed for the compositions containing up to 5% of PMPIA, i.e., in the range of the system existence in the mesophase state. Whereas the strength and the modulus have increased by 10‒30%, the tension at break has been increased by almost 3 times, to reach 150%. The effect of the increase in the deformational properties of the composition, so unusual for polymers, is likely due to the character of the structuring of the system. In the range of the intermediate compositions, where the degree of ordering of the fibrillar morphology of the cellulose matrix is decreased, the tension at break has been sharply reduced. Further increase in ε has been observed only at СPMPIA ≥ 60%. At high content of PMPIA in the composition, the change in the mechanical parameters has been typical, i.e., the values of the strength and the modulus have been increased, the tension at break decreasing [43].
Targeted tuning of cellulose structure is an extremely important issue, especially regarding the fabrication of carbon fiber on its basis and elucidation of the relationship of the structure and properties of the precursor and the respective parameters of the produced black fiber. The accumulated experience has led to a suggestion that the structural transformations should be directed at the creation of high structural uniformity along the chains, with less sharp boundaries of the phase-structure transitions, i.e., the domains of the crystalline phase should be shorter, whereas the short amorphous regions should be more ordered. In this regard, investigation of the possibility of targeted formation of the cellulose structure via the introduction of PAN copolymers in the cellulose solutions is of special interest. It has turned out that the dissolution of PAN copolymers in MMO and their co-dissolution with cellulose in MMO can only be performed via the method of solid-phase low-temperature dissolution under shear.
The PAN copolymers consisting of acrylonitrile (AN), methyl acrylate (MA), and an acidic comonomer are the most common precursors of carbon fiber. The interaction of the PAN‒acrylic acid (AA) copolymer with the acidic fragment of AA (composition: 97% AN, 2% MA, and 1% AA) synthesized in supercritical CO2 medium [46] with MMO during the mechanoactivation has been investigated by means of IR spectroscopy. From the data in Fig. 11 it follows that the spectrum of the activated (PAN‒AA)‒MMO mixture is not a superposition of the spectra of the initial components, PAN‒AA and MMO. Instead of a single band at 1732 cm‒1 as in the PAN‒AA spectrum (Fig. 11, spectrum 1), the spectrum of the activated mixture has contained two bands in this region: 1743 and 1684 cm‒1. The splitting of the C=O stretching band of the carboxylate groups of the copolymer under the action of MMO is most likely due to the formation of two types of the hydrogen complexes between this group of the polymer and the polar N → O bond in MMO [47–49].

The first type of the complex can be explained by the formation of hydrogen bonds between the negatively charged oxygen atom of the N → O group in the MH MMO molecule and the proton of the carboxylate group of the polymer, which leads to the shift of the electron density from the С=О bond, accompanied by the shift of the band at 1732 cm–1 in the IR spectrum towards long-wave region, to 1684 cm–1. At the same time, the oxygen atom of the carbonyl group can interact with the proton at the tertiary carbon atom of the PAN‒AA backbone.
Another type of the complex formation can be realized via the coordination of a strongly polar N → O bond in MH MMO with a moderately polar С=О bond of the carboxylate group of the polymer. The proton of the ОН group can be coordinated with the nitrile group of the adjacent unit. Such type of the coordination leads to the enrichment of the C=O bond with the electrons, owing to the strongly electronegative oxygen atom in MMO, and the shift of the C=O band towards short-wave region, to 1743 cm–1. The coordination of a carboxylate group in the PAN‒AA macromolecule with the MMO molecule (with the formation of either complex a or complex b) inevitably results in the redistribution of the electron density in this group and, hence, additional coordination with the functional groups of an adjacent polymer unit (nitrile group or proton at the tertiary carbon atom of the backbone). Such coordination should induce the conformational rearrangements of this part of the chain as well as redistribution of the electron density in the nitrile groups. The nitrile group band in the spectrum of the activated solid-phase (PAN‒AA)‒MMO mixture (2244 cm–1) has not been noticeably shifted, but relative intensity of this band has been significantly decreased in comparison with the spectrum of the PAN‒AA copolymer. The weakening of the band in the spectra of the activated mixture can be due to a change in the polarization of the bonds in the separate CN groups in the copolymer.
The obtained results have led to a conclusion that the interaction of the carboxylate groups of acrylic acid units in the copolymer and the electron-donor N → O bond of the solvent molecule, leading to the formation of the solid pre-solutions of PAN‒AA in MMO, occur during the solid-phase mechanical activation. At temperature above 120°C, the mere dissolution process is accompanied by the processes of cyclization of the nitrile groups of PAN with the formation of a conjugated bonds system.
