Abstract
The sorption capacity and porous-textural characteristics of synthetic montmorillonite subjected to acid activation in a 3M HCl solution in various modes are studied. Single-phase montmorillonite, corresponding to the chemical formula Na1.0Al1.0Mg1.0Si4O10(OH)2.H2O, is obtained under hydrothermal conditions. It is established that acid treatment of synthetic montmorillonite leads to an increase in its specific surface area (from 170 to 356 m2/g), which is accompanied by a significant decrease in the degree of adsorption of the organic dye methylene blue (from 60 to 25–30% depending on the processing mode) and lead ions (from 35 to 27%) from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
It is well-known that the treatment of clay minerals with acids can lead to a sharp increase in their catalytic and sorption capacity [1–7]. Acid treatment of bentonite clays, i.e., clays consisting of at least 60–70% of minerals of the montmorillonite group is a common technology stage for obtaining clays with a high specific surface area [4]. In relation to this, considerable research has been carried out on the acid activation of clays and various assumptions have been made about the mechanism of acid-clay reactions. However, the question of the interaction of acids with clay minerals, which is related to the different phase and mineral composition of clays from different deposits, remains largely unclear One way to solve this problem is to study the effect of acid treatment on synthetic samples that are 100% montmorillonite.
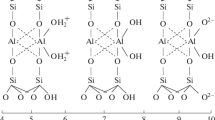
The sorption properties of synthetic montmorillonite Na1.0Al1.0Mg1.0Si4O10(OH)2.H2O, subjected to acid treatment in various modes, are studied. Montmorillonite is synthesized under hydrothermal conditions at 350°C using the previously described technique [8]. According to the chemical analysis, the resulting sample has the following composition (wt %): 55% SiO2, 22.82% Al2O3, 8.04% MgO, and 2.69% Na2O
Acid treatment was carried out in a 3M HCl solution in various modes presented in Table 1.
Figure 1 shows the X-ray diffraction patterns of the original and acid-treated samples of montmorillonite. It can be seen from the presented diffraction patterns that a single-phase sample with the montmorillonite structure was synthesized, as indicated by the presence and position of characteristic reflection peaks (hkl) in region 7° (001), 19° (110), 28° (004), 35° (201), and 60°–62° ((060) and (330)). Acid treatment does not lead to the destruction of the montmorillonite framework, which follows from the preservation of the positions and intensities of the main peaks in the diffraction pattern, with the exception of the reflection (004) and (060), which disappears gradually as the duration and temperature of the acid treatment increase. There is also a shift in the position of the basal reflex d001 to the region of small angles for samples subjected to acid treatment, as well as a significant decrease in its intensity for samples treated with acid at 80°C. This indicates an increase in the interlayer distance of the samples and partial destruction of the packet structure.
In order to assess the effect of acid treatment on the sorption properties of the studied samples of montmorillonite, we studied the adsorption kinetics of the cationic dye methylene blue C16H18N3SCl (MB) and lead ions from aqueous solutions. The studies were carried out using the techniques described in [9, 10].
For the experiments, 20 mg of the sample was dispersed in an aqueous solution of MB with a concentration of 1.5 g/L or an aqueous solution of lead nitrate with a concentration of 50 mg/L at a temperature of (25 ± 1)°C. Experiments were carried out under stirring with regular sampling for 90 min, which corresponded to the moment that equilibrium was established. Each sample was filtered and the concentration of the sorbed component in the filtrate was determined as the arithmetic mean of three measurements. The concentration of MB was determined using UV absorption spectroscopy (LEKISS2109UV spectrophotometer) by the value of optical density at a wavelength of 246 nm [11]. The content of lead ions in the studied solutions was determined by flame photometry on an iCE3000 atomic absorption spectrometer.
The data available in the literature on the adsorption of MB by acid-activated clay minerals containing montmorillonite indicate an increase in their adsorption capacity after acid treatment (for example, [12–14]). As can be seen from the results presented in Fig. 2, this is not the case for synthetic montmorillonite samples.
Treatment of samples in a 3M HCl solution of various durations (from 30 min to 4 h) and in various temperature regimes (20 and 80°C) in all cases leads to a decrease in the adsorption capacity of the samples with respect to MB. An increase in the duration of treatment leads to a significant decrease in the degree of adsorption of MB from aqueous solutions. A similar result was also obtained in the study of the adsorption capacity of acid-activated samples with respect to lead ions. Thus, during the treatment of samples with a solution of lead nitrate with a concentration of 50 mg/L for 60 min, the degree of extraction of lead ions by a sample of montmorillonite was 35%, and acid treatment in various modes led to a gradual decrease in this indicator to 27%. At the same time, the results of studying the samples by the method of low-temperature nitrogen adsorption showed an increase in the specific surface area and a slight increase in the pore diameter.
