Abstract
2-Alkylamino-4-amino-6-arylpyridine-3,5-dicarbonitriles were synthesized by the reaction of primary and secondary amines with 4-amino-6-aryl-2-chloropyridine-3,5-dicarbonitriles. The study of the spectral luminescent properties showed the presence of fluorescence in solutions with a maximum in the region of 399–471 nm and in the solid state with a maximum in the region of 393–502 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Derivatives of 2-aminonicotinonitriles have a wide range of practically important properties, such as biological activity [1–4] or spectral-luminescent properties [5–11]. Examples include effective fluorophores [8, 10] and pH-stable heterocyclic azo dyes [5–7].
We earlier reported the synthesis of 4-amino-6-aryl-2-chloro(bromo)pyridine-3,5-dicarbonitriles 1 by the reaction of arylmethylidene derivatives of malononitrile dimer with hydrogen halides in the presence of oxidizing agents [12–14]. It was found that compounds 1 containing donor substituents in the benzene ring exhibits intense fluorescence in solutions and in the solid state, with the quantum yield reaching 92% [14]. In this work, we present the synthesis and spectral and luminescent properties of 2-alkylamino-4-amino-6-arylpyridine-3,5-dicarbonitriles 2 derived from compounds 1.
RESULTS AND DISCUSSION
Derivatives of 2-aminonicotinonitriles can be obtained using multicomponent cascade transformations [3, 8, 15–17] and rearrangements [18], but the most common method is the substitution of a halogen substituent in the pyridine ring [1, 4, 12, 13, 19–21]. We substituted chlorine in compounds 1 under the action of primary and secondary amines. Pyrrolidine was chosen as the base amine to study the effect of substituents in the benzene ring on the optical properties of the synthesized compounds. In was found that the reaction proceeds best in 1,4-dioxane under heating at 70–80°C for 1 h in the presence of an excess of N,N-diisopropyl(ethyl)amine (DIPEA). The final 2-alkylamino-4-amino-6-arylpyridine-3,5-dicarbonitriles 2a–2e were obtained in yields of 63–94% (Scheme 1).
Chloropyridine 1b was also reacted with various primary and secondary amines, including ciprofloxacin, a fluoroquinolone antimicrobial agent (Scheme 2).
The 1H NMR spectra show proton signals of the aryl substituent and the free amino group (a singlet at 6.94–7.26 ppm) and other amine fragments. The IR spectra display absorption bands of the conjugated cyano groups at 2200–2214 cm–1, as well as the amino groups in the region of 3245–3494 cm–1. The mass spectra of all compounds contain molecular ion peaks.
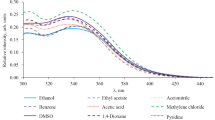
Compounds 2a–2o are white or yellow crystals. Using compound 2f as an example, we studied the solvatochromic properties of the synthesized compounds. It was found that the nature of the solvent has practically no effect on the position of the short-wavelength maximum in the absorption spectra at 271–274 nm (Table 1). The absorption spectrum itself is a superposition of different maxima, the longest wavelength of which should be in the region of 350 nm (Table 1, Fig. 1), corresponding to the optimal excitation wavelength. The position of the fluorescence maximum changes insignificantly, but it correlates well with the dipole moments of the solvents: the shortest wavelength is the maximum in benzene and the long-wavelength maxima are in DMSO and acetonitrile. The highest quantum yields were observed in dichloromethane and benzene.
Further on we studied the spectral-luminescent properties of compounds 2a–2e in dichloromethane. The nature of the substituents in the benzene ring has practically no effect on the position of the absorption maxima of compounds 2a–2e, while the short-wavelength maximum is attenuated in the presence of donor substituents (Table 2, Fig. 2). The methoxyl substituents have almost no effect on the position of the fluorescence maxima, while both the nitro and dimethylamino substituents shift the maximum to the short-wavelength region. Donor substituents in the benzene ring generally increase the fluorescence quantum yield.