MIXED SOLUTIONS OF CELLULOSE AND PAN–AA IN MMO
On the basis of the kinetic features of solid-phase dissolution of cellulose and various PAN copolymers in MMO, namely, the fact that the rate of dissolution of the PAN copolymers significantly exceeds this of cellulose, a method of solid-phase co-dissolution of cellulose and PAN in MMP has been elaborated and the mixed solutions have been obtained [50, 51]. The thermodynamically incompatible solutions are two-phase (emulsions) over the entire range of the components ratio. The high difference in the viscosity and elasticity of the phases, up to several orders of magnitude, results in the formation of numerous morphological forms in the emulsions.
The features of the interactions between cellulose, PAN‒AA, and MMO in the spun fiber have been investigated by means of IR spectroscopy in dependence of the amount of the introduced PAN‒AA.
As seen in the spectra (Figs. 12a, 12b), significant changes in the characteristic absorption bands of cellulose have occurred depending on the amount of the introduced PAN‒AA, especially in the 1200‒1450 cm–1 range sensitive to the crystalline phase of cellulose. The bands assigned to the amorphous phase (1152 and 900 cm–1) have been changed in the intensity as well, depending on the content of PAN‒AA in the fiber. The –ОН and С‒О groups of cellulose are usually strongly associated, and to a certain degree determine the intra- and interchain structuring via the formation of the crystallites and the amorphous regions in the polymer.
At low content of PAN‒AA (0.5 and 2%), the bands at 3300‒3400 cm–1 (‒ОН) and 1025 cm–1 (С‒О) have been insignificantly weakened, the band at 3300‒3400 nm–1 being shifted towards the long-wave range, which points at a higher degree of association of the –ОН groups, possibly also with the nitrile groups of PAN‒AA. With the increase in the PAN‒AA content to 3 and 5%, both bands have been sharply weakened. The band at 3350 cm–1 has been shifted towards short-wave range, and a new band has appeared at 3433 cm–1, assignable to weakly associated –ОН groups. Such behavior of the bands due to the ‒ОН and С‒О groups can be explained only by redistribution of the hydrogen bonds in cellulose and should result in the degree of ordering of the macromolecules. Indeed, the bands responsible for the crystallinity (at 1200‒1450 cm–1) have been significantly strengthened. The O’Connor indexes [52, 53] obtained from the analysis of intensity of these bands have shown that the introduction of up to 2% of the PAN‒AA copolymer in cellulose leads to a change in the degree and type of association between the –ОН groups of cellulose, accompanied by an increase in the number (likely getting shorter) of the crystalline as well as the amorphous parts in the cellulose chains. At the PAN‒AA content of 3%, association of the nitrile groups of PAN with the OH groups of cellulose occurs, which creates a new geometry of the nitrile groups, favoring further cyclization along the PAN‒AA chain. Such cyclization has been the most pronounced in the cellulose spectra at the PAN content of 5%; at the same time, the degree of crystallinity of cellulose has been significantly decreased, and the number of the amorphous regions has been increased.
The fiber has been prepared from the 18% mixed solutions containing 0.5 to 5% of PAN, under stable spinning conditions at 120°C. The results of their structure investigation are presented in Fig. 13. From the presented diffractograms it is to be seen that the structure of the cellulose fiber has been typical of cellulose II: the major reflections have been observed at 2θ = 12° and 20° [45]. The diffractogram of the PAN fiber has shown an equatorial peak at 2θ = 16.5°, corresponding to the interplanar distance of d = 5.37 Å (110), and a broad amorphous halo centered at 2θ = 26°. Structure of the composite fiber containing 5% of PAN has been different from the structure of both cellulose and PAN. The diffractogram has exhibited a redistribution of the intensities of the characteristic peaks of cellulose at 2θ = 12° and 20° and the absence of the characteristic reflection of PAN at 2θ = 16.5°. The amorphous halo has been shifted towards wider angles and has appeared as a weak righthand shoulder at the basement of the characteristic peak of cellulose at 2θ = 20°.
The mechanical properties of the cellulose and composite fibers are collected in Table 2. The change in the mechanical parameters of the composite fibers in comparison with the cellulose ones has been likely due to the above-discussed structural changes of the cellulose matrix induced by the incorporated PAN copolymer additive. For instance, it follows from the data in Table 2 that the introduction of no more than 3% of PAN–AA in the cellulose matrix leads to a slight decrease in the strength and the elasticity modulus of the composite fibers, the deformation properties being improved by more than 4 times. At the PAN–AA content increased to 5%, the interactions between the polymeric macromolecules have dominated in the system, which has led to more pronounced amorphization of cellulose, cyclization of the nitrile groups of PAN, and, hence, even stronger decrease in the fiber strength and increase in the deformation properties.
