Abstract
Using key publications, the review analyzes fundamental methods for synthesizing polymer biocides of varying chemical structure and discusses the mechanism behind the effect of the described formulations and their areas of application. High molecular weight compounds with inherent biocidal activity and opportunities for modification of polymers through chemical transformations or introduction of organic or inorganic additives are considered. The key focus of this review is on the analysis of approaches to the production of antibacterial coatings. Particular attention is given to the prospects of using cationic polyelectrolytes and metallopolymer compounds to which the resistance of pathogenic bacteria is not developed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
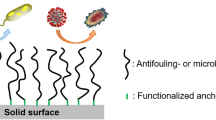
Despite a large amount of known drastic antibiotics and other antibacterial and fungicidal agents, bacterial and fungal infections remain a serious problem for medicine, the food industry, agriculture, maritime transport, and other fields. At present, high priority is given to combating microbiological surface contamination. Antimicrobial (biocidal) properties are imparted to the surface using methods differing in the technology of coating deposition and the efficacy of its biocidal effect.
As evidenced by many studies, treatment of the surface with low molecular weight biocides is ineffective: these coatings are brittle films having a weak adhesion to the surface under treatment and/or easily washed with water; therefore, the technology of multiple biocide deposition is required. The use of polymer materials [1–5] opens wide opportunities for obtaining strong and durable coatings. According to [6], biocidal polymers may be classified as follows:
(1) polymers including bound biocidal organic compounds;
(2) polymers acquiring biocidal activity during their chemical modification;
(3) polymers with inherent biocidal activity;
(4) polymers containing biocidal inorganic additives;
(5) polymer nanocomposites.
This classification is not strict; there are examples of biocidal polymer formulations that can be assigned to several sections of the above classification.
This work will analyze the published data covering biocidal polymers (polymer formulations) mostly in terms of criteria proposed by the authors of [6].
BIOCIDAL MATERIALS BASED ON POLYMER COMPOSITES
This section addresses the characteristic examples of polymer structures obtained by modification of the polymer matrix with biocidal compounds of different chemical nature. The modifying compounds strongly bind to the polymer matrix and exhibit their biological properties being included in the composition of the multicomponent material (composite).
The authors of [7] described formation of a synthetic polymer fiber from waste and secondary raw material of plastics (polypropylene and polystyrene); copper sulfide or silver chloride was simultaneously fed to nozzles. The powders of inorganic salts were introduced into a polymer fiber at the stage of liquid melt solidification. A similar process may also be implemented in the production of film materials or finished injection molded products.
A coating based on the copolymers of styrene and acrylates containing additives of triclosan, an antibacterial and antifungal agent, inhibited the growth of Enterococcus faecalis; as a result, the obtained formulation was recommended for formation of an antimicrobial layer on packaging materials [8].
Aluminosilicate particles were immobilized in the hydroxyethyl cellulose matrix, and the resultant composite was used for the manufacture of polymer film materials [9]; the latter exhibited a fungistatic effect with respect to the Candida fungi.
Nanoparticles based on quaternized polyethylenimine [10] incorporated into medical materials showed antibacterial activity against streptococcus mutants. The highest activity was exhibited by octyl-alkylated polyethylenimine, which fully inhibited the growth of S. mutans in samples three months old.
In the above coatings, the biocidal component cannot migrate along the polymer matrix. In polymer composites with the “immobile” biocide, only biologically active additives distributed on the external surface of the deposited material are active. An increase in the fraction of the incorporated biocide is frequently accompanied by worsening of the physicomechanical properties of the composite polymer coating. This forces us to search for other methods for immobilizing biocidal additives in the polymer matrix which are not so sensitive to the amount of the immobilized material.
POLYMER DONORS OF BIOCIDES
Polymer composites with the “mobile” biocide can gradually evolve biologically active compounds into the environment. These may be composites in which a biocidal substance is randomly distributed in a chemically inert polymer matrix or initially chemically bonded to a polymer but in the course of time is released (split off) under the impact of chemical or physical stimuli.
These polymer biocide donors are described in many reviews and papers, for example, review [11] and original works [12–16]. The role of antimicrobial additives is played by various biocidal agents, such as antibiotics, benzalkonium chlorides, cetylpyridinium chloride, aldehydes, anilides, diamidines, chlorhexidine, triclosan, and N-galamines, as well as silver and copper ions and nanoparticles (metallopolymer compounds are discussed in detail in section Cationic Polymer Biocides). Polymer materials described in these studies were used to form coatings capable of gradually release of biocidal agents into the environment.
The results of impregnation of urinary catheters with several antimicrobial activity agents, such as rifampicin, sparfloxacin, and triclosan, were described in [17]. Antimicrobial catheters prevented the colonization by common uropathogens Proteus mirabilis, Staphylococcus aureus, and Escherichia coli for 7–12 weeks in vitro compared with 1–3 days for commercially available antimicrobial catheters.
For combating Pseudomonas biofilms, the authors of [18] proposed to use the combination of a synthetic polymer containing primary amines, oligo(ethylene glycol) moieties, and hydrophobic groups and an essential oil (carvacrol or evgenol). The latter played the role of an antimicrobial agent that ensured the death of more than 99% of bacteria.
It was reported [19, 20] that N-bromo-hydantoin and N-bromo-5,5'-dimethylhydantoin chemically bonded to polystyrene granules are promising disinfectants with a broad-spectrum antimicrobial activity owing to a gradual release of a strong oxidizer halogen in the surrounding aqueous solution. All the tested materials demonstrated well-defined antimicrobial activity against Escherichia coli and bacteriophages MS2. These results indicate the antimicrobial potential of halogenated cyclic molecules as water disinfectants.
The hydrolytic release of 5-chloro-8-hydroxyquinoline from polymers containing 5-chloro-8-quinolinyl acrylate provides a noticeable antimicrobial activity of the samples [21].
The authors of [22] investigated the activity of a mixture of low molecular weight biocide, benzalkonium chloride, and acrylic (or methacrylic) acid against gram-positive bacteria E. coli, gram-negative bacteria S. aureus, and fungi C. albicans. The polymerization of the mixture was accompanied by a decrease in its antimicrobial activity, which was explained by a reduction in the rate of migration of the active component during solidification of the system (formation of the polymer film)].
Antibacterial films based on copolymers containing a modified antibiotic, ampicillin [23], showed a strong adhesion to stainless steel. Such films may be used for the treatment of medical instruments and devices; the antimicrobial effect of films is related to the occurrence of hydrolytic reactions in the polymer which lead to the controlled release of the antimicrobial agent. It was shown that polymer biocides demonstrate a high activity against gram-positive bacteria Staphylococcus aureus.
Sometimes the antibacterial effect is also exhibited by polymers (films) in the absence of special biologically active additives. For example, this property is typical of films/products produced using melamine-formaldehyde resin [24]; the latter, among other applications, is used as a binder in the manufacture of wood chipboards. The biocidal effect of wood chipboards is associated with the degradation of the resin and the emission of the formaldehyde being formed. However, a high toxicity (carcinogenicity) of formaldehyde makes the melamine-formaldehyde resin inapplicable for use as a polymer biocide donor.