The study of the spectral and luminescent properties of compounds 2c, 2f–2o, obtained from various primary and secondary amines, showed that the short-wavelength absorption maximum shifted hypsochromically in the case of compounds 2i and 2l derived from the primary methyl- and butylamine (Table 3, Fig. 3). The nature of the amine has practically no effect on the position of the fluorescence maximum, except for compounds 2i and 2n, whose maxima appear in a shorter wavelength region, and compound 2o derived from ciprofloxacin, whose absorption spectrum does not fit into the general series because of the inherent absorption of the ciprofloxacin residue. The fluorescence quantum yield, too, is almost insensitive to the nature of the amine, except for compound 2n, whose quantum yield was 11.3%.
The solid-state emission of compounds 2a–2o is weak, and donor substituents generally shift the emission maximum to short-wavelength region, which acceptor substituent, to the long-wavelength region. The nature of the amine only slightly affects the solid-state emission, except that compounds 2i and 2l, derived from primary amines, emit at shorter wavelengths.
EXPERIMENTAL
The IR spectra were recorded on an FSM-1202 Fourier spectrometer for thin films (suspensions in mineral oil). The 1H and 13C NMR spectra were obiained on a Bruker DRX-400 spectrometer in DMSO-d6, internal standard TMS. The mass spectra were measured on a Shimadzu GCMS-QP2020 instrument (EI, 70 eV). The elemental analyses were obtained on an Elementar vario MICRO cube CHN analyzer. The absorption spectra were recorded on a Cary 60 spectrophotometer. The fluorescence spectra were run on a Cary Eclipse instrument. The melting points were determined on an OptiMelt MPA100 automatic melting point apparatus. The reaction progress and the purity of the synthesized compounds were monitored by TLC on Sorbfil PTSKh-AF-A-UV plates, eluent EtOAc, visualization by exposure to UV light, iodine vapor, and high temperature. Compounds 1 were synthesized as described in [14]. Primary and secondary amines, DIPEA, and 1,4-dioxane are commercial products.
4-Amino-2-(pyrrolidin-1-yl)-6-phenyl-3,5-dicarbonitrile (2a). Pyrrolidine (1.1 mmol) and DIPEA (1.1 mmol) were added to a suspension of 0.255 g (1 mmol) of 4-amino-2-chloro-6-phenylpyridine-3,5-dicarbonitrile (1a) in 5 mL of 1,4-dioxane. The reaction mixture was stirred for 1 h at 70–80°C. After completion of the reaction (TLC monitoring), the mixture was cooled, and the precipitate that formed was filtered off and washed with cold distilled water and i-PrOH. When necessary, the product was recrystallized from 1,4-dioxane. Yield 0.263 g (91%), mp 208–209°C. IR spectrum, ν, cm–1: 3476, 3367 (NH2), 2206 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.83–1.98 m (4H, CH2), 3.69–3.78 m [4H, N(CH2)2], 7.07 s (2H, NH2), 7.38–7.61 m (3H, C6H5), 7.72–7.89 m (2H, C6H5). 13C NMR spectrum (DMSO-d6), δ, ppm: 25.5, 49.7, 71.7, 81.0, 117.4, 117.6, 128.8, 129.1, 130.9, 138.4, 157.4, 161.1, 163.6. Mass spectrum, m/z (Irel, %): 289 (49), 260 (100). Found, %: C 70.68; H 5.17; N 24.15. C17H15N5. Calculated, %: C 70.57; H 5.23; N 24.20. M 289.34.
Compounds 2b–2o were prepared in the same way.
4-Amino-2-(4-methoxyphenyl)-6-(pyrrolidin-1-yl)pyridine-3,5-dicarbonitrile (2b). Yield 0.262 g (82%), mp 208–209°C. IR spectrum, ν, cm–1: 3432, 3371 (NH2), 2214 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.82–2.05 m (4H, CH2), 3.65–3.78 m [4H, N(CH2)2], 3.83 s (3H, OCH3), 6.99 s (2H, NH2), 7.01–7.12 m (2H, C6H4), 7.76–7.81 m (2H, C6H4). 13C NMR spectrum (DMSO-d6), δ, ppm: 25.5, 49.6, 56.0, 71.3, 80.2, 114.2, 117.5, 117.9, 130.6, 130.8, 157.3, 161.2, 161.6, 162.8. Mass spectrum, m/z (Irel, %): 319 (55), 290 (100). Found, %: C 67.80; H 5.32; N 22.01. C18H17N5O. Calculated, %: C 67.70; H 5.37; N 21.93. M 319.37.