It is impossible not to consider the influence of the fundamental difference between the processes of orientation of cellulose and PAN occurring during the spinning on the mechanical properties of the composite fiber. During spinning of cellulose from the solutions in MMO, the orientation of macromolecules proceeds in a single stage, in the air gap as the filament passes from the bottom of the spinneret to the surface of the spin bath, while the PAN fibers are oriented during multi-stage drawing. In other words, when spinning the mixed solutions, the multi-stage drawing is lacking to achieve PAN orientation in the droplets of the dispersed phase, and hence the non-oriented copolymer chains can act as defects that worsen the strength but significantly improve the deformability of the composite fibers.
The PAN‒methyl sulfonate (MS) has been further considered as an object of investigation, in which more polar ionic sulfonate groups (capable of formation of high-energy bond with MMO and tune the cellulose structure through them) replace the carboxylic ones. Moreover, the solid-phase activation of these mixtures, unlike the activation of the (PAN‒AA)‒MMO system, has been carried out at higher mechanical stress and shear deformation, as implemented in a twin-screw extruder. The possibility to perform organic synthesis in extruders was demonstrated for the first time in [54, 55].
The IR spectroscopy investigation of the (PAN‒MS)‒MH MMO system has revealed that, in contrast to the mechanical mixtures, the interaction between the sulfonic groups of the polymer and the N → O bond of MH MMO is weakened, the geometry parameters of the sulfonic groups are changed, MH MMO is transformed in a more high-melting crystal hydrate form upon partial dehydration, and the nitrile groups is significantly polarized due to the association with the N → O bond through a water molecule. Schematic illustration of the interaction in the (PAN‒MS)‒MH MMO system in the mechanical and activated mixtures is given below [56‒58].

The redistribution of the hydrogen bonds in the activated (PAN‒MS)–MH MMO system with a loss of the crystal hydrate water molecules from MH MMO should inevitably result in an increase in the temperature of MMO melting and dissolution of PAN‒MS in MH MMO, respectively.
Comparison of the spectra of PAN‒MS and the high-melting MMO with the spectra of the activated mixture and the solution is shown in Fig. 14.
The spectrum of the activated (PAN‒MS)−MMO mixture has exhibited the spectral signs of the water migration, i.e., the crystal hydrate water of the MMO molecules is shifted towards the oxygen atom of the S=O bond in the formed complex. The bands of the nitrile groups have become very strong, and the bands of the sulfonic groups have been even more shifted in comparison with the spectrum of the analogous mixture with the monohydrate, i.e., noncovalent binding of MMO with the polymer through the water molecules have been more pronounced. The band of the nitrile groups have been even more weakened and shifted by 5 cm–1 towards long-wave range. Besides the shifts of the bands evidencing the interaction of the polymer with the solvent, a new band at 1596 cm–1 appeared in the spectrum, reflecting the appearance of conjugation in PAN macromolecules.
The processes of mechanochemical treatment of solid polymers are performed under nonequilibrium conditions and are determined by the energetics of the equipment. The dependences of the degree of interaction between the system components on the mode of extrusion at different extrusion rate and the screw length in a twin-screw extruder have been analyzed by means of IR spectroscopy. A mixture containing cellulose and PAN‒MS (8% each) and 84% of MMO has been used as the test system [59]. For comparison, the cellulose‒(PAN‒MS)‒MMO mechanical mixtures of the same phase composition, obtained via mere mechanical mixing of the solid reagents without a shear impact have been investigated. The spectra of the mixtures have been corrected for baseline and normalized by the intensity of the band of the MMO skeleton vibrations at 1009 cm–1 to assess the influence of the extrusion rate on the spectral changes. Figure 15 displays the spectra of the initial mixture and this activated at different rates of extrusion, in comparison with the spectrum of MMO. It is to be seen that the spectra are not superpositions of the spectra of the initial systems. For better visualization and more complete analysis of the system state depending on its degree of deformational impact, the parts of the obtained spectra have been considered separately (Fig. 16).
Details of the spectra in Fig. 15. See discussion in the text.
The spectra of the systems activated at different extrusion rates have revealed strengthening of the stretching bands of the ‒ОН groups of cellulose at 3368 and 3306 cm–1 (Fig. 16a) and the bands of libration motion of water at MMO bound to PAN‒MS and cellulose, at 779 and 665 cm–1 (Fig. 16c) with an increase in the mixing rate. Intensity of the nitrile group band of PAN‒MS in the spectra of the activated mixtures have been strongly (by more than 4 times) decreased in comparison with the spectrum of the initial mixture (Fig. 16b); simultaneously, the bands at 2197 and 2299 cm–1, assignable to the nitrile groups differing in the polarization degree, have been strengthened. The band of the C=O stretching at 1730 cm–1 (the acrylate units in PAN) has been almost vanished in the spectrum of the activated mixture, yet the bands of amide groups at 1677 cm–1 as well as imide and/or anhydride groups at 1762 and 1790 cm–1 unexpectedly appeared and increased in intensity with an increase in the extrusion rate
Hence, besides the specific interactions between the solid-phase reagents and MMO, deep chemical transformations of the nitrile groups on PAN–MS occur in the solid-phase cellulose–(PAN‒MS)‒MMO system with an increase in the shear deformation. Hydrolysis of the nitrile groups in a liquid phase usually occurs only at elevated temperatures, fully coinciding with the kinetic features of the solid-phase reactions, according to which the rate constant of the reactions in a solid phase under conditions of shear deformation is several orders of magnitude higher than in a liquid phase [2]. The revealed dependences evidenced that an increase in the degree of the deformational impact leads to an increase in polarizability and, hence, reactivity of the functional groups of the system components, i.e., the value of the mechanical loading is correlated with the physico-chemical response.