FUNCTIONALIZED POLYMER BIOCIDES
Biocidal properties are also manifested by some polymers whose macromolecules contain active functional groups, for example, hydroxyl, phenyl, and phosphonium.
As a biocidal component, the authors of [25] used ortho-, meta-, and para-nitro-substituted phenylaminomaleimides synthesized from maleic anhydride and nitro-substituted phenyl hydrazine [25]. The antifungal activity of homopolymers and copolymers with methyl methacrylate was higher compared with traditional antifungal agents.
The biocidal behavior was exhibited by the polymer material synthesized from para-hydroxyphenyl acrylate [26]. Biocidal polymers were synthesized by the copolymerization of N-isopropylacrylamide and methacryloyloxyethyltrialkylphosphonium chlorides with different length of the alkyl substituent of alkyl [27]; the antibacterial activity was enhanced with lengthening of the alkyl substituent and an increase in the fraction of phosphonium groups in the copolymer.
An interesting example of synthesizing biocidal polymer directly in the bulk of the modified sample wаs described in [28, 29]. The authors synthesized six types of acrylate monomers with covalently bonded biologically active moieties (pentachlorophenolyl acrylate, 8-hydroxyquinolyl acrylate). Wood samples (outer young layers of southern pine trunks) were treated with solutions of monomers and а crosslinking agent and polymerized in situ. The polymer-modified samples were resistant against brown rot fungus Gloeophyllum trabeum.
Since the end of the 1980s, preparations based on cationic poly(hexamethylene guanidine) have been used as detergents and anticorrosive disinfectants [30]. These preparations combine the properties of biocides and flocculants and are often used for the treatment of wood and modification of composite materials [31]. The antimicrobial activity and selectivity of functionalized polyguanidine with respect to multidrug resistant Klebsiella pneumoniae was reported in [32]. Slight changes in the hydrophobicity of the polymer decreased its toxicity in vivo owing to self-assembly at high concentrations and at the same time increased the antimicrobial activity. The authors believe that the functionalized polyguanidine shows promise for the in vivo treatment of lung infection caused by Klebsiella pneumoniae.
CATIONIC LOW MOLECULAR WEIGHT BIOCIDES
The disinfecting properties of low molecular weight quaternary ammonium bases, for example, alkylbenzyldimethylammonium chloride (better known as benzalkonium chloride) and cetyltrimethylammonium chloride (centrimonium chloride), are well known. Their antimicrobial activity is a function of N-alkyl chain length and, hence, lipophilicity. Compounds with a chain length of 12–14 methylene groups (n = 12–14) provide the optimum antibacterial activity against gram-positive bacteria, while compounds with n = 14–16 ensure the optimum antibacterial activity against gram-negative bacteria.
Quaternary ammonium compounds compare favorably with traditional disinfectants (sodium hypochlorite, 3-aminopropyl, chlorhexidine) by good solubility in water and high stability and have no damaging effect on the surfaces under treatment. They contain no components aggressive to medical materials and no toxic compounds (e.g., aldehydes and phenols) and have no pungent orders [33].
The development of this direction made it possible to markedly expand the spectrum of antimicrobial compounds; several examples are presented below.
Three commercially available disinfectants based on low molecular weight quaternary ammonium compounds were tested on various hospital bacterium strains (Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae) [34]. Tests showed a higher activity of quaternary ammonium compounds against gram-positive bacteria compared with gram-negative ones.
A series of 24 new quaternary ammonium compounds containing oxadiazole and thiazole heterocycles and hydroxyalkyl substituents was described in [35]. Six compounds demonstrated a well-defined antimicrobial activity against common pathogens, including S. aureus, E. coli, P. aeruginosa, and Canidia albicans, combined with a low cytotoxicity against human cells. The antibacterial mechanism behind the effect of quaternary ammonium compounds consists in their binding to the cellular wall of bacteria followed by wall “piercing” and bacterial cytoplasm leakage.
The authors of [36] synthesized 43 quaternary ammonium compounds with different length of alkyl chains and estimated their antimicrobial activity. The crucial factor for high activity is the lipophilicity of quaternary ammonium compounds: their antimicrobial activity increases with an increase in the length of the alkyl chain and a decrease in the content of oxygen atoms in their molecules.
The synthesis of 36 quaternary ammonium compounds, each of which contained two cationic groups, was reported in [37]. These compounds demonstrated high activity against gram-positive and gram-negative bacteria, including Staphylococcus aureus resistant to antibiotic methicillin. At the same time, no clear correlation was found between the geometry of a linker between cationic groups and the antimicrobial activity of compounds.
The mechanism behind the effect of quaternary ammonium compounds was discussed in the review [38]. The main pathway for the emergence of antimicrobial activity by quaternary ammonium compounds is their incorporation into a cellular membrane which initiates the lysis of cells (see also [35]). However, recent studies revealed that, among bacterial genes, there are genes (usually called “quc” genes) which code the displacement of low molecular weight quaternary ammonium compounds from bacterial cells. “Quc” genes can be transferred horizontally through plasmids to other bacteria and are frequently transferred together with other antibiotic-resistant genes. These processes promote, firstly, a decrease in the concentration of quaternary ammonium compounds within cells and, secondly, the survival of bacteria resistant to quaternary ammonium compounds. Eventually, this leads to a noticeable weakening of the antimicrobial effect of quaternary ammonium compounds. These results force us to take a fresh look at possible strategies for enhancing the antimicrobial effect of quaternary ammonium compounds.
CATIONIC POLYMER BIOCIDES
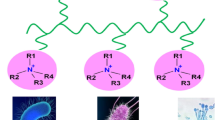
One of the most promising approaches to the manufacture of biocidal films/coatings involves the synthesis of polymers with cationic groups. Cationic polymers when dissolved in water bind to the negatively charged surface and initiate a number of processes [39] which eventually cause a serious impairment of cell function or its death. Upon application of the aqueous solution of a polymer on the surface and subsequent drying, a film with well-defined bactericidal properties is formed [40]. Polymers containing quaternary ammonium groups successfully combine the biological activity of low molecular weight quaternary ammonium compounds and the physicomechanical properties of high molecular weight compounds.
In [41], a cationic polymer was synthesized using spherical microballs with a narrow particle size distribution which were composed of a mixture of two polymers, poly(4-vinylpyridine) and poly(vinylidene fluoride). The quaternization of pyridinium groups by alkyl bromides containing 4–10 carbon atoms in the alkyl radical resulted in the production of cationic microspheres with antibacterial and antifungal properties, as evidenced by experiments with E. coli and A. niger.
The functionalization of polymer materials with bactericidal cationic groups was described in [42]. The procedure included the grafting of 4-vinylpyridine onto a polymer film followed by the quaternization of pyridine groups with hexyl bromide. Pyridinium groups formed on the surface of the film showed the antibacterial activity against E. coli. The maximum activity was achieved at a surface concentration of pyridinium groups of 15 nmol/cm2.
The antimicrobial activity of silicone rubber coated with covalently bonded 3-(trimethoxysilyl)propyldimethyloctadecylammonium chloride was studied in vitro and in vivo in [43]. The as-formed coating decreased the viability of adhesive gram-positive bacteria S. aureus and S. epidermidis to 0% and gram-negative bacteria E. coli and P. aeruginosa to 25%. Here, the presence of plasma proteins insignificantly influenced the activity of the coating.
