Abstract
Metallurgical properties of slag are determined to a great extent by its viscosity. High-temperature viscosity measurements are time-consuming and expensive. It is necessary to develop an accurate viscosity model for blast furnace slag in the SiO2-Al2O3-CaO-MgO system using reliable viscosity data. This paper describes a systemic evaluation procedure to determine the viscosity data to be used for model development. 1780 viscosity data from 10 to 65 wt pct SiO2, 3.5 to 40 wt pct Al2O3, 2 to 60 wt pct CaO, and 2 to 38 wt pct MgO in the SiO2-Al2O3-CaO-MgO system have been accepted for model evaluation after critical reviews. The existing 14 viscosity models in SiO2-Al2O3-CaO-MgO system is also reviewed and evaluated. Based on the structure of alumina-silicate slag and evaluated viscosity data, a new viscosity model has been proposed for the system SiO2-Al2O3-CaO-MgO. A new term “probability,” based on the basic oxide and electronegativity, is introduced to calculate the integral activation energy of slag. The model has been evaluated and compared with existing viscosity models in three different composition ranges in SiO2-Al2O3-CaO-MgO system for different applications. The new model reports an outstanding agreement between predictions and experimental data. The industrial implications of the new model have also been discussed in ironmaking and steelmaking processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
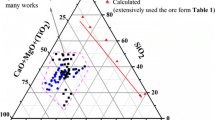
Viscosity is one of the important physicochemical properties of slags in metallurgical process, which significantly impact on operation and fuel usage efficiency.[1] In ironmaking and steelmaking industry, SiO2-Al2O3-CaO-MgO system is one of the major systems for blast furnace (BF) slags and steelmaking slags.[2–4] The approximate composition ranges of typical BF and ladle slags are shown in Table I. It can be seen that ladle slags have higher CaO/SiO2 and Al2O3 than BF slags.[5]
In ironmaking and steelmaking process, proper viscosity of slag leads to (a) fluently flowing in tapping process, (b) efficient desulphurization, (c) controllable accretion formation on the furnace wall, (d) easy separation from hot metal and coke (Ironmaking),[1] and (e) well dispersion of inclusion (Steelmaking).[5] However, high-temperature viscosity measurements are complex, fallibly techniques, cost- and time-consuming. It is necessary to establish a viscosity model to provide reliable information for process optimization.
Successful development of a viscosity model always depends on large numbers of reliable data. The quality and quantity of the data directly influence the viscosity model performance. Abundant viscosity measurements in the SiO2-Al2O3-CaO-MgO system have been carried out at wide ranges of compositions and temperatures.[6–42] A critical and systematic examination of the experimental measurements is essential to adopt reliable data for the development of viscosity model.
The present study aims to establish an accurate viscosity model in SiO2- Al2O3-CaO-MgO quaternary system. A systemic evaluation procedure to select valuable viscosity data were firstly performed, followed by reviewing and evaluating the available viscosity models in SiO2-Al2O3-CaO-MgO system. Using the accepted data above, a new viscosity model is proposed for SiO2-Al2O3-CaO-MgO system and the performance of this model is compared with other existing models.
Review of Experimental Viscosity Data
3135 viscosity data in the SiO2-Al2O3-CaO-MgO system have been critically reviewed from 37 publications.[6–42] The measurements have covered compositions of 10 to 67 wt pct SiO2, 1 to 40 wt pct Al2O3, 1 to 60 wt pct CaO, 1 to 38 wt pct MgO, and temperatures between 1623 K and 1823 K (1350 °C to 1550 °C). Measurement of slag viscosity at high temperatures is difficult and has potential for large uncertainties in the results. Hence, three sequential steps are used to evaluate the experimental data:
-
Review experimental techniques.
-
Check data self-consistency.
-
Cross-reference comparison.
Experimental Techniques in Viscosity Measurements
The improper selection and setting of viscometer will increase measurement uncertainty. Three types of viscometer have been used: rotational viscometer (28 publications),[6–33] oscillation plate viscometer (7 publications),[34–40] and falling-ball viscometer (2 publications).[41,42] It is widely accepted that the rotational viscometer is a more reliable viscosity measurement technique compared to others.[43] Still, few researchers reported detailed setting parameters: spindle weight, distances between the spindle and crucible, and thermal expansion that have studied and reported as uncertainty factor by the present authors.[6] The oscillating plate viscometer suits better for low viscosity within the range of 10−5 to 10−2 Pa s, such as pure liquid metal system.[44] For falling-ball viscometer, it has been found that the thermal expansion of the sensor (ball) significantly increases viscosity measurement uncertainty, ranges from 1 to 100 Pa s, which depends on temperature and falling-ball material.[45] The viscosities measured by falling-ball viscometer were rejected because of large uncertainty. The measurements by oscillating plate and rotational viscometer are reviewed as follows.
Mill et al.[43] reported that improper selection of container/sensor materials can cause significant uncertainty (>50 pct) in viscosity measurement. At high temperatures, the aggressive molten slags may react with the container and sensor materials leading to changes in slag composition or container/sensor geometry. Pt, Pt/Rh alloy, Fe, Mo, and graphite are major materials used for containers and sensors in the reviewed studies.[6–42] Most of researchers used Ar,[6,7,10,11,16–18,20–22,25–27,29–31,33] N2,[9] or CO[8] gas to prevent potential oxidation of crucible/spindle. Bockris et al.[46] reported that graphite material may reduce SiO2 and form SiC at high temperature, which will change slag composition and contribute to experimental uncertainties. The reaction initiated above 1793 K (1520 °C), which was estimated by FactSage 6.2.[47] The viscosity data from graphite container/sensor were carefully reviewed, and high-temperature data were found not reliable and rejected (>1793 K) (1520 °C). When Mo was used for spindle and crucible, it could be oxidized if the system was not sealed properly. The resulted MoO3 would dissolve into the slag and affect the viscosity. For most of the experiments, the conditions were not reported in details and possible oxidation of Mo was not considered. This criterion is not used in the present study. Pt sensor/container were used in air for viscosity measurements.[28,35–37,41] Despite the chemical reactions, the geometry of container/sensor can be physically changed at high temperatures due to thermal expansion and softening. The hardness of metal keeps reducing when temperature approaches the melting point. The melting temperatures of pure Fe and Pt are 1811 K and 2031 K (1538 °C and 1798 °C), respectively. Therefore, the viscosity measurements taken under improper container/sensor materials and temperature are not reliable and will not be accepted for model evaluation.