The evolution of the interactions between the polymers and the solvent during preparation of the solutions and spinning has been considered by means of IR spectroscopy for the composite fiber containing 60% of PAN and 40% of cellulose, spun in different modes at 120°C [59, 60].
Figure 17 displays the IR spectra of two samples recorded at room temperature: this obtained immediately upon preparation of the emulsion (1) and this spun upon heating the emulsion during 30 min (2). The spectra of the composite fibers have retained the characteristic bands of the polymers, but the spectra of the composite fibers of the same composition differing in the history have been significantly different. The spectrum of sample 2 of the fiber (obtained upon heating during 30 min at 120°C) (Fig. 17a, spectrum 2) has exhibited significant decrease in intensity and slight shift of the maximum towards long-wave range of the cellulose OH stretching band at 3378 cm–1.
Comparison of IR spectra of the composite fibers 60% PAN‒MS‒40% cellulose spun at 120°C from 18% solutions immediately upon the transition into the viscous flowing state (1), upon heating during 30 min at 120°C, and PAN‒MS (3) in the regions of the ОН, СН, and ‒C≡N groups stretching (a) and in the region of the С=О and С–О stretching (b).
The said effect has confirmed participation of the hydroxyl groups of cellulose in the formation of new hydrogen bonds, besides these present in the cellulose itself. A strong broad band of the С‒О‒С bond of cellulose at 1038 cm–1 (Fig. 17b) in the spectrum of the composite fiber 1 has been split in comparison with the cellulose spectrum, whereas in the spectrum of fiber 2 this band has become weaker and its degree of splitting has been changed, which also evidences significant degree of association of the OH groups with the functional groups of PAN‒MS. The formation of new hydrogen bonds between cellulose and the ester groups of PAN–MS has been confirmed by a change in relative intensity of the C=O stretching band at 1732 cm–1 (methacrylate groups of PAN‒MS). For example, the band at в образце 1 интенсивность полосы при 1732 cm–1 in the spectrum of sample 1 has weakened (Fig. 17), almost vanishing and observed as a weak shoulder in the new band at 1683 cm–1 in the spectrum of sample 2.
The most reactive functional groups of the PAN‒MS copolymer, methyl sulfonate ones absorbing IR radiation at 800‒1100 cm–1, unfortunately have not been observed in spectra of the composite samples, since they are completely overlapped by strong stretching bands of the cellulose OH groups, and their contribution into the system of the appearing hydrogen bonds is not discussed at this point.
Meanwhile, besides the new band at 1683 cm–1, another band at 1575 cm–1 appeared in the spectrum of fiber 2; they are in good agreement with Amide I and Amide II bands in primary amides [59]. These changes in the spectrum of sample 2 compared to spectrum 1 can be explained by simultaneous formation of new hydrogen bonds and partial hydrolysis of the nitrile groups in PAN–MS during heating of the emulsion:

The amount of the formed amide units can be estimated by comparing the intensities of the Amide I band (1683 cm–1) and the band of the C=O bonds in methacrylate units of PAN–MS at 1732 cm–1. It is known that the intensity of the band at 1680 cm–1 in the spectrum of acrylamide is 2.3 times higher than the intensity of the band at 1735 cm–1 in the spectrum of methyl acrylate; therefore, intensities of these bands being equal, the content of the amide units is 2.3 times lower than the methyl acrylate units. In the spectrum of the composite fibers (Fig. 17b), intensity of the band at 1680 cm–1 (the amide units) is 1.8 times higher than this of the band at 1735 cm–1 (the methyl acrylate units). Since the content of methyl acrylate units in PAN‒MS does not exceed 6%, simple calculation has revealed that the content of the amide groups formed via hydrolysis upon heating of the spinning emulsions during 30 min does not exceed 4.5%.