The authors of [44] synthesized copolymers [3-(methacryloylamino)propyl]-trimethylammonium chloride) and 3-trimethylsilylpropyl methacrylate with cationic groups which were used to obtain antimicrobial coatings, as shown by experiments with bacteria S. aureus and E. coli and fungi C. albicans. The tested copolymers were less toxic to human cells than the commercial low molecular weight antimicrobial agent dimethyloctadecyl[3-(trimethoxysilyl)propyl]аmmonium chloride.
Antimicrobial properties can be imparted to the existing polymer. In [45], gelatin was modified with epoxy organosilicon salt containing quaternary ammonium so that the gelatin skeleton was bonded to two types of groups, silyl and quaternary ammonium. The resulting copolymer demonstrated bactericidal behavior with respect to gram-positive and gram-negative bacteria but had no fungicidal effect on mold.
One of the most common cationic polymers is poly(N,N-diallyl-N,N-dimethylammonium chloride) (PDADMAC) containing quaternary groups. Using this polymer, disinfectant Septol, effective against gram-positive and gram-negative bacteria and fungi, was developed and registered [46]. The disinfectant Septol is noncarcinogenic, does not cause allergy, and possesses no chronic toxicity.
The introduction of other comonomers into a polymer chain makes it possible to tune the properties of the final copolymer, specifically, its adhesive behavior. Simultaneously, the price of the copolymer changes, since the monomer (DADMAC) is the most expensive component of the system. The list of the used comonomers includes acrylamide [47–50], vinyl acetate [51], acrylonitrile [47, 52], (meth)acryloylethyltrimethylammonium chloride [53], dimethylaminoethyl methacrylate [54], N,N-dimethylacrylamide [55], maleic acid [56], and carboxybetaine diallylmethylammonium acetate [57]. For a number of copolymers, the biological (biocidal) activity was tested.
A copolymer consisting of two types of monomers (DADMAC + monoethanolamine vinyl ester) inhibited the growth of both gram-positive (S. aureus) and gram-negative (E. coli) bacteria [58] and demonstrated bactericidal properties against sulfate-reducing bacteria [58].
The antimicrobial activity of free PDADMAC and PDADMAC immobilized in poly(methyl methacrylate) nanoparticles was investigated in [59]. The activity of the free polymer was higher compared with the immobilized one, which was apparently associated with the limited mobility of the polymer upon its binding to nanoparticles.
Since the 2000s, approaches have been under development for the modification of surfaces of various types (plastics, glass, textile) with quaternized polyethylenimine to impart constant microbiocidicity and virucidicity to them [60–62]. These studies were motivated by the need to design active antibacterial food packaging. This direction included studying the possibility of grafting polycations to surfaces and the addition of quaternized PEI in water-insoluble dyes. The resulting materials were effective against various pathogenic bacteria and fungi. The data on the microbiological properties of quaternized PEI are summarized in the review [63]. This polymer is distingusihed by a high level of the antimicrobial activity and the absence of toxicity for mammalian cells and, at the same time, has no negative effect on the structure and mechanical properties of the materials under treatment. These indicators make quaternized PEI an attractive additive for the modification of surfaces of various nature.
Antimicrobial monomers based on quaternized pyridine, phosphocholine, and methacrylic acid quaternary derivatives and the corresponding polymers are reviewed in [64]. A high antimicrobial activity of monomer/polymers is observed, and many examples of their use for obtaining biocidal polymer composites are presented.
The authors of [65] synthesized ionenes, polymers carrying a quaternized nitrogen atom in the main chain, and estimated their biocidal properties. According to [65], ionenes are active against pathogens (E. coli, S. aureus, and C. albicans). Here, the decisive contribution to antimicrobial activity is made by the topology (para/meta isomerism) and flexibility of polymers which determine the possibility to “adjust” macromolecules for the attacked bacterium.
Dendrimers, symmetric treelike macromolecules with regular branches, were used as antimicrobial polymers. Polypropylenimine dendrimers functionalized by quaternary ammonium were described in [66]. The antimicrobial properties of dendrimers were improved with an increase in the molecular weight of the polymer. Dendrimers with bromide anions possessed a higher activity compared with dendrimers in which a chloride anion was used as a counterion. Cationic hyperbranched polymers with an uncontrolled number and length of branches demonstrated a lower antimicrobial activity than dendrimers of the same chemical composition.
The authors of [67] proposed a nontraditional approach to the synthesis of biocidal structures through modification of the surface of lipid bilayer vesicles (liposomes) with quaternized ammonium derivative (quaternary ammonium compound). The modified liposomes suppressed the adhesion of bacteria E. coli and the formation of biofilms and simultaneously decreased the toxicity of the used quaternary ammonium compounds.
The properties of films obtained from homopolymer poly(diethylaminoethyl methacrylate) and copolymer poly(diethylaminoethyl methacrylate-vinylbenzyl chloride) were compared in [68]. The quaternization of the homopolymer and copolymer gave antimicrobial properties against gram-negative and gram-positive bacteria to films. The adhesion and durability of the copolymer films were higher.
The surface of polyurethane modified with the copolymer containing units of N-vinylpyrrolidone and quaternary ammonium salt [69] acquired antimicrobial properties which made it possible to reduce the fraction of surviving bacteria to 40%. The antimicrobial activity was enhanced with an increase in the length of the alkyl chain in the ammonium unit.
When operating under certain special conditions (work in hospitals and rehabilitation centers), it is recommended to treat the wood surface with antimicrobial preparations. In [70], the antimicrobial properties of wood were improved by grafting 2-(dimethylamino)ethyl methacrylate, its polymerization, and quaternization by alkyl halide. E. coli tests showed that the bactericidal effect of the modified wood is higher compared with the wood treated with monomer 2‑(dimethylamino)ethyl methacrylate.
The biocidal effect of cationic polymers is commonly associated with their ability to destroy the cellular wall of bacteria. This mechanism is typical of polymers bearing quaternary ammonium groups [71]. Polymers with guanidine groups can penetrate microbial cells and interact with cytosolic components [72].
A pronounced biocidal effect is observed for by polymers containing phosphonium quaternary salts (e.g., review [73]). The mechanism behind the biocidal effect of these polymers is close to that of the polymers with quaternary ammonium salts. It consists in the binding of a positively charged polymer to a negatively charged cell membrane followed by destruction of the cellular membrane and leakage of cytosol (cell fluid).
In conclusion, let us mention the modification of cationic polymers by their binding to anionic polymers; the final products were called polycomplexes [74]. In [75], a low molecular weight polyguanidine was used as a cationic polymer and carboxymethyl cellulose functioned as an anionic polymer. The antimicrobial activity of guanidine was preserved upon its binding to the polyanion. The antimicrobial activity of chitosan and water-soluble interpolyelectrolyte complexes poly(acrylic acid)–chitosan [76] against P. aeruginosa and P. oleovorans was studied. Interpolyelectrolyte complexes Spec-2 were more effective than chitosans. This can be explained by the activity of amino groups of chitosan and carboxylic acid groups of poly(acrylic acid).
