Abstract
A promising nontoxic and environmentally safe detergent consisting of sodium salts of iminodiacetate derivatives of fatty acid tri- and diglycerides and acetate derivatives of polymucosaccharides was synthesized, and its composition and structure were studied. Fractions consisting of fats, sodium salts of polymucosaccharides, and sodium salts of α-amino acids, obtained from liquid base hydrolyzates of protein-containing waste, served as feedstock. Polymucosaccharide derivatives coordinate tri- and diglyceride derivatives along the chains via hydrogen bonds to form host–guest complexes with the hydrocarbon radicals directed toward different sides. When dissolved, the complexes form thread-like particles incorporating biphilic molecules, wetting agents, and complexones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Modern detergents are formulated from biphilic molecules, wetting agents, and complexones [1–3]. Radicals of biphilic molecules are inserted into insoluble organic contaminations in such a fashion that the ionic groups remain on the surface. Wetting agent molecules are noncovalently bonded with the ionic groups, thus making the complexones able to decompose mineral deposits, favoring flocculation of the contaminations into the aqueous phase. Salts of carboxylic or sulfonic acids or quaternary ammonium bases with the hydrocarbon radicals containing 14 to 22 carbon atoms are used as biphilic molecules; soluble polyhydric alcohols or polysaccharide derivatives, as wetting agents; and salts of nitrilotriacetic or ethylenediaminetetraacetic acid, as complexones [4, 5]. Organic contaminations, as a rule, contain compounds with metal cations bonded to anions of fatty or naphthenic acids, to resins, to porphyrins, etc. They stabilize the structure of floccules, complicating their decomposition with the recovery of useful products (petroleum, industrial oils, fats, etc.), and inhibit biodegradation [6]. The complexones used are incapable of insertion into organic contaminations, whereas wetting agents in many cases are adsorbed on mineral deposits, preventing the access of complexones. Therefore, detergents consisting of biphilic molecules and wetting agents with complexone groups are of particular interest. When radicals of biphilic molecules are inserted into the phase of organic contaminations, the polar groups of the polycomplexones will bind the metal ions in the organic phase, and binding with ionic groups of biphilic molecules will lead to simultaneous decomposition of the deposits.
An example of such detergent is a product termed polycomplexone. It is a mixture of sodium salts of iminodiacetate derivatives of fatty acid tri- and diglycerides and polymucosaccharides. The polycomplexone was obtained from the insoluble fraction isolated from liquid base hydrolyzates of protein-containing waste. The synthesis is performed similarly to the synthesis of carboxyl-containing complexones from amino acids. Polycomplexone-based formulations of technical detergents and household cleaning agents, capable of removing fat, oil, and petroleum contaminations and mineral deposits from surfaces of various materials even at room temperature, have been developed [7, 8]. Up to now, the composition and structure of the polycomplexone have not been determined; therefore, the mechanism of its action as a detergent has not been practically substantiated.
This study was aimed at examining the steps of the polycomplexone synthesis and at determining its composition and structure.
EXPERIMENTAL
We took a sample of the fraction isolated from the base hydrolysis product under the action of steam under the conditions of limited hydration of waste from fleshing of degreased cattle hide (OOO Tsentr). The fraction consisted of sodium salts of α-amino acids (molar content Na = 3.39 mol kg–1) from connective tissue proteins. According to [9], the polycomplexone contains 22.6% polymucosaccharides (mainly hyaluronic acid) from connective tissue glycoproteins and subcutaneous tissue and 36.7% glycerides from connective tissue lipoproteins, namely, triglycerides with unsaturated radicals of oleic, linolenic, and arachidonic acids and diglycerides with saturated radicals of stearic, palmitic, and myristic acids. Pure grade HCl and KOH were used. The polycomplexone was prepared using the commercial installation of OOO Tsentr [10]. X-ray fluorescence analysis was performed with an ARL Perform’X X-ray fluorescence wave spectrometer (Thermo Fisher Scientific, New Wave). The nitrogen content was determined by the Kjeldahl method involving wet ashing of organic substances in the sample with sulfuric acid on heating. The iodine number of fats was determined by the Hanus method based on using iodine bromide, which undergoes quantitative addition to multiple bonds. The Cl– content was determined by the Fajans argentometric method based on direct titration with a standard AgNO3 solution using adsorption indicators. The sodium content was determined by the flame photometric method with the injection of the solution sample in the atomized form into a flame (FPA-1 flame photometric analyzer). The dissociation constant of the acid was determined by potentiometric titration. The mean molecular mass of the fats was determined viscometrically with an Ubbelohde viscometer using dichloroethane as a solvent and glycerol tristearate as a reference. The photomicrograph was taken with a Motic DM-BA 300 microscope. The IR spectrum was taken with a Perkin Elmer 2000 Fourier IR spectrometer.