The amide units formed via hydrolysis of the nitrile groups readily form hydrogen bonds with the hydrophilic OH groups of cellulose. Such noncovalent bonds between the ester or amide groups of PAN and hydroxyl or glycoside groups of cellulose can be formed either directly or, as has been shown by quantum-chemical simulation in [49]. The appearance of a small number of noncovalent sufficiently strong crosslinks between the chains of PAN‒MS and cellulose leads to a change of the conformation of the polymer chains, especially since the parts of the chains not linked by the hydrogen bonds should repel due to the difference in hydrophilicity. The complex structural transformations occurring in the considered composite solutions under different conditions should determine the structure and properties of the obtained hybrid fiber.
The fibers structure has been investigated by means of X-ray diffraction analysis, and the corresponding diffractograms are shown in Fig. 18. For the Lyocell fibers, the principal reflections are observed at 2θ~12.1°, ~20.1°, and ~21.5° and are assigned to the crystallographic planes (101), (101̅), and (002) (shown in black dotted line in the figure), which corresponds to the cellulose II phase (regenerated cellulose), i.e., no structural transformations of cellulose at the molecular level has occurred in the presence of PAN‒MS.
The diffractogram of the PAN‒MS fiber has exhibited two reflections at 2θ = 16.9° (d = 0.524 nm) and 2θ = 29.4° (d = 0.303 nm) and a broad reflection at 25.7°, marked in red dotted line. In the composite fibers, angular position of the characteristic reflections has been preserved, only their intensity has been changed depending on the phase composition of the fiber. Since the fine changes in the intensity of the principal reflections can hardly be tracked from the presented diffractograms and, according to the IR spectroscopy data, a change in the PAN content results in amorphization of cellulose, the indexes of crystallinity and length of crystallites have been determined as function of the amount of PAN in the composite fiber. Figure 19 displays the dependences of the degree of crystallinity and the size of the cellulose crystallites depending on the PAN content in the composite fiber. From the data in Fig. 19a it is to be seen that the introduction of polyacrylonitrile in cellulose induces linear decrease in the crystallinity index.
The size of the cellulose crystallites has been estimated using the Scherrer equation for the reflection corresponding to the (101̅) plane [61]. The structure of cellulose fibers obtained from the solutions in MMO, including the Orcel fiber [10] prepared using the solid-phase MMO process, is traditionally characterized by the increased values of the degree of crystallinity as well as the longitudinal crystallite size. The introduction of PAN‒MS into cellulose has first favored an increase in the crystallite size, while their size has been decreased with an increase in the copolymer fraction above 30%, i.e., the dependence on concentration is extremal. Likely, such behavior of the system is due to the above-discussed effect of intermacromolecular interaction, which does not affect the character of self-ordering of cellulose at PAN content in the mixture up to 30%, whereas its effect is enhanced at the concentration above 30%, leading to amorphization and changing in the macrochains conformation. At the same time, the index of crystallinity has been decreased, resulting in a decrease in the longitudinal size of the crystallites, during the spinning accompanied by the orientation.
Morphology of the composite fiber with high content of PAN is presented in Fig. 20.
In contrast to the Lyocell or Orcel cellulose fibers, in which strong longitudinal orientation of the crystallites and the amorphous regions favors the formation of fibrils with size not exceeding 0.08‒0.1 μm and the packing so dense that they are not manifested in the cross-section of the fiber, the obtained composite fibers have exhibited larger heterogeneous layered texture formed by the microfibers with size about 1 μm rather than the fibrils. The microfibers have been also heterogeneous, consisting of numerous oriented fibrillar subunits. The fiber surface has been grooved due to the microfibers exposed to it, while the cross-section has been in general circular. The phase composition of the formed structures can hardly be determined solidly, yet the cellulose fibrils isolated upon removal of PAN, firstly, have confirmed the electron-donor-acceptor (EDA) nature of the intermacromolecular bonds; secondly, the preserved sufficiently high strength properties have evidenced the validity of the above-suggested mechanism, according to which the PAN macromolecules interact with the surface functional groups of the cellulose structural aggregates.
The data obtained in the discussed studies evidence high efficiency of the solid-phase activation of the considered systems, ensuring high degree of interaction between the polymers. Pyrolysis of the composites of cellulose with PAN‒MS leads to the conversion of the EDA interpolymer bonds into the covalent ones, thus forming in situ the crosslinked copolymers of cellulose and PAN, which can be an important positive factor in the manufacturing of the carbon fibers.
The obtained copolymers of cellulose and PAN‒MS, with a spatially fixed structure, can be potentially used to obtain hydrogels for diverse applications. Evidently, these directions require detailed investigation.