In 37 publications, three publications[18,38,39] reported nonequilibrium viscosity measurements, in which the viscosity data were recorded during continuous-cooling process. The viscosity and internal structure of the molten slag do not correspond to the recorded temperature if the time is not enough for equilibrium. For the same slag, the viscosity measured on continuous-cooling is shown to be lower than the viscosity measured at steady condition at the same temperature.[48] Therefore, nonequilibrium viscosity measurements are not accepted in the database for viscosity model.
Slag compositions, presence of solid, and container/sensor geometry changes can be examined by postexperimental technology. However, none of the slag samples was quenched after viscosity measurements in 36 publications,[7–42] except the measurements by present authors.[6] The EDS, XRF, and ICP analyses were often used to determine slag composition. The techniques of postexperimental analysis are summarized in Table II.
In summary, from 37 publications, the reported methodology is not sufficient to filter out the reliable viscosity data. Therefore, self- and cross-consistency of viscosity data should be checked.
Data Consistency
Liquidus temperature of the slag is an important indicator to discover inappropriate measurements of the viscosity. The phase diagrams of the system SiO2-Al2O3-CaO-MgO[43] have been reported and they are used together with FactSage 6.2[47] to predict the liquidus temperature of slag. The viscosity of bulk slag with solid precipitations is much higher than that in fully liquid condition. For example, it can be seen from Figure 1 that the viscosities measured at lower temperatures of two sets data are much higher than the rest of data in the same set at high temperatures. As indicated in the figure, these high viscosities were measured at the temperatures below their liquidus. The last point in each set is rejected due to the presence of solid phase.
It is well known that slag viscosity and temperature follows the Arrhenius-type equation[49]:
where η is viscosity in Pa s, A is the pre-exponential factor, E is the activation energy in J/mol, and T is the absolute temperature in K.
According to Eq. [1], the natural logarithm of viscosity has a linear correlation to reciprocal of absolute temperature. Figure 2 shows examples of viscosity measurements with high and low consistencies. The data from Machin et al.[37] show a good linear relationship. In comparison, the data from Muratov and Kulikov[24] and Yakushev et al.[40] have a low reliability and they are excluded from the database. In Yakushev et al.’s data,[40] three data points at high temperatures increased dramatically indicating the viscosities were measured below liquidus. Due to insufficient information of postexperiment analysis from published papers, the reasons for other nonlinear results are not clear. Data linearity is a good indication to evaluate the measurements reliability in the absence of enough experimental conditions.
Cross-Reference Comparison
The viscosities measured from different researchers at close compositions were carefully compared to cross check the reliability of the data. As shown in Figure 3, there were four sets of viscosity measurements[9,11,34,37] in the same composition and three sets of data[9,11,37] are close. Data from Kita et al.[34] are excluded from the database as they are significantly different from others.
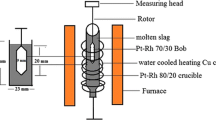
In case the available viscosity data are not consistent and the information reported is not enough for the evaluation, the viscosities at this composition were measured by the present authors using a recently developed technique at the University of Queensland. Figure 4 is an example, where it can be seen that the results of Park et al.[26] are confirmed by the authors’ measurements and Kim et al.’s[16] data are not accepted. The methodology was described in details in a previous publication.[6] The main feature of this technique is the possibility of quenching the slag sample immediately after the viscosity measurement. Electron probe X-ray microanalysis (EPMA) with wave spectrometers was used for microstructural and elemental analyses of the quenched samples. In addition, the possible errors associated with the high-temperature viscosity measurements have been analyzed and significantly minimized, which include effects of bob weight, distances to crucible, and thermal expansion during rotational viscometer measurements.[6]
The experimental conditions on high-temperature viscosity measurements have been critically reviewed and summarized from 37 publications.[6–42] As shown in Table II, the data measured in graphite crucible, such as Gualityi[9] and Gupta and Seshadri,[10] were mostly rejected. The data of three authors, Kim and Seo,[18] Sheludyakov et al.,[38] and Tsybulnikov et al.[39] were fully rejected because measurements were carried out at nonequilibrium condition. The viscosity data of Kato and Minowa[41] and Taniguchi[42] were also fully rejected because of large uncertainty at the falling-ball viscosmeter. Around one-third of Machin et al. ’s[35–37] data were rejected, due to most of measuring temperatures were below liquidus temperatures of the slags. In summary, 1780 viscosity measurements from 10 to 65 wt pct SiO2, 3.5 to 40 wt pct Al2O3, 2 to 60 wt pct CaO, and 2 to 38 wt pct MgO were accepted and utilized for viscosity model development in SiO2-Al2O3-CaO-MgO system.
Review of Viscosity Models relevant to BF Slags
Abundant viscosity models related to SiO2-Al2O3-CaO-MgO slags have been proposed in last decades[20,51–64] that correlate slag viscosity as a function of temperature and bulk composition. The parameters of mathematical equations were calculated from the physical properties of slag, for example, optical basicity and viscosity. In the present study, 14 existing[51–64] viscosity models relevant to SiO2-Al2O3-CaO-MgO system are reviewed and evaluated using the accepted viscosity database from previous section.