The sample composition was studied by matrix-assisted laser desorption/ionization mass spectrometry with a Bruker autoflex speed TOF/TOF device equipped with a solid-state laser (λ = 355 nm) in the linear mode with recording positive ions in the ranges 200–1500 and 500–5000 Da. 2,5-Dihydroxybenzoic acid was used as a matrix; it was dissolved in methylene chloride (30 mg mL–1). Solutions of the matrix and analytes (2 mg mL–1 in toluene) were applied in succession in the order matrix–analyte–matrix after drying of the previous layer. The data were processed using Bruker Compass 1.4 program package.
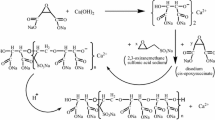
The polycomplexone was synthesized in several steps. In the first step, the waste from fleshing of degreased cattle hide was treated with steam with the addition of formaldehyde. After the reaction with formaldehyde was complete, treatment of the mixture with steam was continued to remove the unchanged formaldehyde. The weight fraction of the steam-distilled substances was 49.3%, their mean molecular mass was ~1184 g mol–1, Niod = 0, NА = 1.44 mol kg–1 in terms of the dry substance.
In the second step, sodium monochloroacetate was added to the product, and the mixture was kept at room temperature for 3 days, after which the solid product (polycomplexone) was separated from the solution. The total content of Cl– ions in the reaction mixture was 84.5 mol, and the content of iminodiacetic acid anions was 6.8 mol.
RESULTS AND DISCUSSION
The polycomplexone is a yellow-gray pasty mass (Tables 1, 2). The polycomplexone was separated into components by isolation from reaction mixtures at pH of the isoelectric point. Concentrated HCl was added at room temperature with continuous vigorous stirring to the polycomplexone to рН 4. The sample separated into two fractions: a fat-like gray mass (I) and a yellow-brown liquid, which was evaporated to obtain a loose powder (II).
When studying fraction I, we proceeded from the fact that it is a mixture of products formed by successive transformations of tri- and diglycerides. A decrease in Niod to zero in the first step indicates that the amino acid salt radical underwent addition to the double bond with the participation of formaldehyde, with the incorporation of OH group and sodium amino acid radical (via –СН2– group) into the chain. The mean molecular mass of the amino acid sodium salt is about 120 g mol–1. The mean composition corresponds to the formula С3.8H11O2.2N1.2Na. The molecular mass of the product increased to 1184 g mol–1 (by the value close to the molecular mass of the amino acid radical), suggesting the occurrence of the addition. One more radical of the amino acid salts is incorporated probably via the Mannich reaction involving the labile hydrogen atom of the СН group of the glyceride radical. In the second step, all the sodium amino acid groups transformed into sodium iminodiacetate groups. Hence, the obtained fraction I is a mixture of triglyceride derivatives with the radicals consisting of 18 and 20 carbon atoms and several sodium iminodiacetate groups, and also of tri- and diglyceride derivatives with the radicals consisting of 18, 16, and 14 carbon atoms and one sodium iminodiacetate group. The synthesis scheme is shown in Fig. 1.
The elemental composition of fraction I (Table 1) was determined by X-ray fluorescence analysis, and the composition of structural fragments of the components, by MALDI TOF mass spectrometry (Fig. 2) [11]. In interpretation of the spectrum, the mean composition of the sodium iminodiacetate radical was С6.8H14O4.2N1.2 with the molecular mass of approximately 185. Peaks at m/z 1650–1620 and 1400–1320 belong to triglyceride derivatives with linolenic and arachidonic acid radicals and three or four iminodiacetate groups; the peaks at m/z about 1232, to triglyceride derivatives with oleic acid radicals and two iminodiacetate groups; the peaks at m/z about 1096, 1060, and 967, to diglyceride derivatives with stearic, palmitic, and myristic acid radicals and one iminodiacetate group; and the peaks at m/z about 816, 792, and 760, to diglyceride derivatives with stearic, palmitic, and myristic acid radicals and one iminodiacetate groups. A group of peaks at m/z 590–510 belongs to degradation products formed by cleavage of bonds in ester groups in tri- and diglyceride derivatives of stearic, palmitic, and myristic acids with one iminodiacetate group. These data reflect the composition of fats in the initial fraction.