BIOCIDAL MATERIALS BASED ON METALLOPOLYMER COMPOUNDS
Silver, copper, and their compounds are the most important components of biocidal materials; since the beginning of time, they have been used to treat bactericidal infections [77]. Silver compounds are toxins for germs, because metal ions interact with phosphorus-containing and sulfur-containing compounds of vital enzymes and inactivate them [78–82]. Another important damaging factor is the generation of active forms of oxygen under the effect of silver ions [78, 81, 83, 84]. Copper compounds also demonstrate well-defined fungicidal and antibacterial effects [85–88]. With the advent of antibiotics, the use of compounds of these metals decreased; however, many pathogenic bacteria can develop resistance to various antibiotics. Owing to the development of methods for the synthesis of metal nanoparticles, the return of silver compounds as antibacterial agents became urgent, since in the case of metallopolymer nanocomposites the effect of bacteria adaptation was absent [14, 85, 89–91]. In recent years, studies on the possibility of using nanotechnologies to fight biofilms of bacteria resistant to antibiotics have been developed extensively [92].
Despite a pronounced biocidal effect, silver ions have a limited application as antimicrobial agents owing to their rapid binding or inactivation by various compounds present in a medium. This limitation can be overcome by using silver nanoparticles as an antimicrobial agent owing to the continuous release of silver ions provided by nanoparticles [93, 94]. The binding of metal ions to functional groups of metallopolymer complexes also ensures their controlled sorption and prolonged release [95, 96]. Interpolyelectrolyte complexes (IPECs) may contain a relatively large amount of metal ions (up to 50 wt %) which determines broad possibilities offered by their use for the synthesis of metallopolymer complexes [95–97].
The design of antibacterial coatings is a modern strategy for preventing bacterial colonization and formation of biofilms. Polymer composite coatings were obtained by the aerosol-assisted plasma deposition from hexamethyldisiloxane solution containing silver nitrate under atmospheric pressure on the surface of PET films [98]. Nanocapsules containing silver in the core and a polymer in the shell were produced. Testing of capsule-containing coatings with the controlled release of Ag+ ions revealed their high antibacterial activity (E. coli and S. aureus). Attempts were also made to introduce metal ions into water-soluble dyes to impart biocidal properties to surfaces to be painted [91]. Examples illustrating the development of antibacterial agents containing copper and silver compounds are also discussed in the section Biocidal Materials Based on Polymer Composites.
Silver nanoparticles are generally more effective that silver ions [82–84, 90, 91] owing to the combination of effects related to the release of Ag+ ions from nanoparticles and the direct interaction of nanoparticles with cellular membranes [81, 83, 89, 94, 99–102]. Nanoparticles not only interact with the membrane surface but also can penetrate inside bacteria [82, 83, 90, 101], because compared with metal ions nanoparticles they more easily pass through biological barriers and cellular membranes. From the point of view of designing biocidal materials, it is important to take into account that the structure of the polymer matrix, which serves as a coating of nanoparticles, strongly influences the absorption of silver nanoparticles by cells [103–105]. The shape and sizes of nanoparticles largely determine the efficiency of their absorption by cells [83, 89, 103, 106, 107]. The assembly of nanoparticles by the reduction of metal ions is used most frequently for the synthesis of metal nanostructures in polymer systems, because this method makes it possible to finely tune their sizes under varied synthetic conditions [108–111]. The synthesis of nanoparticles under conditions of different interaction of functional groups of macromolecules is an alternative way to control the sizes of nanoparticles [110–114]. Publications of the past two decades show that soluble metallopolymer nanocomposites can be synthesized and subsequently deposited on the surface [89, 91, 115–119]. However, there are also illustrative examples which indicate that approaches to the synthesis of biocide nanoparticles immediately in polymer films and coatings are developed [86, 87, 97, 120–125].
Considerable attention is given to the opportunity of using natural polymers (such as glucose, starch, and chitosan) for the synthesis of nanocomposites [89, 115–118], since natural polysaccharides can act as a “green” stabilizing agent for ultradisperse particles. In AgNO3 aqueous solutions containing glucose and starch, hybrid materials with silver nanoparticles were obtained which could be integrated in medical applications [89]. In this case, glucose functioned as a reducer and starch played the role of the stabilizing matrix.
In past decades, polymer systems based on chitosan were widely applied for the synthesis of metallopolymer nanocomposites because the use of matrices based on this polymer with the inherent antibacterial activity leads to a synergistic biocidal effect for nanocomposites [118]. Moreover, silver nanoparticles coated with this polysaccharide provide more effective damage of DNA and cause the apoptosis of cells [104]. It was found that polymer silver nanoparticles coated with quaternized chitosan and tested as biocides against Bacillus subtilis manifest a higher antimicrobial activity against Bacillus subtilis compared with silver nanoparticles coated with the reference antimicrobial polyvinylpyrrolidone [105]. It was shown that the samples break the respiratory chain of bacterial cells and the cellular wall and disrupt the function of cellular membranes.
Nanocomposites silver–chitosan can be synthesized according to ecological approaches using a chitosan suspension as a stabilizer and reducer in the absence of other chemical compounds [115]. To prepare pure nanocomposites, silver nanoparticles with sizes of 7–30 nm were obtained under exposure to γ radiation under conditions available for production (in the presence of air oxygen) using chitosan as a stabilizer [117]. The resulting silver nanoparticles were stable for more than 3 months and exhibited antimicrobial activity against colibacillus and Staphylococcus aureus. Nanocomposites can be used in antimicrobial materials, including antimicrobial food packaging. It was also shown that stable copper nanoparticles with antibacterial activity against gram-negative and gram-positive bacteria can be synthesized in chitosan solutions [126].
In addition, great opportunities are used for the development of biocidal formulations from synthetic polymers [91, 119–121, 125, 127, 128].
Considerable attention is paid to biocidal and fungicidal materials manufactured by introducing silver nanoparticles into polymer-based water-soluble dyes which can be applied on various surfaces. Wall paint based on nanosilver prevented the formation of mold inside buildings and the growth of algae on outer walls [129]. Minimum inhibitory concentration tests quantitatively showed that Ag nanoparticles are more effective than Ag+ ions against representatives of gram-positive/gram-negative bacteria and saprotrophic fungi [91]. Antifungal/antibacterial effects against Aspergillus niger, Penicillium phoeniceum, and Staphylococcus aureus on the surface of cotton fabric in a water-soluble dye were confirmed; the growth of Bacillus subtilis and Escherichia coli was also suppressed.
Polyvinyltriazole is a nontoxic polymer matrix. Using polyvinyltriazole and its macromolecular complexes, materials containing silver and copper nanoparticles were obtained by chemical or radiation-chemical reduction [119, 121]. It was shown that composites with silver nanoparticles exhibit biocidal activity against S. aureus and E. сoli strains.
To fight biofilm infections, micellar particles of Soluplus® (copolymer polyvinylcaprolactam–poly(vinyl acetate)–poly(ethylene glycol)) containing silver nanoparticles were synthesized and showed high efficiency against Staphylococcus epidermidis strains [130].