Features of the Existing Viscosity Model
It is widely accepted that molten slag viscosity is determined by its internal structure. In SiO2-Al2O3-CaO-MgO system, (SiO4)4− tetrahedral forms the slag network, hence increases viscosity. Mg2+ and Ca2+ perform as network modifiers to reduce the slag viscosity. Al3+ can form (AlO4)5− tetrahedral structure similar to (SiO4)4− (network former). However, (AlO4)5− requires Mg and Ca cations to balance the electrical charge. If amount of Ca and Mg cations are insufficient, Al3+ behaves as network modifier (same as Mg2+ and Ca2+).[50]
The reviewed models can be categorized based on model structure, parameters, and consideration of the silicate structures. In different stages of the model developments, the understanding of alumina-silicate structure was different:
-
(I)
Al2O3 as an amorphous oxide was not considered in the model development, such as Gan and Lai's[58] model.
-
(II)
Al2O3 was considered as network former and introduced into the viscosity model. This includes the models of Urbain,[51] Riboud et al.,[52] Iida et al.,[53] Mill and Sridhar,[54] Shankar,[55] Ray and Pal,[56] Hu et al.,[57] Tang et al.,[59] Li et al.,[20] Suzuki and Jak,[60] and FactSage.[61,62]
-
(III)
If basic oxides, e.g., CaO and MgO are insufficient, the excess Al2O3 will behave as basic oxides. This was considered by the models of Shu[63] and Zhang et al.[64]
Researches established the viscosity models through different mathematical equations. The most popular equation was Arrhenius-type equation, which was utilized by Li et al.,[20] Urbain,[51] Riboud et al.,[52] Mill and Sridhar,[54] Shankar,[55] Ray and Pal,[56] Hu et al.,[57] FactSage,[61,62] Shu,[63] and Zhang et al.[64]:
where η is viscosity in Pa s, T is temperature in K, E A is the slag activation energy in J/mol, A is pre-exponential factor, R is gas constant [8.314 J/(mol K)], X can be 0, 0.5, and 1 from different researchers.
Vogel–Fulcher–Tammann (VFT) as a classic equation for glass-forming liquid (Eq. [3]) was firstly proposed by Gan et al.[58] to predict slag viscosity of molten slag of SiO2-Al2O3-CaO-MgO system:
where η is viscosity in Pa s, T is temperature in K, and A, B, C are model parameters.
Iida et al.[53] and Tang et al.[59] proposed their own equations for viscosity prediction, which are shown in Eqs. [4] and [5], respectively.
where η is viscosity in Pa s, A and E are model parameters, η 0 is the viscosity of nonnetwork forming melts, and B i is slag basicity determined using Iida equation.[53]
where η is viscosity in Pa s, T is temperature in K, and NBO/T is defined as the ratio of nonbridging oxygen to total silicate ion.
Researchers proposed different mathematical formulas to correlate the slag structures with compositions. Urbain[51] firstly used weight ratio of \( \left( {W_{\text{CaO}} + W_{\text{MgO}} } \right)/W_{{{\text{Al}}_{2} {\text{O}}_{3} }} \) to describe the basicity of slag and predict viscosity. Riboud et al.[52] revised Urbain’s work to predict the mold slag, which includes the CaO-SiO2-Al2O3-MgO system. Mills and Sridhar[54] proposed viscosity models using optical basicity to describe the viscosity tendency with slag composition. Based on Mill’s model, Shankar,[55] Ray et al.,[56] and Hu et al.[57] revised the model structures and parameters to improve the precision and accuracy on BF slags containing minor elements. Suzuki and Jak[60] decently investigated the impact of minor units within silicate structure to activation energy, which included over 100 equations and parameters for quaternary system. In addition, the core factor “bond fraction” of Suzuki's model[60] can only be calculated by FactSage Software. FactSage viscosity model[61,62] uses “Q-Species” from FactSage thermodynamic database to calculate the viscosity. Shu[63] and Zhang et al.[64] established viscosity models with consideration of three type’s oxygen O, O−, and O2−. However, the calculation of oxygen concentration is lack of theory support and relies on assumption.[63,64] The features of the existing viscosity models are summarized in Table III.
Model Performance Evaluation
14 structural models were reviewed and evaluated in the present study using the accepted viscosity database. Equation [6] is used to calculate difference between the measured and the calculated viscosity values. The evaluation results have been summarized in Table III.
where Δ is the average deviation, \( \eta_{exp} \) is the experimental viscosity, \( \eta_{\text{Calc}} \) is the calculated viscosity, and n is the number of data.
It can be seen that the relative error ranges are between 28 and 70 pct in full composition range. The models of Urbain[51] and Zhang et al.[64] reported the lowest relative errors 30.2 and 28.5 pct, respectively.
Four viscosity models with relative good performances are selected for further comparison with the experimental data. As shown in Table IV, viscosities of two synthetic BF slags were measured by the present authors to evaluate the viscosity models.[6] It can be seen from Figures 5(a) and (b) that, in general, the four models can reasonably reproduce the measurements. FactSage[61,62] tends to underestimate the viscosities and other three models[51,60,64] overestimate the viscosities. Zhang et al.'s model[64] has the best performance reporting 15 pct deviations to the experimental data of these compositions.