As shown by рH-metric titration of a 1% solution of fraction I (Fig. 3), this fraction contains iminodiacetate groups with pН-metric titration of a 1% solution of fraction I (Fig. 3), this fraction contains iminodiacetate groups with рK about 8.2–8.3 and 9.1–9.2. Their molar ratio is from 1 : 1.47 to 1 : 1.52. For N-alkyliminodiacetates, рK of dissociation of the betaine hydrogen ion is 9.1–9.6; oxygen-containing radicals such as ester groups located close to the nitrogen atom decrease the betaine basicity. Therefore, it can be assumed the iminodiacetate groups in the fraction are substituents both in the glyceride radical and in hydrocarbon radicals of unsaturated acids.
When studying fraction II, we proceeded from the fact that NaCl formed by neutralization of iminodiacetate and carboxy groups passed into the liquid phase in the course of separation of the polycomplexone into two fractions. The elemental analysis of fraction II (Table 1) shows that the iminodiacetate derivatives of the polysaccharides have the empirical formula C26H35O19.4N3.4.
The potentiometric titration of the solution of fraction II (Fig. 3) gives the range of pH 7.5–11.1, characteristic of the iminodiacetic acid monosodium salt. The titration results in combination with the data on the composition of the iminodiacetate radical show that each radical contains two iminodiacetate groups. The scheme of the synthesis of this fraction is shown in Fig. 4.
Structure of the polycomplexone. The structure of the polycomplexone was determined from the IR spectra [12] of fractions I and II (Fig. 5). In the spectrum of the polycomplexone in the range 3600–3150 cm–1, there is a complex band with a maximum at 3362 cm–1 and a broad shoulder at 3326 cm–1. This range is characteristic of vibrations of bound water and OH groups in polysaccharides. The band at 3016 cm–1 can be assigned to N–H+ vibrations in iminodiacetate groups. The bands at 1723, 1232, 1150, and 1092 cm–1 correspond to the С=О and С–О–С vibrations in ester groups. The band with a maximum at 1644 cm–1 and a shoulder at 1610–1595 cm–1 can be assigned to bending vibrations of bound water molecules and antisymmetric vibrations of carboxy groups, and the band near 1418 cm–1, to symmetric vibrations of carboxy groups. The band at 939 cm–1 and a shoulder at 916 cm–1 belong to vibrations of undistorted and distorted glycoside groups [6].
In the spectrum of fraction I (Fig. 5), bands at 3098 and 3036 cm–1 appeared; they correspond to N–H+ vibrations in iminodiacetate groups as substituents in hydrocarbon and glyceride radicals. The positions of the vibration bands of ester groups changed: The С=О vibration band shifted to 1723 cm–1, and the С–О–С vibration bands, to 1294, 1170–1160, and 1100–1092 cm–1. In the spectrum of fraction II (Fig. 5), the ОН vibration band in polysaccharides shifted to 3319 cm–1, and the shoulder, to 3217 cm–1 (with a decrease in its intensity); the shoulder in the region of 3050 cm–1, belonging to N–H+ vibrations, disappeared. The carboxy group vibrations are observed at approximately 1634 and 1458 cm–1. The structure of vibration bands in the range 910–925 cm–1 changed.
In the polycomplexone molecule, there are, on the average, two molecules of tri- or diglyceride derivatives per three cellobiose units of polymucosaccharide derivatives.
The shift of bands in the IR spectra suggests the presence of a developed system of hydrogen bonds in the polycomplexone. Under their influence, triglyceride derivatives are coordinated by polymucosaccharide derivatives to form host–guest molecular complexes. The IR data confirm the presence of intermolecular hydrogen bonds in the polycomplexone between monoprotonated iminodiacetate, carboxy, carbamide, hydroxy, and glycoside groups in polymusosaccharide derivatives and monoprotonated iminodiacetate and ester groups in triglyceride derivatives. The hydrocarbon radicals in these complexes are oriented toward different sides along polysaccharide chains. These data are confirmed by photomicrographs of the polycomplexone solution (Fig. 6), in which threadlike particles are clearly seen. The structure is determined by the fact that polymucosaccharide macromolecules contain 6400–6500 cellobiose radicals. In iminodiacetate derivatives of polymucosaccharides, hydroxy, carboxy, and iminodiacetate groups are densely distributed along the chains.