There are many studies addressing the synthesis of antibacterial materials in matrices of polymer fibers, tissues, coatings, and films. Silver nanoparticles were synthesized in autoclaves by reducing ions under hot steaming conditions in polyacrylonitrile fibers [131]. Antibacterial materials showed a high efficiency (99%) against Escherichia coli and Staphylococcus aureus bacteria after 20-fold wash tests. Silver nanoparticles were synthesized in propylamine-substituted poly(vinyl alcohol) films using chemical and green methods (through the reduction of ions by starch). The composite material synthesized in [132] demonstrated a high antibacterial activity and excellent mechanical characteristics. According to [132], this material is promising for use as coatings and medical plasters.
IPECs and interpolymer complexes are commonly used for the manufacture of films and coatings from composites with copper and silver nanoparticles since they provide a good opportunity to control the interaction of functional groups of polyanions and polycations with the surfaces of metal nanoparticles and the ability to efficiently stabilize nanoparticles [97, 108, 122, 133, 134].
Composites with silver nanoparticles were prepared in the films of IPECs based on synthetic polymers (PAA-PEI) [108, 120, 124, 135] using radiation-induced reduction of silver ions. In complexes of natural polymers with different combinations of polycations (chitosan, cationic starch, cationic beta-cyclodextrin) and polyanions (pectin, carboxymethyl cellulose, anionic starch), metallopolymer nanocomposites were obtained by the thermochemical reduction of silver ions or reduction by ascorbic acid [122]. For nanoparticles in pectin–polyethylenimine films, silver ions were reduced using ascorbic acid, hydrazine, or sodium borohydride [123]. Nanocomposites with a smaller average size of nanoparticles possessed a higher antimicrobial activity against S. aureus and E. coli strains. Testing of the materials based on interpolyelectrolyte complexes of pectin and polyethylenimine and copper nanoparticles revealed their high antimicrobial activity against S. aureus and E. coli strains [86, 87].
The localization of nanoparticles on the surface of the matrix ensures the accessibility of metal nanostructures for reagents or detectable compounds. This result shows promise for the creation of antibacterial water purification systems and biocidal materials. From this point of view, the development of approaches to the synthesis of structures in which metal nanoparticles are localized near the surface of polymer films is of fundamental interest. The use of sodium borohydride as a reducing agent, which cannot penetrate deep into a matrix owing to electrostatic repulsion, provided conditions for the predominant formation of silver nanoparticles near the surface of an ion-exchange polymer gel containing sulfo groups (ion-exchange resin Purolite C100E [136]). Composites with the localization of copper nanoparticles on the surface of interpolymer films were obtained by exposure of interpolyelectrolyte complexes PAA–PEI–Cu2+ and interpolymer complexes poly(1-vinyl-1,2,4-triazole)-PAA–Cu2+ to X-ray radiation [120, 121]. A contrast between absorption of X-ray radiation by a water-alcohol medium and interpolymer complexes with copper ions ensures favorable conditions for the formation of metal nanostructures in the near-surface layer of films.
The alternate adsorption of polycations and polyanions is a commonly used method for obtaining IPEC ultrathin coatings. Various combinations of synthetic and natural macromolecules of polyanions (poly(acrylic acid), pectin, poly(styrenesulfonic acid), hyaluronic acid, humic acid) and polycations (polyethylenimine, poly(allylamine hydrochloride), poly-diallyldimethylammonium chloride, chitosan) were used for the synthesis of metallopolymer complexes and nanocomposites in IPEC matrices [86, 97, 122, 124, 125, 134, 137–141]. However, the properties of the interpolymer complex PAA–PEI that can form tertiary metallopolymer complexes with a high content of metal ions were studied in the most detail. The reduction of metal ions by chemical and radiation-chemical methods made it possible to obtain interpolyelectrolyte coatings containing copper and silver nanoparticles [120, 124, 125]. The testing of coatings containing silver nanoparticles demonstrated their antibacterial properties against E. coli and S. Auerus. These properties were preserved [124] after a fivefold wash cycle.
CONCLUSIONS
The analysis of the published data showed that polymer systems offer great potential for the manufacture of biocidal materials of various types. Using high molecular weight compounds, not only soluble antibacterial agents but also biocidal films and coatings can be synthesized. Formulations based on nontoxic synthetic or natural polymers remove many constraints on the development of materials for food production, transport, and storage. In recent years, increasing attention has been focused on the manufacture of biocidal coatings to combat bacterial films, because most microorganisms occur in the form of organized communities.
Approaches directed at both the synthesis of structures with inherent biocidal activity and the possibility of incorporating organic or inorganic antibacterial additives are progressing intensely. Of particular attention are biocidal polymer compounds to which, as opposed to antibiotics, the resistance of pathogenic bacteria is not developed. From this point of view, commercially available PDADMAC and other cationic polymers can serve as a basis for the creation of a family of biocidal polymer formulations. Metallopolymer compounds and nanocomposites are capable of controlled and gradual release of biologically active compounds into the environment and are used for the manufacture of various types of biocidal materials.
At present, most studies are focused on the development of medical preparations. At the same time, much less attention is given to the manufacture of biodical materials for the food industry. For the protection of industrial premises and storage facilities, dyes based on polymers including organic and inorganic biocidal compounds with metal ions and nanoparticles are generally used. Meanwhile, coatings based on polyelectrolytes are a promising basis for the manufacture of biocidal materials, since in many cases they possess inherent bactericidal activity. The functional groups of polyelectrolytes and IPECs effectively bind and controllably release metal ions and low molecular weight biocides and stabilaze inorganic nanoparticles. Thus, polymer materials with ionogenic groups show promise as a basis for the design of long-acting antibacterial systems. In contrast to low molecular weight compounds, the use of polymer matrices opens wide opportunities for tuning adhesion interactions with surfaces of different types in order to produce strong and durable coatings.
REFERENCES
E. R. Kenawy, S.D. Worley, and R. Broughton, Biomacromolecules 8, 1359 (2007).
L. Timofeeva and N. Kleshcheva, Appl. Microbiol. Biotechnol. 89, 475 (2011).
A. Jain, L. S. Duvvuri, S. Farah, N. Beyth, A. J. Domb, and W. Khan, Adv. Healthcare Mater. 3, 1969 (2014).
A. Chen, H. Peng, I. Blakey, and A. K. Whittaker, Polym. Rev. 57, 276 (2017).
T. Wei, Q. Yu, and H. Chen, Adv. Healthcare Mater. 8, 1970007 (2019).
A. Munoz-Bonilla and M. Fernandez-Garcia, Prog. Polym. Sci. 37, 281 (2012).
S. V. Bordunov, O. V. Galtseva, N. M. Natalinova, A. A. Rogachev, and R. Z. Zhang, IOP Conf. Ser.: Mater. Sci. Eng. 168, 012087 (2017).
D. W. Chung, S. E. Papadakis, and K. L. Yam, Int. J. Food Sci. Technol. 38, 165 (2003).
E. V. Garas’ko, A. N. Rodionova, O. V. Alekseeva, N. A. Bagrovskaya, and A. V. Agafonov, Usp. Sovrem. Estestvozn., No. 11, 20 (2015).