Construction of New Viscosity Model
Silicate Structure
The viscosity of molten slag closely related to its structure, which is dependent on composition and temperature. It is widely accepted that basic oxides tend to break Si-O-Si bond in silicate network and forms Si-O− intermediate (also known as nonbridging oxygen). Also, amphoteric oxide Al2O3 can form (AlO4)5− unit to connect with (SiO4)4− network, which require cations (Ca2+ or Mg2+) charge compensation. As shown in Figure 6, the major role of Ca2+/Mg2+ is to break the (SiO4)4− network and compensate the (AlO4)5− charges. This intermediate Ca(Mg)-SiO4 structure unit has one free positive charge, which is able to break another SiO4 or compensate the AlO4 charges.
Al2O3 can behave as either acidic oxide or basic oxide depending on the concentrations of basic oxides. If sufficient Ca2+ and Mg2+ cations are present to balance the (AlO4)5− charges, Al2O3 acts as an acidic oxide which is incorporated into the silicate network in tetrahedron coordination. In the case of insufficient basic oxides, Al3+ will behave the same as Ca2+ or Mg2+ to break the (SiO4) network.[70]
In the present SiO2-Al2O3-CaO-MgO system, due to electrical force between charges, it is presumed that when Ca2+/Mg2+ concentration is low, they have higher priority to balance the (AlO4)5− charges than breaking the Si-O covalent bonds.[50]
Temperature Dependence
The temperature dependence of viscosity can be described by the Arrhenius-type equation (Eq. [7]).[49]
where η is the viscosity in Pa s, T is the absolute temperature in K, A is the pre-exponential factor, E A represents the integral activation energy in J/mol.
Pre-exponential Factor A
As shown in Eq. [8], a linear relationship between pre-exponent factor A and activation energy E A was proposed by Urbain.[51] The activation energy EA and pre-exponential factor A can be determined by plotting ln(η) against 1/T under the same composition.
This linear correlation has been widely applied in different viscosity models, such as the Shankar’s[55] and Hu et al.’s models.[57] In the present study, from accepted viscosity data, the linear correlation is confirmed as shown in Figure 7. ln(A) and E A have a linear relationship with R 2 = 0.948, which will be used in construction of the present viscosity model. m and n values in Eq. [8] are 0.4144 and 4.1485, respectively.
Network Modifier Probability
With the study of silicate-based mineralogy, Ramberg[71] suggests that the silicate structure (polymerized level of SiO4 network) is dependent on basic oxide concentrations, atomic radius, and electronegativity. Review of the viscosity measurements in SiO2-CaO-MgO ternary system by Licko and Danek[72] shows that the replacement of CaO by MgO will reduce the slag viscosity. As shown in Figure 8(a), at 1773 K (1500 °C), the increasing MCaO/MMgO ratio cause viscosity reduction at both 40 and 50 mol pct SiO2 conditions. The viscosity measurements by Bockris et al.[46] in SiO2-CaO and SiO2-MgO binary systems support this conclusion as shown in Figure 8(b). At 2123 K (1850 °C), at the same basic oxide concentration, the viscosity of SiO2-CaO system is larger than SiO2-MgO system. Through continuously basic oxide additions, the viscosity differences between SiO2-CaO and SiO2-MgO system decreases. In the existing viscosity models of SiO2-Al2O3-CaO-MgO system, few researchers discussed the distribution of cations (Ca2+ or Mg2+) in SiO4 and AlO4 network structure except for Zhang's model.[64]
In the present study, “probability (P)” is introduced to describe the fraction of cations (Ca2+ or Mg2+) to break the Si-O network and the rest (1 − P) will be used for charge compensation of (AlO4)5− tetrahedral units. It is related to the electronegativity of Ca2+, Mg2+, (SiO4)4−, and (AlO4)5− and their molar fractions. At low concentration of CaO/MgO, there is a high probability to compensate the (AlO4)5− charges. When the concentration of CaO/MgO increases, the probability of breaking Si-O will raise.
where M is molar fraction of metal oxide; χ is electronegativity of structure units in slag system.
The electronegativity χ of Ca2+, Mg2+, AlO4, and SiO4 units are determined using Revised–Mulliken Electronegativity,[73] which is derived from first ionization energy and electron affinity of the atom. The values of electronegativity are shown in Table V.
where I is the ionization energy (kJ/mol) and E is electron affinity (kJ/mol).
Activation Energy E A
In the present study, E A is defined as the integral activation energy of silicate slag, which is composed of four metal oxides and can be expressed as
where E i is activation energy of i component (i = SiO2, Al2O3, CaO and MgO), which is calculated using Eqs. [13] through [16].
In SiO2-Al2O3-CaO-MgO system, as a network modifier, three structure units are relevant to CaO including free oxygen O2−, SiO4-Ca-SiO4, and SiO4-Ca-AlO4. As defined before, P Ca represents the probability of one Ca2+ cation connecting with one SiO4 tetrahedron, and (1 − P Ca) is the probability to compensate the (AlO4)− charges. Therefore, the probabilities of SiO4-Ca-SiO4 and SiO4-Ca-AlO4 can be calculated by P 2Ca and P Ca × (1 − Pca), respectively. As Eq. [13] shows, the integral activation energy of CaO is calculated by sum of energy contributions of each structural unit multiplied by its probability. The \( E_{\text{Ca}}^{0} \) is the constant representing O2− from CaO. Similarly, the calculation of MgO integral energy is expressed in Eq. [14].