4-Amino-2-(3,4-dimethoxyphenyl)-6-(pyrrolidin-1-yl)pyridine-3,5-dicarbonitrile (2c). Yield 0.255 g (73%), mp 195–196°C. IR spectrum, ν, cm–1: 3494, 3397 (NH2), 2208 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.86–2.01 m (4H, CH2), 3.70–3.77 m [4H, N(CH2)2], 3.80 s (3H, OCH3), 3.83 s (3H, OCH3), 6.98 s (2H, NH2), 7.06 d (1H, C6H3, J 8.3 Hz), 7.41–4.46 m (1H, C6H3), 7.47 d (1H, C6H3, J 2.1 Hz). Mass spectrum, m/z (Irel, %): 349 (78), 320 (100). Found, %: C 65.41; H 5.42; N 19.96. C19H19N5O2. Calculated, %: C 65.32; H 5.48; N 20.04. M 349.39.
4-Amino-6-(4-dimethylaminophenyl)-2-(pyrrolidin-1-yl)pyridine-3,5-dicarbonitrile (11d). Yield 0.219 g (66%), mp 183–184°C. IR spectrum, ν, cm–1: 3450, 3342 (NH2), 2210 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.79–2.04 m (4H, CH2), 2.84 s [6H, N(CH3)2], 3.69–3.77 m [4H, N(CH2)2], 6.69 d (2H, C6H4, J 8.7 Hz), 6.94 s (2H, NH2), 7.78–7.83 m (2H, C6H4). 13C NMR spectrum (DMSO-d6), δ, ppm: 25.5, 49.6, 56.0, 71.3, 80.2, 114.2, 117.5, 117.9, 130.6, 130.8, 157.3, 161.2, 161.6, 162.8. Mass spectrum, m/z (Irel, %): 332 (19). Found, %: C 68.58; H 6.10; N 25.32. C19H20N6. Calculated, %: C 68.65; H 6.06; N 25.28. M 332.41.
4-Amino-2-(4-nitrophenyl)-6-(pyrrolidin-1-yl)pyridine-3,5-dicarbonitrile (2e). Yield 0.267 g (80%), mp 236–237°C. IR spectrum, ν, cm-1: 3422, 3346 (NH2), 2204 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.88–2.00 m (4H, CH2), 3.71–3.79 m [4H, N(CH2)2], 7.24 s (2H, NH2), 7.88–8.17 m (2H, C6H4), 8.35 d (2H, C6H4, J 8.6 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 25.5, 49.7, 72.2, 81.5, 117.0, 117.1, 124.0, 130.5, 144.2, 148.9, 157.3, 160.9, 161.5. Mass spectrum, m/z (Irel, %): 334 (64) [M]+. Found, %: C 61.17; H 4.15; N 25.05. C17H14N6O2. Calculated, %: C 61.07; H 4.22; N 25.14. M 334.34.
4-Amino-2-(4-methoxyphenyl)-6-(piperidin-1-yl)pyridine-3,5-dicarbonitrile (2f). Yield 0.293 g (88%), mp 201–202°C. IR spectrum, ν, cm–1: 3462, 3330 (NH2), 2208 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.58–1.70 m (6H, CH2), 3.74–3.79 m [4H, N(CH2)2], 3.83 s (3H, OCH3), 7.06 d (2H, C6H4, J 8.9 Hz), 7.16 s (2H, NH2), 7.80 d (2H, C6H4, J 8.9 Hz). Mass spectrum, m/z (Irel, %): 333 (100) [M]+. Found, %: C 68.52; H 5.69; N 20.94. C19H19N5O. Calculated, %: C 68.45; H 5.74; N 21.01. M 333.40.