The conducted set of studies has allowed, having considered the features of the behavior of the studied cellulose–MMO systems with various polymers in the processes of mechanical activation by shear deformation, transformation into solution, and spinning as well as structural features of the hybrid fibers, to identify the main factors that determine the directions of cellulose structure regulation. For example, the principal special feature of the cellulose (PMPIA, LC polymers, polyurethane)‒MMO system is the energetically strong interaction of the polymers with MMO, leading to the formation of the MMO‒polymer crystal solvates and determining further structural transformations of the systems following the process stages. The polymers in the emulsion are not in direct interaction, they interact through the solvent molecules, thus creating certain steric obstacles and directing the cellulose macromolecules structuring via a mechanism analogous to the formation of mesomorphic ordering of cellulose in the presence of MMO molecules (incomplete phase separation during spinning).
When PAN copolymers differing in the reactivity towards MMO and cellulose to the cellulose‒MMO system, the solid-phase mechanoactivation and further stages of the process are accompanied by specific interactions between the system components and structural-chemical transformations. As has been considered in sufficient detail, potential energy of the forming specific interactions between cellulose and PAN‒AA at the content of the latter up to 3% is not enough for significant change in the mechanism of structuring of cellulose and PAN‒AA. The resulting hybrid fibers, while maintaining strength characteristics, acquire high deformation properties.
In the cellulose–(PAN–MS)–MMO system, as in the cellulose‒PMPIA–MMO one, the PAN‒MS macromolecules demonstrate high affinity to water and MMO> However, in contrast to the system with PMPIA (in which, according to the IR spectroscopy studies of the mechanical mixture of the starting polymers, no direct bonds between the polymers are formed), new hydrogen bonds between the OH groups of cellulose and the functional groups of PAN are formed in the cellulose‒(PAN‒MS) system. These facts mean that the conditions of mechanical activation of cellulose and PAN lead to certain re-association of the OH bonds of cellulose from the interaction with other hydroxyl groups of cellulose to the complementary groups (amide, ester, and carboxylic ones) in the macromolecules of transformed PAN‒MS in a solution in MMO.
In summary, the performed series of investigations have extended the existing views on the reactivity of solid organic substances under conditions of shear deformation and have resolved the following important aspects of the cellulose issue:
—to establish that the main regulator of the cellulose structure in the solutions in MMO is the high affinity of the introduced additives of polar synthetic polymers to MMO, due to which the polymers in solution interact with cellulose only through the solvent molecules, thereby disrupting the algorithm of the structure formation of cellulose macromolecules, realizing a mesomorphic state at intermediate stages;
—to propose mechanisms of interaction of PAN copolymers of various nature with MMO, as well as cellulose and PAN copolymers with MMO at various stages of obtaining the mixture compositions, from the stage of mechanical activation to transition into the fluid state and spinning;
—to prove the EDA interaction and structural-chemical transformations between the components of the cellulose–PAN copolymers–MMO system at all stages of the process of obtaining the hybrid fibers, due to which the multicomponent system acquires a pseudo crosslinked character, the depth and degree of structural transformations being predetermined by the nature of PAN;
—to demonstrate that the noncovalent bonds between the associates of cellulose and the PAN copolymers are converted into the covalent ones, to form in situ the crosslinked copolymers of cellulose and PAN, which should be considered during formation of carbon fibers based on them.
REFERENCES
A. A. Berlin, S. A. Vol’fson, and N. S. Enikolopyan, Kinetics of Polymerization Processes (Khimiya, Moscow, 1978) [in Russian].
N. S. Enikolopov, Usp. Khim. 60 (3), 586 (1991).
N. S. Enikolopov, S. A. Wolfson, A. I. Nepomnjaschie, V. G. Nikolskie, V. A. Teleshov, and V. A. Filmakova, US Patent No. 4607797 (1986).
N. S. Enikolopyan, E. L. Akopyan, A. S. Kechek’yan, V. G. Nikol’skii, and N. M. Styrikovich, Vysokomol. Soedin., Ser. A 26 (11), 2362 (1984).
N. S. Enikolopyan, E. L. Akopyan, and V. G. Nikol’skii, Dokl. Akad. Nauk SSSR 266 (4), 889 (1982).
N. F. Franks and J. K. Varga, US Patent No.4290815 (1979).
A. F. Turbak and R. Hammer, in Solvent Spun Rayon, Modified Cellulose Fibers and Derivatives, Ed. by A. F. Turbak (ACS Symposium Series, Washington, 1977), p. 12.
S. M. Hudson and J. A. Cuculo, J. Macromol. Sci. 18 (1), 1 (1980).
C. C. McCorsley and J. K. Varga, US Patent No. 4246211 (1981).
L. K. Golova, Ross. Khim. Zh. 46 (1), 49 (2002).
V. A. Zhorin, N. A. Mironov, V. G. Nikol’skii, and N. S. Enikolopyan, Vysokomol. Soedin., Ser. A 22 (2), 397 (1980).
N. S. Enikolopyan and M. L. Fridman, Dokl. Akad. Nauk SSSR 290 (2), 379 (1986).
L. K. Golova, V. G. Kulichikhin, and S. P. Papkov, Polym. Sci., Ser. A 28 (9), 1795 (1986).
L. K. Golova, N. V. Vasil’eva, O. E. Borodina, S. Z. Rogovina, S. N. Zelenetskii, and S. P. Papkov, RF Patent No. 2075560 (1994).