As an amphoteric oxide, Al2O3 shows both negative and positive impacts on activation energy. There are four possible structure units for aluminum cations: network modifier unit (O2−), 3(SiO4)-Al, and network former unit (AlO4-Ca-AlO4 and AlO4-Mg-AlO4). The charge balanced AlO4-Ca/Mg structure units give a positive contribution to the integral activation energy. The (1 − P Ca) and (1 − P Ca) are used to describe the probability of Ca2+/Mg2+ participating on alumina network. One Ca2+/Mg2+ cation is able to balance two (AlO4)5− structure units, and therefore, the probabilities of AlO4-Ca-AlO4 and AlO4-Mg-AlO4 are calculated by (1 − P Ca)2 and (1 − P Mg)2. 3(SiO4)-Al represents the network breaking effect of Al3+ cation and shows a negative contribution to activation energy. The Al3+, which is not charge compensated, can be described as (1 − (1 − P Ca) × (1 − P Mg)). One Al3+ can compensate three (SiO4)4− units. Therefore, the probability of Al3+ connecting with 3 (SiO4)4− can be written as (1 − (1 − P Ca) × (1 − P Mg))3. The \( E_{\text{Ar}}^{0} \) is a constant activation energy representing free O2− from Al2O3. However, due to charge compensation, most of O2− contributes into (AlO4)5− network, which reflects small activation energy in Table VI. Therefore, the calculation of Al2O3 integral energy is expressed in Eq. [15].
Silica is assumed to be fully polymerized and only exists one structure unit (SiO4)4−. It is considered to be the base structure unit of silicate and the parameter for \( E_{{{\text{SiO}}_{4} }} \) is a constant as shown in Table VI. The calculation of SiO2 integral energy is expressed in Eq. [16].
The overall activation energy of all structure units is optimized from collected viscosity data in the SiO2-Al2O3-CaO-MgO system. From the parameters in Table VI, the major structural unit in network breaking is Si-Ca(Mg)-Si. The free O2− and Si-Ca(Mg)-Al have less significant impacts on the activation energy. In addition, CaO has higher priority to compensate the AlO4 charges and lower priority for SiO4 charges, which is demonstrated by the optimized parameters.
Performances of the New Model
The performance of the current model is evaluated by comparison with other models using the viscosity data in the SiO2-Al2O3-CaO-MgO system. The mean deviation Δ is calculated using Eq. [6].
SiO2-Al2O3-CaO-MgO system
The evaluations of the model performances were carried out with respect to the following data: (i) all viscosity data in the SiO2-Al2O3-CaO-MgO system; (ii) slag composition in the blast furnace : 30 to 40 wt pct SiO2, 10 to 20 wt pct Al2O3, 30 to 45 wt pct CaO, and 5 to 10 wt pct MgO; and (iii) slag composition in the ladle: 10 to 25 wt pct SiO2, 20 to 30 wt pct Al2O3, 40 to 50 wt Pct CaO, and 5 to 10 wt pct MgO.
The results for model comparison are shown in Figure 9. It can be seen that the present model performs very well in all composition ranges, with the mean deviation 21.4 pct in the full composition, 12.5 pct in the BF slag composition, and 15.5 pct in the ladle slag composition range.
A detailed comparison is conducted using three most accurate models: present model, Zhang's model,[64] and Urbain's model[51] at the viscosity range of 0 to 5 Pa s. It can be seen from Figure 10, the present model has overall superior performance than both Zhang's[64] and Urbain's[51] models. The mean deviation is 12.5, 19.4, and 19.3 pct for the present model, Zhang's model, and Urbain's model, respectively. At high value ranges (>2 Pa s), the present model prediction distributed on both sides of the experiment viscosity. In contrast, the Urbain's and Zhang's models tend to underestimate the experimental data. Furthermore, the temperature dependence of present model was evaluated and compared with Zhang's model.[64] As shown in Figure 11, at two different temperature ranges [1698 K to 1823 K (1425 °C to 1600 °C) and 1923 K to 1993 K (1650 °C to 1720 °C)], the present model shows a good agreement with the experimental data.[7,10] Zhang's model[64] well reproduces the experimental data at high temperature ranges with viscosity values lower than 1 Pa s, but shows lower predictions comparing with the data from Gupta and Seshardri.[10]
Predictions of viscosity trend
The impacts of CaO and MgO on viscosity are investigated using model prediction and experimental data. At fixed SiO2, Al2O3 and temperature [1773 K (1500 °C)], as shown in Figure 12, the replacement of MgO by CaO content was evaluated under two compositions: (1) high acidic oxide (44 wt pct SiO2, 15 wt pct Al2O3) and (2) low acidic oxide (33 wt pct SiO2 and 5 wt pct Al2O3). In both conditions, through CaO replacement, the slag viscosities decrease and decrement slope continuously reduced. Because of charge compensation impact of SiO4 and AlO4 units, the viscosity decrement is more sensitive at low acidic oxide concentrations. The model predictions agree well with experimental data by Gultyai[9] and Hofmann.[12]
Subternary and subbinary systems
The present model can also be used to predict the low-order silicate systems containing CaO, MgO, and Al2O3. As shown in Figure 13, the linear relationship between activation energy EA and pre-exponential factor B can also be applied for lower-order systems with different m and n values in Eq. [8]. For each binary or ternary system, the individual m and n values were used to minimize the prediction deviation. The values of m, n, and prediction deviation for each system are summarized in Table VII.
The linear relationship between E A and ln(A) for SiO2-Al2O3-CaO from Hofmann,[12] Bills,[74] Johannsen and Brunion,[14] Machin et al.,[35–37] Urbain et al.,[75] Yasukouchi et al.,[77] Tunezo and Kawai,[78] Zhang and Chou,[79] Toplis and Dingwell[80] and SiO2-Al2O3-MgO systems from Johannsen and Brunion,[14] Lyutikov and Tsylev,[76] Toplis and Dingwell[80]
The experimental viscosity data for the systems of SiO2-Al2O3-CaO, SiO2-Al2O3-MgO, SiO2-CaO, SiO2-MgO, and SiO2-Al2O3 are evaluated with calculated values by the present model. The error deviations of different systems are shown in Table VII which shows the predicted viscosities by the present model reasonably agree with reported data. Higher error deviations are reported in two ternary systems indicating that current model needs to be improved to better describe amphoteric behavior of Al2O3 in extreme conditions (very high Al2O3 concentration). Note that all available viscosity data in the ternary and binary systems have been used.