4-Amino-2-(4-methoxyphenyl)-6-(piperazin-1-yl)pyridine-3,5-dicarbonitrile (2g). Yield 0.217 g (65%), mp 191–192°C. IR spectrum, ν, cm–1: 3394, 3326, 3244 (NH2, NH), 2209 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 2.77–2.82 m [4H, HN(CH2)2], 3.70–3.75 m [4H, N(CH2)2], 3.83 s (3H, OCH3), 7.06 d (2H, C6H4, J 8.8 Hz), 7.19 s (2H, NH2), 7.80 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 46.1, 49.2, 55.8, 73.0, 81.4, 114.1, 116.9, 117.4, 130.3, 130.8, 160.8, 161.3, 161.6, 162.4. Mass spectrum, m/z (Irel, %): 334 (5), 266 (100). Found, %: C 64.75; H 5.49; N 25.07. C18H18N6O. Calculated, %: C 64.66; H 5.43; N 25.13. M 334.38.
4-Amino-2-(4-methoxyphenyl)-6-morpholinopyridine-3,5-dicarbonitrile (2h). Yield 0.308 g (92%), mp 205–206°C. IR spectrum, ν, cm–1: 3397, 3337 (NH2), 2200 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 3.69–3.72 m [4H, O(CH2)2], 3.77–3.80 m [4H, N(CH2)2], 3.83 s (3H, OCH3), 7.06 d (2H, C6H4, J 8.9 Hz), 7.26 s (2H, NH2), 7.82 d (2H, C6H4, J 8.9 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 48.2, 55.8, 66.4, 73.6, 82.1, 114.1, 116.7, 117.3, 130.1, 130.8, 160.9, 161.2, 161.6, 162.5. Mass spectrum, m/z (Irel, %): 335 (78), 278 (100). Found, %: C 64.57; H 5.05; N 20.81. C18H17N5O2. Calculated, %: C 64.47; H 5.11; N 20.88. M 335.37.
4-Amino-6-(4-methoxyphenyl)-2-(methylamino)pyridine-3,5-dicarbonitrile (2i). Methylamine hydrochloride (1.1 mmol) and DIPEA (2.5 mmol) were used in the synthesis. Yield 0.218 g (78%), mp 232–233°C. IR spectrum, ν, cm–1: 3432, 3351, 3250 (NH2, NH), 2208 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 2.92 d (3H, CH3NH, J 4.5 Hz), 3.83 s (3H, OCH3), 7.05 d (2H, C6H4, J 8.9 Hz), 7.09 s (2H, NH2), 7.43 q (1H, CH3NH, J 4.4 Hz), 7.81 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 28.8, 56.0, 71.4, 81.0, 114.2, 115.8, 117.8, 130.8, 130.9, 159.4, 160.2, 161.6, 164.2. Mass spectrum, m/z (Irel, %): 279 (100) [M]+. Found, %: C 64.44; H 4.74; N 25.13. C15H13N5O. Calculated, %: C 64.51; H 4.69; N 25.07. M 279.30.
4-Amino-6-(4-methoxyphenyl)-2-(dimethylamino)pyridine-3,5-dicarbonitrile (2j). Dimethylamine hydrochloride (1.1 mmol) and DIPEA (2.5 mmol) were used in the synthesis. Yield 0.217 g (74%), mp 214–215°C. IR spectrum, ν, cm–1: 3393, 3340 (NH2), 2210 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 3.27 s [6H, (CH3)2N], 3.83 s (3H, OCH3), 7.05 d (2H, C6H4, J 8.9 Hz), 7.09 s (2H, NH2), 7.81 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 40.7, 56.0, 71.5, 80.7, 114.2, 117.4, 117.8, 130.5, 130.9, 160.1, 161.5, 161.7, 162.3. Mass spectrum, m/z (Irel, %): 293 (59), 264 (100). Found, %: C 65.60; H 5.19; N 23.80. C16H15N5O. Calculated, %: C 65.52; H 5.15; N 23.88. M 293.33.