According to the study of the samples by X-ray diffraction, the crystal structure of the samples was not destroyed. The results of chemical analysis showed the complete leaching of sodium ions from the structure of the samples. Thus, the sorption capacity of montmorillonite is most likely mainly due to cation exchange, while in studies of adsorption by natural bentonite clays, it is often concluded that the pore size, which increases during acid activation, affects the adsorption of MB. Moreover, the greater the concentration of the acid and the duration of the treatment the higher the sorption capacity of the samples. Thus, in [12], the maximum value of adsorption by MB was recorded for a sample treated with 30% H2SO4 for 6 h, and the adsorption capacity of such a sample increased by a factor of 2.9 compared to an unmodified sample of natural clay. The difference in the behavior of acid-activated natural bentonite clays and synthetic montmorillonites is probably due to the absence of impurity phases in the test sample. The increase in the adsorption capacity of natural clay minerals after their acid treatment may be related not to an increase in the porosity of montmorillonite, as previously assumed, but with the dissolution and removal of various impurity phases, leading to the production of a monomineral product, the destruction of the secondary structure (cryptostructure), and an increase in the access of the adsorbed component toward the adsorbent surface.
REFERENCES
Tarasevich, Yu.I. and Ovcharenko, F.D., Adsorbtsiya na glinistykh mineralakh (Adsorption on Clay Minerals), Kiev: Naukova Dumka, 1975.
Shattar, S.F.A., Zakaria, N.A., and Foo, K.Y., One step acid activation of bentonite derived adsorbent for the effective remediation of the new generation of industrial pesticides, Sci. Rep., 2020, vol. 10, p. 20151.
Komadel, P., Acid activated clays: Materials in continuous demand, Appl. Clay Sci., 2016, vol. 131, pp. 84–99.
Pankina, G.V., Chernavskii, P.A., Lokteva, E.S., and Lunin, V.V., Optimization of acidic treatment of bentonitic clays from the national layers, Moscow Univ. Chem. Bull., 2010, vol. 65, no. 2, pp. 57–61.
Duadova, A.L., Mezhidov, V.Kh., and Viskhanov, S.S., Acid modification of bentonites of various chemical composition, Izv. Vyssh. Uchebn. Zaved., Sev.-Kavk. Reg., Tekh. Nauki, 2015, no. 1, pp. 118–123.
Finevich, V.P., Allert, N.A., Karpova, T.R., and Duplyakin, V.K., Composite nanomaterials on the basis of acid-activated montmorillonites, Russ. J. Gen. Chem., 2007, vol. 77, no. 12, pp. 2265–2271.
Mostalygina, L.V., Chernova, E.A., and Bukhtiyarov, O.I., Acid activation of bentonite clay, Vestn. Yuzhno-Ural. Gos. Univ., 2012, no. 24, pp. 57–61.
Golubeva, O.Yu., Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite, Microporous Mesoporous Mater., 2016, vol. 224, pp. 271–276.
Golubeva, O.Yu., Alikina, Yu.A., and Kalashnikova, T.A., Influence of hydrothermal synthesis conditions on the morphology and sorption properties of porous aluminosilicates with kaolinite and halloysite structures, Appl. Clay Sci., 2020, vol. 199, p. 105879.
Golubeva, O.Yu., Maslennikova, T.P., Ul’yanova, N.Yu., and Dyakina, M.P., Sorption of lead(II) ions and water vapors by synthetic hydro- and aluminosilicates with layered, framework, and nanotube morphology, Glass Phys. Chem., 2014, vol. 40, no. 2, pp. 250–255.
Ghosh, D. and Bhattacharyya, K.G., Adsorption of methylene blue on kaolinite, Appl. Clay Sci., 2002, vol. 20, pp. 295–300.
Kormosh, E.V., Modification of montmorillonite-containing clays for complex wastewater treatment, Cand. Sci. (Chem.) Dissertation, Belgorod, 2009.
Sarma, G.K., SenGupta, S., and Bhattacharyya, K.G., Blue adsorption on natural and modified clays, Sep. Sci. Technol., 2011, vol. 46, pp. 1602–1614.
Gil, A., Mouzdahir, Y.El., Elmchaouri, A., Vicente, M.A., and Korili, S.A., Equilibrium and thermodynamic investigation of methylene blue adsorption on thermal- and acid-activated clay minerals, Desalin. Water Treatm., 2013, vol. 51, pp. 13–15.
Funding
This study was carried out as part of of a state task of the Institute of Silicate Chemistry, Russian Academy of Sciences (project no. 0081-2022-0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Golubeva, O.Y., Brazovskaya, E.Y. & Alikina, Y.A. Effect of Acid Activation on the Sorption Properties of Synthetic Montmorillonite. Glass Phys Chem 48, 673–675 (2022). https://doi.org/10.1134/S1087659622600466
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659622600466