Industrial Implications
Blast Furnace Slags for Ironmaking process
Examples of the industrial applications using the developed viscosity model are demonstrated in this section. Figure 8 shows effect of (W CaO/\( W_{{{\text{SiO}}_{2} }} \)) on viscosity of blast furnace slag at 15 wt pct Al2O3 and various MgO concentrations at 1773 K (1500°C). It can be seen that predictions agree well with the data of Kim et al.,[16] Gultyai,[9] and Machin and Hanna[35]. At a given Al2O3 and MgO concentration, the addition of CaO continuously decreases the slag viscosity. Also, it indicates that at a given W CaO/\( W_{{{\text{SiO}}_{2} }} \), the slag viscosities decrease with increasing MgO concentration. The effect of MgO seems to be more significant at low W CaO/\( W_{{{\text{SiO}}_{2} }} \). MgO is usually added in the BF operation as flux. Reduction of MgO can decrease the direct cost in material and also fuel consumptions. It can be seen from Figure 14 that reduced MgO will increase the slag viscosity. To keep the slag viscosity at a low-level, W CaO/\( W_{{{\text{SiO}}_{2} }} \) needs to be increased. However, liquidus temperature has to be controlled to avoid formation of solid phase at operating temperature.
The present viscosity model can only predict viscosities for single liquid phase. It is essential to make sure the slag is liquid before the viscosity is calculated by the viscosity model. It is therefore necessary to present isoviscosity lines on the phase diagram. As an example, the isoviscosity lines are calculated using the present viscosity model for blast furnace slags at 1773 K (1500 °C) and 15 wt pct Al2O3. In Figure 15, all viscosities are presented within the fully liquid region. It can be seen from the figure that clearly, the viscosity is mainly dependent of SiO2 concentration. The isoviscosity lines are almost parallel to the CaO-MgO axis, which has bias down to the MgO direction. It is indicated that the replacement of CaO by MgO will slightly decrease the slag viscosity at fixed SiO2 concentration. This behavior is consistent with the fact that the viscosity parameters of E Mg is higher than E Ca as network modifier, which also matches the conclusion from review of binary viscosity data of SiO2-CaO and SiO2-MgO systems.
Ladle Slags for Steelmaking Process
In steelmaking process, the desired viscosity of ladle slag (0.2 to 0.4 Pa s) is lower than BF final slag (0.4 to 0.6 Pa s).[74] Figure 16 shows effects of temperature and slag basicity on viscosity at 30 wt pct Al2O3 and 5 wt pct MgO. The present model can well predict Song et al.’s data[30] with average deviation 15 pct. At fixed Al2O3 and MgO concentrations, the viscosities decrease significantly with increasing W CaO/\( W_{{{\text{SiO}}_{2} }} \) ratio and the decrement is more significant at low temperatures. For example, the viscosity is decreased by approximately 0.13 Pa s at 1723 K (1450 °C) when W CaO/\( W_{{{\text{SiO}}_{2} }} \) is increased from 3 to 5.5. At 1823 K (1550 °C), the decrement of the viscosity is only approximately 0.05 Pa s when W CaO/\( W_{{{\text{SiO}}_{2} }} \) is increased from 3 to 5.5.
Effects of W CaO/\( W_{{{\text{SiO}}_{2} }} \) and temperature on slag viscosities with fixed 5 wt pct MgO and 30 wt pct Al2O3 by present model in comparisons with data from Song et al.[30]
Conclusions
Viscosity data and models in the system SiO2-Al2O3-CaO-MgO have been critically reviewed and evaluated. 3135 viscosity data for 607 compositions have been collected from 37 papers and carefully examined based on (1) experimental techniques, (2) data consistency, and (3) cross-reference comparisons. 1780 viscosity measurements from 10 to 65 wt pct SiO2, 3.5 to 40 wt pct Al2O3, 2 to 60 wt pct CaO, and 2 to 38 wt pct MgO composition have been accepted to the database for viscosity model evaluation and development. 14 structure-based viscosity models have been critically reviewed for their structures, parameters, applicable slag systems, and prediction performance. By comparing with the accepted viscosity data, it has been found that the relative error ranges between 28.5 and 70 pct in full composition range. All information will be utilized for further development of the viscosity model to improve the prediction performance.
An accurate viscosity model has been developed in the system SiO2-Al2O3-CaO-MgO using a large number of critically reviewed experimental data. A new term ‘probability’ based on composition and electronegativity was introduced to describe the distribution of cations within the acidic oxide. The new model can accurately predict viscosities for blast furnace slags and steel refining slags in the system SiO2-Al2O3-CaO-MgO. The model developed also has good performance for the subsystems SiO2-Al2O3-CaO, SiO2-Al2O3-MgO, SiO2-Al2O3, SiO2-CaO, and SiO2-MgO.
References
A.K. Biswas: Principles of Blast Furnace Ironmaking, 2nd ed., Cootha Publication House, Brisbane, Australia, 1981, p. 329.
C. Wu, Y.Q. Sum, D.X. Luo, and Y.X. Lu: Journal of Wuhan University of Science and Technology, 2013, vol.36, pp. 254-57.
J.W. Matousek: The Minerals, Metals & Materials Society, 2015, vol.67, pp. 1216-22.
L. Zhou, X.H. Wang, and J. Wang: J. Iron Steel Res., 2014. vol.21, pp. 70-3.
K.C. Mills and S. Sridhar: Ironmaking Steelmaking, 1999, vol. 26, pp. 262-68.