4-Amino-2-[butyl(methyl)amino]-6-(4-methoxyphenyl)pyridine-3,5-dicarbonitrile (2k). Yield 0.285 g (85%), mp 143–144°C. IR spectrum, ν, cm–1: 3420, 3332 (NH2), 2201 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 0.91 t (3H, CH3, J 7.4 Hz), 1.27–1.32 m (2H, CH2), 1.59–1.65 m (2H, CH2), 3.27 s (3H, CH3N), 3.59–3.75 m (2H, CH2N), 3.83 s (3H, OCH3), 6.92–7.13 m (4H, NH2, C6H4), 7.81 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 14.3, 20.0, 29.9, 39.0, 51.6, 56.0, 71.1, 80.5, 114.2, 117.4, 117.8, 130.5, 130.8, 159.4, 161.6, 161.7, 162.3. Mass spectrum, m/z (Irel, %): 335 (14), 293 (100). Found, %: C 67.95; H 6.25; N 20.97. C19H21N5O. Calculated, %: C 68.04; H 6.31; N 20.88. M 335.41.
4-Amino-2-(butylamino)-6-(4-methoxyphenyl)pyridine-3,5-dicarbonitrile (2l). Yield 0.218 g (68%), mp 204–205°C. IR spectrum, ν, cm–1: 3474, 3351, 3245 (NH2, NH), 2212 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 0.89 t (3H, CH3, J 7.4 Hz), 1.26–1.32 m (2H, CH2), 1.45–1.68 m (2H, CH2), 3.42–3.47 m (2H, CH2NH), 3.83 s (3H, OCH3), 6.97–7.15 m (4H, NH2, C6H4), 7.47 t (1H, CH2NH, J 5.7 Hz), 7.81 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 14.4, 20.1, 31.9, 41.0, 56.0, 71.2, 80.8, 114.2, 115.8, 117.8, 130.8, 159.6, 159.8, 161.6, 164.0, 164.3. Mass spectrum, m/z (Irel, %): 321 (39), 279 (100). Found, %: C 67.38; H 6.02; N 21.71. C18H19N5O. Calculated, %: C 67.27; H 5.96; N 21.79. M 321.38.
4-Amino-2-(dibutylamino)-6-(4-methoxyphenyl)pyridine-3,5-dicarbonitrile (2m). Yield 0.286 g (76%), mp 119–120°C. IR spectrum, ν, cm–1: 3418, 3346 (NH2), 2206 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 0.90 t (6H, 2CH3, J 7.4 Hz), 1.27–1.35 m (4H, 2CH2), 1.52–1.75 m (4H, 2CH2), 3.54–3.71 m [4H, (CH2)2N], 3.83 s (3H, OCH3), 6.96 s (2H, NH2), 7.04 d (2H, C6H4, J 8.9 Hz), 7.80 d (2H, C6H4, J 8.8 Hz). Mass spectrum, m/z (Irel, %): 377 (12), 335 (100). Found, %: C 69.89; H 7.26; N 18.61. C22H27N5O. Calculated, %: C 70.00; H 7.21; N 18.55. M 377.49.
4-Amino-2-(diallylamino)-6-(4-methoxyphenyl)pyridine-3,5-dicarbonitrile (2n). Yield 0.217 g (63%), mp 114–115°C. IR spectrum, ν, cm–1: 3462, 3342 (NH2), 2203 (C≡N). 1H NMR spectrum (DMSO-d6), δ, ppm: 3.35 d [4H, (CH2)2N, J 1.4 Hz], 3.82 s (3H, OCH3), 5.15–5.28 m (4H, =CH2), 5.86–6.00 m (2H, =CH), 7.06 d (2H, C6H4, J 8.8 Hz), 7.11 s (2H, NH2), 7.81 d (2H, C6H4, J 8.8 Hz). 13C NMR spectrum (DMSO-d6), δ, ppm: 52.1, 56.1, 71.9, 81.3, 114.4, 117.2, 117.6, 118.1, 130.4, 130.9, 134.3, 159.2, 161.6, 161.8, 162.5. Mass spectrum, m/z (Irel, %): 345 (33). Found, %: C 69.63; H 5.49; N 20.23. C20H19N5O. Calculated, %: C 69.55; H 5.54; N 20.28. M 345.41.
