Abstract—
The morphology of particles of aluminum-magnesium montmorillonite of different chemical compositions, synthesized under conditions of stepwise hydrothermal heat treatment of gels in the range from 200 to 300°C is studied. The morphologies of particles synthesized under the conditions of static and stepwise heat treatment are compared. The possibility of obtaining particles with spherical and spongy morphologies with different porosities, depending on the heat treatment conditions and the chemical composition of montmorillonite, is shown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Montmorillonite is a clayey mineral subclass of layered silicates, which has the ability to strongly swell and has pronounced sorption properties [1, 2]. Under conditions of directed hydrothermal synthesis, compounds with the montmorillonite structure of the given chemical composition and with certain porous-textural characteristics can be obtained. Thus, earlier in [3, 4], it was demonstrated that it was possible to synthesize aluminosilicates with the structure of montmorillonite, corresponding to the general chemical formula Na2x(Al2(1–x),Mg2x)Si4O10(OH)2.nH2O (0 < x < 1), with different degrees of isomorphic substitution of magnesium for aluminum in octahedral layers. Montmorillonite was obtained at temperatures ranging from 200 to 350°C, autogenous pressure ranging from 20 to 70 MPa, and duration of synthesis ranging from 5 to 288 h. It was found that the temperature and duration of synthesis have the greatest effect on the crystallization of montmorillonite. Sample particle size in a plane perpendicular to the c axis was 20 ± 3 nm and did not depend on the synthesis conditions or the chemical composition of the samples. Depending on the synthesis conditions, during the static heat treatment of the initial gels, both delaminated samples in the form of nanolayers, in some cases twisting into tubes, and samples with a packet structure were obtained.
The study of the influence of the conditions for the synthesis of layered silicates with the kaolinite structure showed the possibility of obtaining them with different morphologies [5, 6]. Thus, by changing the conditions of the hydrothermal treatment of the initial gels, samples of kaolinite with spherical, tubular, lamellar, and nanospongy morphologies were obtained. Samples with different morphologies differed significantly in terms of their physicochemical properties, sorption capacity, and biological activity [5, 6].
The aim of this study is to study the possibility of obtaining montmorillonite with different particle morphologies. The synthesis was carried out from dried gels of the corresponding compositions. Three compositions of montmorillonite were chosen as the test samples, corresponding to the chemical formula Na2x(Al2(1–x),Mg2x)Si4O10(OH)2.nH2O, differing in the degree of substitution of magnesium in the octahedral layers for aluminum: with x = 1 (Mg3Si4O10(OH)2· nH2O, analogue of natural saponite), x = 0.9 (Na1.8Al0.2Mg1.8Si4O10(OH)2·H2O), and x = 0.5 (Na1.0Al1.0Mg1.0Si4O10(OH)2·H2O)). The hydrothermal treatment of the gels was carried out in an aqueous medium in steel autoclaves with platinum crucibles using a stepwise heat treatment mode: 200°C (24 h)–250°C (24 h)–300°C (24 h).
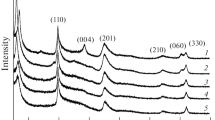
Figure 1 shows the X-ray diffraction patterns of the synthesized samples (X-ray diffractometer D8-Advance, Bruker). Single-phase samples with the structure of montmorillonite were obtained, as indicated by the position of the characteristic reflection peaks hkl 19° (110), 28° (004), 35° (201), 60°–62° ((060), and (330)). The nature of the diffraction patterns indicates changes in the structure of the samples as the degree of substitution of magnesium atoms for aluminum increases and the transition of the dioctramedral structure of the samples (2θ = 60.8°, d = 1.48 Å, (060)) to the trioctrahedral structure (2θ = 62.3°, d = 1.52 Å, (330)).
Figure 2 shows the results of studying the morphology of the synthesized samples by scanning electron microscopy (Carl Zeiss Merlin instrument (Oberkochen, Germany)). For comparison, electron micrographs of the samples synthesized under the conditions of static heat treatment of gels of the corresponding composition at 350°C for 3 days are presented. It follows from the obtained results that the stepwise heat treatment of gels corresponding to the composition of saponite Mg3Si4O10(OH)2·nH2O leads to the formation of a sample with closed pores, which will probably reduce the sorption capacity of the sample. Stepped heat treatment of the gel corresponding to the composition of Na1.8Al0.2Mg1.8Si4O10(OH)2·H2O leads to the formation of both thin layers and spherical particles. The possibility of forming spherical particles for layered silicates with the montmorillonite structure is described for the first time. For samples synthesized from gels of Na1.0Al1.0Mg1.0Si4O10(OH)2·H2O under conditions of stepwise heat treatment, the formation of spongy structures with open porosity is characteristic. The same morphology is also characteristic of montmorillonite of the same composition obtained by static heat treatment. Such structures are likely to have the highest sorption capacity.
Thus, the results of studying the influence of the heat treatment mode of the initial gels on the morphology of montmorillonite particles formed from them showed the possibility of obtaining particles with different morphologies. The particle morphology is determined both by the heat treatment regime and by the chemical composition of montmorillonite.
REFERENCES
Bergaya, F. and Lagaly, G., General introduction: Clays, clay minerals, and clay science, in Developments in Clay Science, Bergaya, F., Benny, K.G., and Theng, G.L., Eds., Amsterdam: Elsevier, 2006, vol. 1, Chap. 1, pp. 1–18.
Vezentsev, A.I., Korol’kova, S.V., and Bukhanov, V.D., Textural characteristics and sorption properties of natural and magnesium-substituted montmorillonite-containing clay, Nauch. Vedom., Ser. Estestv. Nauki, 2010, no. 9 (80), pp. 119–123.
Golubeva, O.Yu., Ul’yanova, N.Yu., Kostyreva, T.G., Drozdova, I.A., and Mokeev, M.V., Synthetic nanoclays with the structure of montmorillonite: Preparation, structure, and physico-chemical properties, Glass Phys. Chem., 2013, vol. 39, no. 5, pp. 533–539.
Golubeva, O.Yu., Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite, Microporous Mesoporous Mater., 2016, vol. 224, pp. 271–276.
Alikina, Yu.A., Kalashnikova, T.A., and Golubeva, O.Yu., Sorption capacity of synthetic aluminosilicates of the kaolinite group of various morphology, Glass Phys. Chem., 2021, vol. 47, no. 1, pp. 42–48.
Golubeva, O.Yu., Alikina, Yu.A., Khamova, T.V., Vladimirova, E.V., and Shamova, O.V., Aluminosilicate nanosponges: Synthesis, properties, and application prospects, Inorg. Chem., 2021, vol. 60, no. 22, pp. 17008–17018.
Funding
This study was supported by the Russian Science Foundation (project no. 21-73-30019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflicts of interest.
Rights and permissions
About this article
Cite this article
Golubeva, O.Y. Effect of Heat Treatment on the Morphology of Montmorillonite Particles. Glass Phys Chem 48, 680–682 (2022). https://doi.org/10.1134/S108765962260048X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S108765962260048X