L. K. Golova, RF Patent No. 1645308 (1991).
L. K. Golova, Khim. Volokna, No. 1, 13 (1996).
H. Chanzy, P. Noe, M. Paillet, and P. Smith, Appl. Polym. Sci., Polym. Symp., No. 37, 239 (1983).
H. Chanzy and S. Nawrot, J. Polym. Sci., Polym. Phys. Ed. 20, 1909 (1982).
V. A. Platonov, Yu. Ya. Belousov, I. D. Zenkov, N. S. Pozhalkin, and V. G. Kulichikhin, Khim. Volokna, No. 1, 27 (1983).
N. V. Bleishmidt, V. E. Dreval’, O. E. Borodina, L. K. Golova, and V. G. Kulichikhin, Vysokomol. Soedin., Ser. A 39 (9), 1511 (1997).
V. G. Kulichikhin, V. E. Dreval’, A. M. Shatalova, L. K. Golova, and A. Yu. Bilibin, Polym. Sci., Ser. A 44 (12), 1336 (2002).
A. Y. Bilibin, V. V. Zuev, and S. S. Skorokhodov, Makromol. Chem. Rapid. Commun. 6, 601 (1986).
E. E. Palchikova, I. S. Makarov, M. I. Vinogradov, L. K. Golova, G. K. Shambilova, and V. G. Kulichikhin, Polym. Sci., Ser. B 63 (6), 833 (2021).
I. D. Zenkov, L. K. Golova, and O. E. Borodina, in Proceedings of the All-Union Scientific Conference on Chemistry, Technology, and Use of Cellulose and Its Derivatives, Mytishchi, 233 (1990).
T. I. Borisova, N. V. Afanas’eva, L. L. Burshtein, O. E. Borodina, and L. K. Golova, Vysokomol. Soedin., Ser. A 35 (8), 1326 (1993).
M. M. Iovleva, V. N. Smirnova, Yu. Ya. Belousov, and S. S. Papkov, Vysokomol. Soedin., Ser. A 28 (4), 749 (1986).
M. M. Iovleva, Vysokomol. Soedin., Ser. A 31 (4), 808 (1989).
S. P. Papkov and V. G. Kulichikhin, Liquid-Crystalline State of Polymers (Khimiya, Moscow, 1977) [in Russian].
S. P. Papkov, Yu. Ya. Belousov, and V. G. Kulichikhin, Khim. Volokna, No. 3, 8 (1983).
R. Gilbert and P. A. Patton, Prog. Polym. Sci. 9, 115 (1983).
V. G. Kulichikhin and L. K. Golova, Khim. Drev., No. 3, 9 (1985).
P. Navard and J. M. Haudin, Brit. Polym. J., No. 12, 1137 (1980).
J. F. Blachot, N. Brunet, P. Navard, and J. Y. Cavaillé, Rheol. Acta. 37 (2), 107 (1998).
L. K. Golova, O. E. Borodina, G. Ya. Rudinskaya, and S. P. Papkov, Khim. Volokna, No. 2, 52 (2001).
L. K. Golova, O. E. Borodina, L. K. Kuznetsova, T. A. Lyubova, and T. B. Krylova, Khim. Volokna, No. 4, 14 (2000).
M. M. Iovleva, A. Sh. Goikhman, S. I. Banduryan, and S. P. Papkov, Vysokomol. Soedin., Ser. B 25 (11), 803 (1983).
L. K. Golova, N. V. Vasil’eva, O. E. Borodina, L. P. Mil’kova, S. P. Papkov, in Abstracts of International Conference on Lyotropic Crystals, Ivanovo, Russia, 1993, p. 45 [in Russian].
L. K. Golova, N. V. Vasil’eva, O. E. Borodina, and S. P. Papkov, in Abstracts of All-Russian Symposium on Liquid-Crystalline Polymers, Chernogolovka, Russia, 1995, p. 72 [in Russian].
O. A. Khanchich, L. K. Golova, O. E. Borodina, T. V. Krylova, and D. V. Loshadkin, Polym. Sci., Ser. A 43 (7), 766 (2002).
B. Wunderlich and J. Grebowicz, Adv. Polym. Sci. 60–61, 2 (1984).
L. K. Golova, I. S. Makarov, E. P. Plotnikova, G. Sh. Shambilova, A. K. Tereshin, and V. G. Kulichikhin, Polym. Sci., Ser. A 51 (3), 283 (2009).