I. Yudovin-Farber, N. Beyth, E. I. Weiss, and A. J. Domb, J. Nanopart. Res. 12, 591 (2010).
J.-B. D. Green, T. Fulghum, and M. A. Nordhaus, Chem. Rev. 109, 5437 (2009).
R. G. Penn, in Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance, Ed. by G. E. McDonnell (ASM Press, Washington, DC, 2007), vol. 28, No. 3, p. 369.
A. M. Milstone, C. L. Passaretti, and T. M. Perl, Clin. Infect. Dis. 46, 274 (2008).
M. Rai, A. Yadav, and A. Gade, Biotechnol. Adv. 27, 76 (2009).
D. R. Monteiro, L. F. Gorup, A. S. Takamiya, A. C. Ruvollo, E. R. Camargo, and D. B. Barbosa, Int. J. Antimicrob. Agents 34, 103 (2009).
M. Zilberman and J. J. Elsner, J. Controlled Release 130, 202 (2008).
L. E. Fisher, A. L. Hook, W. Ashraf, A. Yousef, D. A. Barrett, D. J. Scurr, X. Y. Chen, E. F. Smith, M. Fay, C. D. J. Parmenter, R. Parkinson, and R. Bayston, J. Controlled Release 202, 57 (2015).
R. Namivandi-Zangeneh, Y. Yang, S. Xu, E. H. H. Wong, and C. Boyer, Biomacromolecules 21, 262 (2019).
S. Farah, O. Aviv, N. Laout, S. Ratner, and A. J. Domb, J. Controlled Release 216, 18 (2015).
S. Farah, O. Aviv, M. Daif, K. R. Kunduru, N. Laout, S. Ratner, N. Beyth, and A. J. Domb, J. Polym. Sci., Part A: Polym. Chem. 54, 596 (2016).
M. Bankova, N. Manolova, N. Markova, T. Radoucheva, K. Dilova, and I. Rashkov, J. Bioact. Compat. Polym. 12, 294 (1997).
J. Wang, J. Xue, X. Dong, Q. Yu, S. N. Baker, M. Wang, and H. Huang, Int. J. Pharm. 575, 119005 (2020).
A. Prudencio, N. D. Stebbins, M. Johnson, M. J. Song, B. A. Langowski, and K. E. Uhrich, J. Bioact. Compat. Polym. 29, 208 (2014).
A. Kandelbauer and P. Widsten, Prog. Org. Coat. 65, 305 (2009).
N. P. S. Chauhan, J. Chaudhary, P. Chaudhary, and B. L. Hiran, Oxid. Commun. 35, 907 (2012).
J. H. Kim, E.-S. Park, J. H. Shim, M.-N. Kim, W.‑S. Moon, K.-H. Chung, and J.-S. Yoon, J. Agric. Food Chem. 52, 7480 (2004).
T. Nonaka, L. Hua, T. Ogata, and S. Kurihara, J. Appl. Polym. Sci. 87, 386 (2003).
R. E. Ibach and R. M. Rowell, Holzforschung, 55, 358 (2001).
R. E. Ibach and R. M. Rowell, Holzforschung, 55, 365 (2001).
I. I. Vointseva and P. A. Gembitskii, Polyguanidines—Disinfectants and Polyfunctional Additives to Composite Materials (LKM-press, Moscow, 2009) [in Russian].
K. Efimov, P. Gembitskii, and A. Snezhko, Dezinfekts. Delo 4, 32 (2000).
C. Yang, W. Lou, G. Zhong, A. Lee, J. Leong, W. Chin, B. Ding, C. Bao, J. P. K. Tan, and Q. Pu, Acta Biomater. 94, 268 (2019).
J. Stefanska, A. Pietruczuk-Padzik, M. Struga, M. Borkowski, and S. Tyski, Polym. J. Microbiol. 62, 359 (2013).
A. Ramzi, B. Oumokhtar, Yassine Ez zoubi, Touria Filali Mouatassem, Moussa Benboubker, and Abdelhakim El Ouali Lalami, BioMed Research International 2020, 6509740 (2020). https://doi.org/10.1155/2020/6509740
X. Xie, W. Cong, F. Zhao, H. Li, W. Xin, G. Hou, and C. Wang, J. Enzyme Inhib. Med. Chem. 33, 98 (2018).
O. Soukup, M. Benkova, R. Dolezal, R. Sleha, D. Malinak, S. Salajkova, A. Markova, M. Hympanova, L. Prchal, and L. Ryskova, Eur. J. Med. Chem. 206, 112584 (2020).
A. J. Leitgeb, J. A. Feliciano, H. A. Sanchez, R. A. Allen, K. R. Morrison, K. J. Sommers, R. G. Carden, W. M. Wuest, and K. P. C. Minbiole, ChemMedChem 15, 667 (2020).
M. C. Jennings, K. P. C. Minbiole, and W. M. Wuest, ACS Infect. Dis. 1, 288 (2015).
L. Parhamifar, H. Andersen, L. P. Wu, A. Hall, D. Hudzech, and S. M. Moghimi, Nonviral Vectors Gene Ther. 88, 353 (2014).
G. Cardenas, P. Anaya, C. von Plessing, C. Rojas, and J. Sepulveda, J. Mater. Sci.: Mater. Med. 19, 2397 (2008).
F. X. Hu, K. G. Neoh, L. Cen, and E. T. Kang, Biotechnol. Bioeng. 89, 474 (2005).
L. Cen, K. G. Neoh, and E. T. Kang, Langmuir 19, 10295 (2003).
B. Gottenbos, H. C. van der Mei, F. Klatter, P. Nieuwenhuis, and H. J. Busscher, Biomaterials 23, 1417 (2002).
H. R. Li, H. Q. Bao, K. X. Bok, C. Y. Lee, B. Li, M. T. Zin, and L. F. Kang, Biomater. Sci. 4, 299 (2016).
J. Li, Z. Sha, W. Zhang, F. Tao, and P. Yang, J. Biomater. Sci., Polym. Ed. 27, 1017 (2016).
L. Fedorova, I. Tsvirova, V. Misin, and G. Zaidlin, Dezinfekts. Delo 4, 40 (2000).
W. H. Schuller, J. A. Price, S. T. Moore, and W. M. Thomas, J. Chem. Eng. Data 4, 273 (1959).
C. Wandrey, J. Hernández-Barajas, and D. Hunkeler, in Radical Polymerisation Polyelectrolytes, Ed. by I. Capek (Springer, Berlin; Heidelberg, 1999), p. 123.
Y. W. Lian, Z. Li, Z. B. Liu, J. Xie, and K. S. Zhao, Colloids Surf., A 490, 343 (2016).
S. S. Sana, S. K. Arla, V. Badineni, and V. K. N. Boya, SN Appl. Sci. 1, 12 (2019).
S. Janietz, M. Hahn, and W. Jaeger, Acta Polym. 43, 230 (1992).
L. A. Volkov, M. E. Kuntsevich, M. P. Zverev, K. K. Egorov, M. I. Cherkashin, and V. M. Misin, A. S. 1311221.
L. W. Becker and E. H. Larson, US Patent No. 4617362-A (1986).
Y. L. Zhang, L. Xu, M. Yi, M. L. Zhai, J. R. Wang, and H. F. Ha, Eur. Polym. J. 42, 2959 (2006).
K. Z. Abdiyev, Z. Toktarbay, A. Z. Zhenissova, M. B. Zhursumbaeva, and R. N. Kainazarova, Polym. Sci., Ser. B 57, 217 (2015).