M. Chen, D. Zhang, M. Kou, and B. Zhao: ISIJ Int., 2014, vol. 54, pp. 2025-30.
L. Forsbacka, L. Holappa, T. Iida, Y. Kita, and Y. Toda: Scand. J. Metall, 2003, vol. 32, pp. 273-80.
Y.M. Gao, S.B. Wang, C. Hong, X.J. Ma, and F. Yang: International Journal of Minerals, Metallurgy, and Materials, 2014, vol. 21, pp. 353-62.
I. Gultyai: Izv. Akad. Nauk SSSR, 1962, vol. 5, pp. 52-65.
V.K. Gupta and V. Seshadri: Trans. Indian Inst. Met., 1973, vol. 26, pp. 55-64.
J.W. Han, E.H. Kwon, S.S. Han, J.H. Chi, B.S. Kim, and J.C. Lee: Mater. Sci. Forum, 2003, vol. 439, pp. 149-55.
E.E. Hofmann: Berg- und hüttenmännische monatshefte, 1959, vol. 106, pp. 397-407.
E.E. Hofmann: Stahl und Eisen, 1959, vol. 79, pp. 846-53.
F. Johannsen and H. Brunion: Zeitschrift fur Erzbergbau und Metallhutten-Wesen, 1959, vol. 12, pp. 272-79.
Y. Kawai: The science reports of the Research Institutes, Tohoku University, Physics, 1952, vol. A, pp. 615–21.
H. Kim, H. Matsuura, F. Tsukihashi, W. Wang, D.J. Min, and I. Sohn: Metall. Mater. Trans. B, 2012, vol. 44, pp. 5-12.
J.R. Kim, Y.S. Lee, and D.J. Min: ISSTech. Conference, 2003, Indianapolis, USA, p. 515.
S.H. Kim and J.D. Seo: Iron & Steelmaker, 1999, vol. 26, pp. 51-7.
T. Koshida, T. Ogasawara, and H. Kishidaka: Tetsu to Hagane, 1981, vol. 67, pp. 1491-97.
P.C.Li and X.J. Ning: Metall. Mater. Trans. B., 2016, vol. 47, pp. 446-57
Y.S. Lee, J.H. Park, D.J. Min, S.H. Yi, and W.W. Huh: Ironmaking Conf. Proc., 2002, vol. 61, pp. 155-65.
Y.S. Lee, D.J. Min, S.M. Jung, and S.H. Yi: ISIJ Int., 2004, vol. 44, 1283-89.
U. Mishra, B. Thakur, and M. Thakur: SEAISI Quarterly, 1994, vol. 23, pp. 72-82.
A.M. Muratov and I.S. Kulikov: Izvestiya Akademii Nauk SSSR. Metally., 1965, vol.1, pp. 57-62.
M. Nakamoto, T. Tanaka, J. Lee, and T. Usui: ISIJ Int., 2004, vol. 44, pp. 2115-19.
H. Park, J.Y. Park, G.H. Kim, and I. Sohn: Steel Res. Int., 2012, vol. 83, pp. 150-56.
N. Saito, N. Hori, K. Nakashima, and K. Mori: Metall. Mater. Trans. B, 2003, vol. 34, pp. 509-11.
C. Scarfe, D. Cronin, J. Wenzel, and D. Kauffman: Am. Mineral., 1983, vol. 68, pp. 1083-88.
A. Shankar, M. Görnerup, A.K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2007, vol. 38, pp. 911-15.
M. Song, Q. Shu, and D. Sichen: Steel Res. Int., 2011, vol. 82, pp. 260-68.
X.L. Tang, Z.T. Zhang, M. Guo, M. Zhang, and X.D. Wang: J. Iron Steel Res. Int., 2011, vol. 18, pp. 1-17.
G.P. Vyatkin, N.L. Zhilo, and M.Y. Ostroukhov: Izvestiya Vysshikh Uchebnykh Zavedenii. Chernaya Metallurgia., 1962, vol. 5, pp. 25-9.
L.Yao, S.Ren, X.Q.Wang, Q.C.Liu, L.Y.Dong, J.F.Yang, and J.B.Liu: Steel Res.Int., 2016. Vol. 87, pp. 241-49.
Y. Kita, A. Handa, and T. Iida: Journal of High Temperature Society of Japan, 2001, vol. 27, pp. 144-50.
J.S. Machin and D.L. Hanna: J. Am. Ceram. Soc., 1945, vol. 28, pp. 310-16.
J.S. Machin and T.B. Yee: J. Am. Ceram. Soc., 1954, vol. 37, pp. 177-86.
J.S. Machin, T.B. Yee, and D.L. Hanna: J. Am. Ceram. Soc., 1952, vol. 35, pp. 322-25.
L.N. Sheludyakov, E.T. Sarancha, and A.A. Vakhitov: Trans. Inst. Khim. Nauk, Akad. Nauk Kaz. SSR, 1967, vol. 15, pp. 158–63.
A.I. Tsybulnikov, G.A. Toporishchev, G.A. Vachugov, E.D. Mokhir, and V.V. Vetysheva: Izvestiya Vysshikh Uchebnykh Zavedenii. Chernaya Metallurgia, 1973, vol. 2, pp. 5–9.
A.M. Yakushev, V.M. Romashin, and V.A Amfiteatrov: Izvestiya Vysshikh Uchebnykh Zavedenii. Chernaya Metallurgia, 1977, vol. 55–58.