L. K. Golova, I. S. Makarov, E. V. Matukhina, and V. G. Kulichikhin, Polym. Sci., Ser. A 52 (11), 1209 (2010).
L. Golova, I. Makarov, L. Kuznetsova, E. Plotnikova, and V. Kulichikhin, in Cellulose—Fundamental Aspects, Ed. by T. G. M. Van De Ven (InTech, Rijeka, 2013), p. 303.
L. K. Golova, I. S. Makarov, M. I. Vinogradov, L. K. Kuznetsova, and V. G. Kulichikhin, Polym. Sci., Ser. A 60 (6), 756 (2018).
I. S. Makarov, L. K. Golova, L. K. Kuznetsova, A. V. Rebrov, A. K. Berkovich, I. Yu. Skvortsov, and V. G. Kulichikhin, Russ. J. Gen. Chem. 87, 1351 (2017).
A. V. Shlyahtin, I. E. Nifant’ev, V. V. Bagrov, D. A. Lemenovskii, A. N. Tavtorkin, and P. S. Timashev, Green Chem. 16, 1344 (2014).
V. Kulichikhin, L. Golova, I. Makarov, G. Bondarenko, V. Makarova, S. Ilyin, I. Skvortsov, and A. Berkovich, Eur. Polym. J. 92, 326 (2017).
I. S. Makarov, L. K. Golova, G. N. Bondarenko, T. S. Anokhina, E. S. Dmitrieva, I. S. Levin, V. E. Makhatova, N. Z. Galimova, and G. K. Shambilova, Membranes 12, 297 (2022).
M. M. Feldstein, T. I. Kiseleva, G. N. Bondarenko, J. V. Kostina, P. Singh, and G. W. Cleary, J. Appl. Polym. Sci. 112, 1142 (2009).
V. G. Kulichikhin, L. K. Golova, I. S. Makarov, M. I. Vinogradov, A. K. Berkovich, and Y. V. Golubev, J. Text. Eng. Fash. Technol 3, 593 (2017).
I. S. Makarov, L. K. Golova, L. K. Kuznetsova, A. V. Shljakhtin, I. E. Nifant’ev, and V. G. Kulichikhin, RF Patent No. 2541473 (2015).
M. L. Nelson and R. T. O’Connor, J. Appl. Polym. Sci. 8, 1311 (1964).
M. L. Nelson and R. T. O’Connor, J. Appl. Polym. Sci. 8, 1325 (1964).
V. G. Kulichikhin, L. K. Golova, Yu. A. Egorov, M. I. Vinogradov, K. V. Zuev, M. V. Azanov, L. R. D’yachenko, D. V. Shul’zhenko, and I. Yu. Bessonova, RF Patent No. 2787619 (2022).
V. G. Kulichikhin, L. K. Golova, K. V. Zuev, A. A. Shabeko, M. V. Azanov, L. R. D’yachenko, D. V. Shul’zhenko, and I. Yu. Bessonova, RF Patent No. 214665 (2022).
V. G. Kulichikhin, L. K. Golova, I. S. Makarov, G. N. Bondarenko, V. V. Makarova, S. O. Ilyin, I. U. Skvortsov, and A. K. Berkovich, Eur. Polym. J. 92, 326 (2017).
L. K. Golova, I. S. Makarov, M. I. Vinogradov, L. K. Kuznetsova, and V. G. Kulichikhin, Polym. Sci., Ser. A 60 (6), 756 (2018).
L. K. Golova, G. N. Bondarenko, I. S. Makarov, L. K. Kuznetsova, M. I. Vinogradov, and V. G. Kulichikhin, Polym. Sci., Ser. A 62 (6), 597 (2020).
M. I. Vinogradov, I. S. Makarov, L. K. Golova, G. N. Bondarenko, and V. G. Kulichikhin, Polym. Sci., Ser. A 65 (3), 280 (2023).
M. I. Vinogradov, L. K. Golova, I. S. Makarov, G. N. Bondarenko, I. S. Levin, N. A. Arkharova, and V. G. Kulichikhin, Materials 16, 5843 (2023).
W. Gindl and J. Keckes, Polymer 46, 10221 (2005).
ACKNOWLEDGMENTS
S.Z. Rogovina and S.N. Zelenetskii contributed much to successful development of the study.
Funding
This study was performed in the scope of the State Task to Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors declare that they have no conflicts of interest.
Additional information
Translated by E. Karpushkin
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Golova, L.K., Bondarenko, G.N., Makarov, I.S. et al. Mechanochemical Solid-Phase Dissolution of Cellulose and Synthetic Polymers in N-Methylmorpholine N-oxide and Its Use in Fiber Spinning. Polym. Sci. Ser. C (2024). https://doi.org/10.1134/S1811238224600113
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1811238224600113