7-{4-[4-Amino-3,5-dicyano-6-(4-methoxyphenyl)pyridine-2-yl]piperazin-1-yl}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (2o). Yield 0.544 g (94%), mp 275–276°C (decomp.). IR spectrum, ν, cm–1: 3430, 3325 (NH2), 2210 (C≡N), 1662 (C=O). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.15–1.22 m (2H, CH2), 1.31–1.37 m (2H, CH2), 3.50–3.62 m (4H, CH2N), 3.85 s (3H, OCH3), 4.02–4.10 m (4H, CH2N), 7.07 d (2H, C6H4, J 8.0 Hz), 7.24 s (2H, NH2), 7.57 s (2H, C6H2), 7.79–7.96 m (3H, C6H2, C6H4), 8.65 s (1H, =CH), 14.97 br.s (1H, COOH). 13C NMR spectrum (DMSO-d6), δ, ppm: 8.2, 26.1, 31.3, 36.5, 47.5, 49.6, 56.0, 67.0, 73.9, 82.4, 106.9, 107.4, 111.6, 111.8, 114.4, 116.9, 117.4, 119.3, 130.3, 131.0, 139.8, 145.4, 148.7, 161.1, 161.3, 161.8, 162.7, 166.6, 177.0. Mass spectrum, m/z (Irel, %): 579 (7) [M]+. Found, %: C 64.36; H 4.58; N 16.82. C31H26FN7O4. Calculated, %: C 64.24; H 4.52; N 16.92. M 579.59.
CONCLUSIONS
2-Alkylamino-4-amino-6-arylpyridine-3,5-dicarbonitriles 2 were synthesized and their spectral-luminescent properties were studied. Compounds 2 fluoresce in solutions with a maximum in the range of 399–471 nm and a quantum yield of up to 12.3%; they scarcely fluoresce in the solid state. The replacement of the chlorine atom in compounds 1 by an amine significantly reduces the luminescence quantum yield. It was found that donor substituents in the benzene ring generally cause a bathochromic shift of the fluorescence maximum. The absorption and solid-state fluorescence maxima of compounds 2 derived from primary amines are shifted to a shorter wavelength region.
REFERENCES
Soliman, E.A., Panda, S.S., Aziz, M.N., Shalaby, E.S.M., Mishriky, N., Asaad, F.M., and Girgis, A.S., Eur. J. Med. Chem., 2017, vol. 138, p. 920. https://doi.org/10.1016/j.ejmech.2017.07.025
Chen, W., Guo, N., Qi, M., Dai, H., Hong, M., Guan, L., Huan, X., Song, S., He, J., Wang, Y., Xi, Y., Yang, X., Shen, Y., Su, Y., Sun, Y., Gao, Y., Chen, Y., Ding, J., Tang, Y., Ren, G., Miao, Z., and Li, J., Eur. J. Med. Chem., 2017, vol. 138, p. 514. https://doi.org/10.1016/j.ejmech.2017.06.053
Mansour, S.Y., Sayed, G.H., Marzouk, M.I., and Shaban, S.S., Synth. Commun., 2021, vol. 51, p. 1. https://doi.org/10.1080/00397911.2020.1870698
Betti, M., Catarzi, D., Varano, F., Falsini, M., Varani, K., Vincenzi, F., Pasquini, S., Di Cesare Mannelli, L., Ghelardini, C., Lucarini, E., Dal Ben, D., Spinaci, A., Bartolucci, G., Menicatti, M., and Colotta, V., J. Med. Chem., 2019, vol. 62, p. 6894. https://doi.org/10.1021/acs.jmedchem.9b00106
Zhao, X.L., Geng, J., Qian, H.F., and Huang, W., Dyes Pigm., 2017, vol. 147, p. 318. https://doi.org/10.1016/j.dyepig.2017.08.020
Zhao, X.L., Jun, T., Feng, Y.N., Qian, H.F., and Huang, W., Dyes Pigm., 2017, vol. 145, p. 315. https://doi.org/10.1016/j.dyepig.2017.06.030