A. V. Lezov, G. E. Polushina, A. A. Lezov, P. S. Vlasov, and N. S. Domnina, Polym. Sci., Ser. A 53, 93 (2011).
A. A. Lezov, A. V. Lezova, P. S. Vlasov, S. A. Samokhvalova, V. B. Rogozhin, G. E. Polushina, and N. V. Tsvetkov, J. Polym. Res. 26, 97 (2019).
Y. Dauletov, N. Nuraje, K. Abdiyev, Z. Toktarbay, and M. Zhursumbaeva, J. Surfactants Deterg. 22, 1129 (2019).
L. M. Sanches, D. F. S. Petri, L. D. D. Carrasco, and A. M. Carmona-Ribeiro, J. Nanobiotechnol. 13 (58), 1 (2015).
A. M. Larson and A. M. Klibanov, Annu. Rev. Chem. Biomol. Eng. 4, 171 (2013).
A. M. Klibanov, J. Mater. Chem. 17, 2479 (2007).
H. Liu, I. Elkin, J. Chen, and A. M. Klibanov, Biomacromolecules 16, 351 (2015).
M. Chrószcz and I. Barszczewska-Rybarek, Polymers 12, 2551 (2020).
J. Xue, J. Wang, D. Feng, H. Huang, and M. Wang, Molecules 25, 4738 (2020).
R. J. Kopiasz, W. Tomaszewski, A. Kuźmińska, K. Chreptowiczmn, J. Mierzejewska, T. Ciach, and D. Jańczewski, Macromol. Biosci. 20, 2000063 (2020).
C. Z. Chen, N. C. Beck-Tan, P. Dhurjati, T. K. van Dyk, R. A. LaRossa, and S. L. Cooper, Biomacromolecules 1, 473 (2000).
C. V. Montefusco-Pereira, B. Formicola, A. Goes, F. Re, C. A. Marrano, F. Mantegazza, C. Carvalho-Wodarz, G. Fuhrmann, E. Caneva, and F. Nicotra, Eur. J. Pharm. Biopharm. 149, 12 (2020).
E. Çitak, H. Testici, M. Gürsoy, E. Sevgili, H. T. Dağı, B. Öztürk, and M. Karaman, J. Vac. Sci. Technol., A 38, 043203 (2020).
L. Howard, R. Almousa, and D. Xie, Emergent Mater. 33, 340 (2018).
H. P. Yu, Y. C. Fu, G. Li, and Y. X. Liu, Holzforschung 67, 455 (2013).
J. Oh, S.-J. Kim, M.-K. Oh, and A. Khan, RSC Adv. 10, 26752 (2020).
A. J. Domb, N. Beyth, and S. Farah, MRS Online Proc. Libr. 1569, 97 (2013).
Y. Xue, H. Xiao, and Y. Zhang, Int. J. Mol. Sci. 16, 3626 (2015).
V. A. Izumrudov and A. V. Sybachin, Polym. Sci., Ser. A 48, 1098 (2006).
L. Y. Qian, C. Dong, X. T. Liang, B. H. He, and H. N. Xiao, Holzforschung 68, 103 (2014).
H. Ortega-Ortiz, B. Gutierrez-Rodriguez, G. Cadenas-Pliego, and L. I. Jimenez, Braz. Arch. Biol. Technol. 53, 623 (2010).
H. J. Klasen, Burns 26, 117 (2000).
Y. Matsumura, K. Yoshikata, S.-I. Kunisaki, and T. Tsuchido, Appl. Environ. Microbiol. 69, 4278 (2003).
S. Y. Liau, D. C. Read, W. J. Pugh, J. R. Furr, and A. D. Russell, Lett. Appl. Microbiol. 25, 279 (1997).
A. D. Russell and W. B. Hugo, Prog. Med. Chem. 31, 351 (1994).
C. Volker, M. Oetken, and J. Oehlmann, Rev. Environ. Contam. Toxicol. 223, 81 (2013).
D. McShan, P. C. Ray, and H. T. Yu, J. Food Drug Anal. 22, 116 (2014).
Z. Wang, T. Xia, and S. J. Liu, Nanoscale 7, 7470 (2015).
H. J. Johnston, G. Hutchison, F. M. Christensen, S. Peters, S. Hankin, and V. Stone, Crit. Revs Toxicol. 40, 328 (2010).
C. Buzea, I. I. Pacheco, and K. Robbie, Biointerphases 2 (4), 17 (2007).
V. Demchenko, S. Riabov, N. Rybalchenko, L. Goncharenko, S. Kobylinskyi, and V. Shtompel, Eur. Polym. J. 96, 326 (2017).
V. L. Demchenko, V. I. Shtompel, S. V. Riabov, L. A. Goncharenko, S. M. Kobylinskyi, and M. V. Iurzhenko, Appl. Nanosci. 10, 5479 (2020).
R. D. Glover, J. M. Miller, and J. E. Hutchison, ACS Nano 5, 8950 (2011).
V. K. Sharma, R. A. Yngard, and Y. Lin, Adv. Colloid Interface Sci. 145, 83 (2009).
J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramirez, and M. J. Yacaman, Nanotecnology 16, 2346 (2005).
I. T. Garipov, R. R. Khaydarov, O. U. Gapurova, I. L. Efimova, and S. Y. Evgrafova, J. Sib. Fed. Univ., Biol. 12, 266 (2019).
B. Malaekeh-Nikouei, B. S. F. Bazzaz, E. Mirhadi, A. S. Tajani, and B. Khameneh, J. Drug Delivery Sci. Technol. 60, 15 (2020).
J. S. Kim, E. Kuk, K. N. Yu, J. H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Y. Hwang, Y. K. Kim, Y. S. Lee, D. H. Jeong, and M. H. Cho, Nanomedicine (N. Y., NY, U. S.) 3, 95 (2007).
C. Beer, R. Foldbjerg, Y. Hayashi, D. S. Sutherland, and H. Autrup, Toxicol. Lett. 208, 286 (2012).
A. A. Zezin, Polym. Sci., Ser. A 61, 754 (2019).
A. B. Zezin, S. V. Mikheikin, V. B. Rogacheva, M. F. Zansokhova, A. V. Sybachin, and A. A. Yaroslavov, Adv. Colloid Interface Sci. 226, 17 (2015).
D. V. Pergushov, A. A. Zezin, A. B. Zezin, and A. H. E. Müller, in Polyelectrolyte Complexes in the Dispersed Solid State I, Ed. by M. Müller (Springer-Verlag, Berlin; Heidelberg, 2013), p. 173.
L. Wang, C. Lo Porto, F. Palumbo, M. Modic, U. Cvelbar, R. Ghobeira, N. De Geyter, M. De Vrieze, S. Kos, G. Sersa, C. Leys, and A. Nikiforov, Mater. Sci. Eng., C 119, 11 (2021).