CONCLUSION
The results of this study show that the polycomplexone is a detergent combining the properties of biphilic molecules, wetting agents, and complexones of new generation. A decrease in the metal content in petroleum recovered from the solution after treatment of the tanks with polycomplexone solutions to remove petroleum sludge indicate that flocculation of the contaminations using the polycomplexone leads to the breakdown of the metal compounds in the organic phases.
The properties of the polycomplexone are determined by the composition of the feedstock, fraction recovered from liquid base hydrolyzates of protein-containing waste. This fraction contains difficultly available and expensive compounds such as hyaluronic acid and triglycerides with linolenic and arachidonic acid radicals. The polycomplexone can become an available and widely used detergent, because the procedure for its production from protein-containing waste is economically feasible and environmentally safe. The use of the polycomplexone in synthesis of safe technical detergents and household chemicals will allow the petroleum consumption for their production to be significantly reduced.
REFERENCES
Kumar, S. and Bhattarai, A., Modern Trends Sci. Technol., 2013, pp. 147–158.
Ponnusamy, T., Dubal, S.A., and Momin, S.A., J. Dispersion Sci. Technol., 2008, vol. 29, no. 8, pp. 1123–1128. https://doi.org/10.1080/01932690701817842
Oliveira, A.G. and Chaimovi, H., J. Pharm. Pharmacol., 1993, vol. 45, pp. 850–861.
O’Lenick, A.J., Cosmet. Toilet. Mag., 2005, vol. 120, no. 11, pp. 63–64. https://doi.org/10.13140/RG.2.1.3084.2082
Khan, N. and Brettmann, B., Polymers, 2019, vol. 11, no. 1, pp. 51–78. https://doi.org/10.3390/polym11010051
Sandrin, T.R. and Maier, R.M., Environ. Health Perspect., 2003, vol. 111, no. 8, pp. 1093–1101. https://doi.org/10.1289/ehp.5840
Maksimov, A.L., Tsivadze, A.Yu., Fridman, A.Ya., Novikov, A.K., Petrukhina, N.N., Shabanov, M.P., Polyakova, I.Ya., Gorbunov, A.M., and Naranov, E.R., in Trudy kongressa s mezhdunarodnym uchastiem i konferentsii molodykh uchenykh “Fundamental’nye issledovaniya i prikladnye razrabotki protsessov pererabotki i utilizatsii tekhnogennykh obrazovanii” (Proc. Congr. with Int. Participation and Young Scientists’ Conf. “Basic Research and Applied Developments in the Field of Processes for Reprocessing and Utilization of Manmade Formations”), Yekaterinburg: Ural’skoe Otdel. Ross. Akad. Nauk, 2019.
Altunina, L.K. and Svarovskaya, L.I., Petrol. Chem., 2012, vol. 52, no. 2, pp. 130–132. https://doi.org/10.1134/S0965544112010033
Kaspar’yants, S.A., Sovremennye predstavleniya o strukture i svoistvakh kollagena (Modern Views on the Structure and Propertes of Collagen), Moscow: Mosk. Veterinarnaya Akad., 1981.
Tsivadze, A. and Fridman, A., Wasteless processing of renewable protein and carbohydrate-containing waste into consumer good, Handbook of Ecomaterials, Leticia Myriam, T.-M., Kharissova, O.V., and Kharisov, B.I., Eds., Berlin: Springer, 2018
Castellino, S., Groseclose, M.R., and Wagner, D., Bioanalysis, 2011, vol. 3, no. 21, pp. 2427–2441. https://doi.org/10.4155/bio.11.232
Mossoba, M.M., Milosevic, V., Milosevic, M., Kramer, J.K., and Azizian, H., Anal. Bioanal. Chem., 2007, vol. 389, no. 1, pp. 87–92. https://doi.org/10.1007/s00216-007-1262-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
FUNDING
The study was financially supported by the Russian Foundation for Basic Research (project no. 18-29-24137).
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tsivadze, A.Y., Fridman, A.Y., Maksimov, A.L. et al. A Detergent Prepared from Iminodiacetate Derivatives of Fats and Polymucosaccharides from Base Hydrolyzates of Protein-Containing Waste. Russ J Appl Chem 93, 333–339 (2020). https://doi.org/10.1134/S1070427220030039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220030039