Zhao, X.L., Geng, J., Hu, B., Xu, D., and Huang, W., Dyes Pigm., 2018, vol. 155, p. 1. https://doi.org/10.1016/j.dyepig.2018.03.014
Hagimori, M., Nishimura, Y., Mizuyama, N., and Shigemitsu, Y., Dyes Pigm., 2019, vol. 171, p. 107705. https://doi.org/10.1016/j.dyepig.2019.107705
de Souza, J.M., Abdiaj, I., Chen, J., Hanson, K., de Oliveira, K.T., and McQuade, D.T., Org. Biomol. Chem., 2021, vol. 19, p. 1991. https://doi.org/10.1039/d0ob02591g
Ershov, O.V., Ievlev, M.Y., Belikov, M.Y., Naidenova, A.I., Maksimova, V.N., and Tafeenko, V.A., RSC Adv., 2017, vol. 7, p. 34886. https://doi.org/10.1039/c7ra06217f
Ershov, O.V., Mikhailov, D.L., Bardasov, I.N., Ievlev, M.Y., and Belikov, M.Y., Russ. J. Org. Chem., 2017, vol. 53, p. 886 https://doi.org/10.1134/S1070428017060124
Bardasov, I.N., Mihailov, D.L., Alekseeva, A.U., Ershov, O.V., and Nasakin, O.E., Tetrahedron Lett., 2013, vol. 54, p. 21. https://doi.org/10.1016/j.tetlet.2012.10.015
Bardasov, I.N., Alekseeva, A.U., and Ershov, O.V., Tetrahedron Lett., 2018, vol. 59, p. 1398. https://doi.org/10.1016/j.tetlet.2018.02.069
Ershova, A.I., Alekseeva, A.U., Ershov, O.V., Ievlev, M.Y., and Bardasov, I.N., Dyes Pigm., 2022, vol. 197, p. 109914. https://doi.org/10.1016/j.dyepig.2021.109914
Baghery, S., Zolfigol, M.A., and Maleki, F., New J. Chem., 2017, vol. 41, p. 9276. https://doi.org/10.1039/c7nj01934c
Ramanathan, M., Wan, J., Liu, Y.H., Peng, S.M., and Liu, S.T., Org. Biomol. Chem., 2020, vol. 18, p. 975. https://doi.org/10.1039/c9ob02427a
Krasavin, M., Sapegin, A., and Dorogov, M., Tetrahedron Lett., 2015, vol. 56, p. 56. https://doi.org/10.1016/j.tetlet.2014.09.067
Dyachenko, I.V., Dyachenko, V.D., Dorovatovskii, P.V., Khrustalev, V.N., and Nenaydenko, V.G., Russ. J. Org. Chem., 2018, vol. 54, p. 1681 https://doi.org/10.1134/S1070428018110106
Yang, C., Zhang, F., Deng, G.J., and Gong, H., J. Org. Chem., 2019, vol. 84, p. 181. https://doi.org/10.1021/acs.joc.8b02588
Keylor, M.H., Niemeyer, Z.L., Sigman, M.S., and Tan, K.L., J. Am. Chem. Soc., 2017, vol. 139, p. 10613. https://doi.org/10.1021/jacs.7b05409
Fedoseev, S.V., Ershova, A.I., Lipin, K.V., Mel’nik, E.A., and Ershov, O.V., Russ. J. Org. Chem., 2021, vol. 57, p. 1361 https://doi.org/10.1134/S1070428021080170
Funding
The work was performed in the framework of the state assignment from the Ministry of Education and Science of the Russian Federation (project no. 0849-2020-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated from Zhurnal Organicheskoi Khimii, 2022, Vol. 58, No. 11, pp. 1181–1191 https://doi.org/10.31857/S0514749222110064.
Rights and permissions
About this article
Cite this article
Bardasov, I.N., Alekseeva, A.U. & Ershov, O.V. Synthesis and Optical Properties of 2-Alkylamino-4-amino-6-arylpyridine-3,5-dicarbonitriles. Russ J Org Chem 58, 1600–1609 (2022). https://doi.org/10.1134/S1070428022110069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022110069