L. Kvitek, A. Panacek, J. Soukupova, M. Kolar, R. Vecerova, R. Prucek, M. Holecova, and R. Zboril, J. Phys. Chem. C 112, 5825 (2008).
D. He, J. J. Dorantes-Aranda, and T. D. Waite, Environ. Sci. Technol. 46, 8731 (2012).
B. Reidy, A. Haase, A. Luch, K.A. Dawson, and I. Lynch, Materials 6, 2295 (2013).
Y. F. Li and C. Y. Chen, Small 7, 2965 (2011).
A. R. Gliga, S. Skoglund, I. O. Wallinder, B. Fadeel, and H. L. Karlsson, Part. Fibre Toxicol. 11 (11), 1 (2014).
M. Ahamed, M. Karns, M. Goodson, J. Rowe, S. M. Hussain, J. J. Schlager, and Y. L. Hong, Toxicol. Appl. Pharmacol. 233, 404 (2008).
J. Wang, M. Sui, Z. Ma, H. Li, and B. Yuan, RSC Adv. 9, 25667 (2019).
W. Liu, Y. Wu, C. Wang, H. C. Li, T. Wang, C. Y. Liao, L. Cui, Q. F. Zhou, B. Yan, and G.B. Jiang, Nanotoxicology 4, 319 (2010).
S. George, S. Lin, Z. Ji, C. R. Thomas, L. Li, M. Mecklenburg, H. Meng, X. Wang, H. Zhang, and T. Xia, ACS Nano 6, 3745 (2012).
A. A. Zezin, Polym. Sci., Ser. C 58, 118 (2016).
J. Belloni, Catal. Today 113, 141 (2006).
Y. Xia, Y. Xiong, B. Lim, and S. E. Skrabalak, Angew. Chem., Int. Ed. Engl. 48, 1 (2009).
Polyelectrolytes and Nanoparticles, Ed. by J. Koetz and S. Kosmella (Springer-Verlag, Berlin; Heidelberg, 2007).
I. M. Papisov and A. A. Litmanovich, Colloids Surf., A 151, 399 (1999).
J. H. Dai and M. L. Bruening, Nano Lett. 2, 497 (2002).
T. C. Wang, M. F. Rubner, and R. E. Cohen, Langmuir 18, 3370 (2002).
Y. K. Twu, Y. W. Chen, and C. M. Shih, Powder Technol. 185, 251 (2008).
H. Z. Huang and X. R. Yang, Carbohydr. Res. 339, 2627 (2004).
R. Yoksan and S. Chirachanchai, Mater. Chem. Phys. 115, 296 (2009).
R. K. Preethika, R. Ramya, M. Ganesan, S. Nagaraj, and K. Pandian, Nanosyst.: Phys., Chem., Math. 7, 759 (2016).
G. F. Prozorova, A. S. Pozdnyakov, N. P. Kuznetsova, S. A. Korzhova, A. I. Emel’yanov, T. G. Ermakova, T. V. Fadeeva, and L. M. Sosedova, Int. J. Nanomed. 9, 1883 (2014).
A. A. Zezin, D. I. Klimov, E. A. Zezina, K. V. Mkrtchyan, and V. I. Feldman, Radiat. Phys. Chem. 169, 108076 (2020).
E. A. Zezina, A. I. Emel’yanov, A. S. Pozdnyakov, G. F. Prozorova, S. S. Abramchuk, V. I. Feldman, and A. A. Zezin, Radiat. Phys. Chem. 158, 115 (2019).
V. Demchenko, S. Riabov, S. Sinelnikov, O. Radchenko, S. Kobylinskyi, and N. Rybalchenko, Carbohydr. Polym. 242, 116431 (2020).
V. Demchenko, S. Riabov, S. Kobylinskyi, L. Goncharenko, N. Rybalchenko, A. Kruk, O. Moskalenko, and M. Shut, Sci. Rep. 10, 7126 (2020).
D. I. Klimov, E. A. Zezina, V. Lipik, S. S. Abramchuk, A. A. Yaroslavov, V. I. Feldman, A. V. Sybachin, V. V. Spiridonov, and A. A. Zezin, Radiat. Phys. Chem. 162, 23 (2019).
J. Dai and M. L. Bruening, Nano Lett. 2, 497 (2002).
A. Manikandan and M. Sathiyabama, J. Nanomed. Nanotechnol. 6, 1 (2015).
H. J. Lee and S. H. Jeong, Text. Res. J. 74, 442 (2004).
H. J. Lee and S. H. Jeong, Text. Res. J. 75, 551 (2005).
R. R. Khaydarov, R. A. Khaydarov, Y. Estrin, S. Evgrafova, T. Scheper, C. Endres, and S. Y. Cho, in Nanomaterials: Risks and Benefits, Ed. by I. Linkov and J. A. Steevens (Springer Nature, Heidelberg, 2009), p. 287.
C. Takahashi, T. Yamada, S. Yagi, T. Murai, and S. Muto, Mater. Sci. Eng. 121, 111718 (2021).
G. Zhang, Y. Xiao, Q. Yin, J. Yan, C. Zang, and H. Zhang, Nanoscale Res. Lett. 16, 36 (2021).
M. Iqbal, H. Zafar, A. Mahmood, M. B. K. Niazi, and M. W. Aslam, Polymers 12, 2112 (2020).
F. H. Schacher, T. Rudolph, M. Drechsler, and A. H. E. Müller, Nanoscale 3, 288 (2011).
A. Bakar, O. Guven, A. A. Zezin, and V. I. Feldman, Radiat. Phys. Chem. 94, 62 (2014).
K. V. Mkrtchyan, A. A. Zezin, E. A. Zezina, S. S. Abramchuk, and I. A. Baranova, Russ. Chem. Bull. 69, 1731 (2020).
J. Bastos-Arrieta, M. Munoz, P. Ruiz, and D. N. Muraviev, Nanoscale Res. Lett. 8, 1 (2013).
M. L. Bruening, D. M. Dotzauer, P. Jain, L. Ouyang, and G. L. Baker, Langmuir 24, 7663 (2008).
G. Liu, D. M. Dotzauer, and M. L. Bruening, J. Membr. Sci. 354, 198 (2010).
I. G. Panova, A. V. Sybachin, V. V. Spiridonov, K. Kydralieva, S. Jorobekova, A. B. Zezin, and A. A. Yaroslavov, Geoderma 307, 91 (2017).
I. Panova, A. Drobyazko, V. Spiridonov, A. Sybachin, K. Kydralieva, S. Jorobekova, and A. Yaroslavov, Land Degrad. Dev. 30, 337 (2019).
J. Macanás, L. Ouyang, M. L. Bruening, M. Muñoz, J. C. Remigy, and J. F. Lahitte, Catal. Today 156, 181 (2010).
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 075-15-2020-775).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Soboleva
This paper was prepared for publication in the thematic issue Polymers and the Environment (Series C).
Rights and permissions
About this article
Cite this article
Misin, V.M., Zezin, A.A., Klimov, D.I. et al. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B 63, 459–469 (2021). https://doi.org/10.1134/S1560090421050079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090421050079