M. Kato and S. Minowa: Trans. Iron Steel Inst. Jpn., 1969, vol. 9, pp. 31-38.
H. Taniguchi: Contrib. Mineral. Petrol., 1992, vol. 109, pp. 295-303.
VDEh: Slag Atlas, 2nd edn, Verlag Stahleisen, Dusseldorf, 1995, p. 351.
G. Leblanc, R. Secco, and M. Kostic: The Measurement, Instrumentation and Sensors Handbook, 1999, Springer, Berlin
E.F. Riebling: Rev. Sci. Instrum., 1963, vol. 34, pp. 568-72.
J.O.M. Bockris, J.D. Mackenzie, and J.A. Kitchener: Trans. Faraday Soc., 1955, vol. 51, pp. 1734-48.
C. Bale, P. Chartrand, S. Degterov, G. Eriksson, K. Hack, R. Ben Mahfoud, J. Melançon, A. Pelton, and S. Petersen: Calphad, 2002, vol. 26, pp. 189-228.
M.Chen, D.W. Zhang, and B. Zhao: Proceeding of 4th Australia-China-Japan Joint Symposium on Iron and Steel-making, 2012, Shenyang, China. pp. 115–27.
S. Sathivel, J. Huang, and W. Prinyawiwatkul: J. Food Eng., 2008, vol. 84, pp. 187-93.
I. Sohn and D.J. Min: Steel Res.Int., 2012, vol. 83, pp. 611-30.
G. Urbain: Steel Res.Int., 1987, vol. 58, pp. 111-6.
P. Riboud, Y. Roux, L. Lucas, and H. Gaye: Fachberichte Huttenpraxis Metallweiterverarbeitung, 1981, vol. 19, pp. 859-69.
T. Iida, H. Sakai, Y. Kita, and K. Shigeno: ISIJ Int., 2000, vol. 40, pp. S110-14.
K.C. Mills and S. Sridhar: National Physical Lab, 1992.
A. Shankar: Ph.D. Thesis, Royal Institute of Technology, Stockholm, 2007.
H.S. Ray and S. Pal: Ironmaking Steelmaking., 2004, vol. 31, pp. 125-30.
X.J. Hu, Z.S. Ren, G.H. Zhang, L.J. Wang, and K.C. Chou: International Journal of Minerals, Metallurgy, and Materials, 2012, vol. 19, pp. 1088-92.
L. Gan and C. Lai: Metall. Mater. Trans. B, 2013, vol. 45, pp. 875-88.
X.L. Tang, M. Guo, X.D. Wang, Z.T. Zhang, and M. Zhang: Beijing Keji Daxue Xuebao, 2010, vol. 32, pp. 1542-46.
M. Suzuki and E. Jak: Metall. Meter. Trans. B, 2013, vol. 44, pp. 1451-65.
A.N. Grundy, H. Liu, I.H. Jung, S.A. Decterov, and A.D. Pelton: Int. J. Mater. Res., 2008, vol. 99, pp. 1185-94.
A.N. Grundy, I.H. Jung, A.D. Pelton, and S.A. Decterov: Int. J. Mater. Res., 2008, vol. 99, pp. 1195-209.
Q. Shu: Ironmaking and Steelmaking., 2015, vol. 42, pp. 641-47.
G.H. Zhang, K.Mills and C. Chou: Steel Res.Int., 2013, vol. 84, pp. 631-7.
J.Frenkel: Acta phys.-chim. URSS., 1935, vol. 3, pp.913-38.
J.A.Duffy and M.D.Ingram: J.Inorg.Nucl.Chem., 1975, vol. 37, pp. 1203-6.
H. Vogel: Phys. Z., 1921, vol. 22, pp. 645-46.
G.S.Fucher: J.Am.Ceram.Soc., 1925, vol. 8, pp. 339-55.
G.Tammann and W. Hesse: Z. Anorg. Allg. Chem., 1926, vol. 156, pp. 245-57.
J.N. Tiwary, S. Sarkar, B. Mishra, and U.K. Mohanty: Emerging Mater. Res., 2013, vol. 2, pp. 152-162.
H. Ramberg: The Journal of Geology, 1952, vol. 1, pp. 331-55.
T. Licko and V. Danek: Physics and chemistry of glasses, 1986, vol. 27, pp. 22-26.
S.G.Bratsch: J.Chem.Educ., 1988, vol. 65, pp. 223-35.
P.M.Bills: J.Iron Steel Inst., 1963, vol. 201, pp. 133-40.
G. Urbain, Y. Bottinga, and P. Richet: Geochimica et Cosmochimica Acta., 1982, vol. 46, pp. 1061-72.
R.A. Lyutikov and L.M. Tsylev: Izv. Vkad. Nauk. SSSR Otd. TSch. Nauk. Metall. Gorn. Delo, 1963, vol. 1, p. 41.
T. Yasukouchi, K. Nakashima and K. Mori: Tetsu-to-Hagane, 1999, vol. 85, pp. 571-7.
S. Tunezo and Y. Kawai: The Research Insitute of Mineral Dressing and Metallurgy, 1951, pp. 492–501.
G.H. Zhang and K.C. Chou: ISIJ Int., 2013, vol. 53, pp. 177-80.
M.J.Toplis and D.B.Dingwell: Geochim. Cosmochim. Acta, 2004, vol. 68, pp. 5169-88.
Acknowledgments
The authors would like to acknowledge the financial support from Shougang Group, China and Rio Tinto Iron Ore, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted March 12, 2016.
Rights and permissions
About this article
Cite this article
Han, C., Chen, M., Zhang, W. et al. Evaluation of Existing Viscosity Data and Models and Developments of New Viscosity Model for Fully Liquid Slag in the SiO2-Al2O3-CaO-MgO System. Metall Mater Trans B 47, 2861–2874 (2016). https://doi.org/10.1007/s11663-016-0744-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0744-4