Abstract
Triads L–SNP–L, containing two ligands, phenolphthalein or 1,3,5,7-tetramethyl-8-(4-hydroxyphenyl)-4,4-difluoro-4-boron-3a,4a-diazaindacene (BODIPY), axially bound to Sn(IV)octaethylporphyrinate, were synthesized. The sensitivity of the obtained triads to changes in the acidity of the medium has been studied. Photoexcitation of the BODIPY-SnP-BODIPY triad leads to photoinduced energy transfer from the BODIPY donor fragments to the porphyrinate acceptor. When the triad is excited at the wavelength λexc = 490 nm, in addition to BODIPY fluorescence, fluorescence sensitized by the porphyrin fragment is recorded, and when the triad is excited at a wavelength λexc = 400 nm, porphyrinate fluorescence flares up compared to initial SnP. In the triad with phenolphthalein molecules, the fluorescent properties of both the ligand and porphyrinate are quenched, however, sensitivity to changes in the solution pH increases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The variety of organic fluorescent receptors is connected with a wide range of tasks and conditions of their use. The sensitivity of fluorescence to the physical properties of the medium in which the fluorophore molecules are located makes it possible to use some of them as detectors of various parameters of the intracellular medium [1–23]. Changes in the viscosity and pH of blood, plasma, or lymphatic fluids can indicate the presence of diseases such as diabetes, hypertension, heart attack, and aging. Monitoring of intracellular viscosity, intracellular and intramitochondrial pH values, and also the difference in electrical potentials on membranes allows us to investigate the mechanisms of energy transformation in cells and to monitor the course of various biological processes in real time, for example, photoinduced cell death during photodynamic therapy [24–29].
Such xanthene dyes as fluorescein and its derivativesare widely used in biological research as pH indicator labels. The strong dependence of their fluorescence on pH is caused by the presence of several acid-base and keto-enol forms with different photophysical properties: quantum yield and fluorescence lifetime [21–23]. Another fluorophore, 1,3,5,7‒tetramethyl-8-(4-hydroxyphenyl)-4,4-difluoro-4-boron-3a,4a-diazaindacene (BODIPY), has high thermal and photochemical stability, high fluorescence quantum yield, good solubility, and chemical resistance [1–3, 5–18]. The newest direction in the study of dyes is the production of new fluorescent materials based on them [30–34]. The combination of Sn(IV) porphyrinates and fluorophores makes it possible to obtain conjugates having both rotary and pH-indicator properties at the same time.
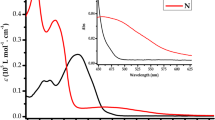
Modification of porphyrin molecules in most cases leads to significant changes in their spectra. Compared with the electronic absorption spectra of dihydroxy-Sn(IV)2,3,7,8,12,13,17,18-octaethylporphyrinate (SnP) 1 in N,N-dimethylformamide (DMF), characteristic changes were recorded in the spectra of its complexes with fluorophores, 1,3,5,7-tetramethyl-8-(4-hydroxyphenyl)-4,4-difluoro-4-boron-3a,4a-diazaindacene (BODIPY) 2, and phentolphthalein (Pht) 4, i.e. triads 3 and 5. The BODIPY-SNP-BODIPY triad 3 is characterized by only small hypochromic shifts of the absorption bands QI and QII by 3 and 4 nm, respectively, and the Soret bands by 5 nm. In the electronic absorption spectra of individual fluorophore 2, a rather intense absorption band, λ = 499 nm, with a shoulder in the region of 470 nm is observed (Fig. 1).

In the electronic absorption spectra of triad 5 with phenolphthalein, Pht-SnP-Pht, a hypsochromic shift of the Qx bands and the Soret band by 1 nm in comparison with the spectrum of compound 1 was recorded (Fig. 2), in addition, the shoulder at λ = 387 nm disappears. Individual phenolphthalein 4 in DMF has one low-intensity absorption band, λ = 425 nm.
The fluorescence spectra, and also the calculated fluorescence quantum yields of triads 3, 5 and fragments 1, 2, and 4 in DMF are shown in Fig. 3 and 4 and Table 1.
The known diaxial complexes of Sn(IV) porphyrinates with various phenolic derivatives have a two-band fluorescence spectrum: two distinct emission bands Qx (0,0) and Qx (0,1) correspond to the transition S1→S0. The fluorescence quantum yield and lifetime depend significantly on the nature of the porphyrin macrocycle, the nature of axial ligands, and the medium. In the vast majority of cases, the replacement of hydroxyl groups in the axial positions of Sn(IV) porphyrinate with phenol derivatives leads to a decrease in the macrocycle fluorescence from several percent to its complete quenching.
The introduction of phenolphthalein 4 molecules into tin porphyrinate 1 leads, as in the case of the introduction of other xanthene dyes [30, 32], to a significant quenching of fluorescence. The quantum yield of Pht-SnP-Pht triad 5 (φf 0.001) is 50 times less than that of compound 1 (Fig. 3). The introduction of phenolphthalein 4 leads to a stronger quenching of fluorescence (50 times) compared with the introduction of fluorescein (4 times) [30]. Apparently, the decrease in the quantum yield of fluorescence in triad 5 is associated with an increase in energy consumption for nonradiative relaxation.
The fluorescence of compounds containing several structural elements, as a rule, depends on the excitation wavelength. The observed low-intensity absorption band of the phenolphthalein solution in DMF is close to the Soret band of triad 5 (Fig. 2), the fluorescence of the porphyrin fragment and the fluorescence of the phenolphthalein fragment in triad 5 are indistinguishable, that is, for Pht–SnP–Pht, there is no dependence of fluorescence on the excitation wavelength (Fig. 3). In turn, for triad 3 the dependence of fluorescence on the excitation wavelength is strongly pronounced.
The analysis of the data for BODIPY–SnP–BODIPY 3 triad indicates that when it is irradiated, energy is transferred from the donor to the acceptor, that is, from axial ligands to Sn(IV) porphyrinate, and an increase (λexc = 400 nm) or the appearance (λexc = 490 nm) of fluorescence is observed in the spectra of the porphyrin fragment (Fig. 4). With selective excitation of both BODIPY and macrocycle, in the triad photoinduced energy is transferred as shown in schemes 1 and 2.
When the triad is excited at a wavelength of 490 nm, the BODIPY fragment is also excited, i.e. an excited singlet state [D*–A–D*] is formed (Scheme 1). The return to the ground state from the state [D*–A–D*] can occur both through the emission of BODIPY fluorescence and through a rapid photoinduced energy transfer from the donor-BODIPY to the acceptor-porphyrinate with the formation of the excited singlet state [D–A*–D] with an excited central fragment, which can partially turn into the ground state due to porphyrin fluorescence observed in the form of nonradiative quenching, and also, according to [19, 20], partially undergo an intercombination transition to form an excited porphyrin triplet state (Fig. 4). Our data on the increase in the fluorescence lifetime of individual compound 2 (1.74) up to 3.43 in triad 3 indirectly support the latter variant.
When triad 3 is excited at a wavelength of λexc = 490 nm, fluorescence spectra characteristic of molecule 2, but of lower intensity, are recorded (Fig. 4c). Fluorescence of 1,3,5,7-tetramethyl-8-(4-hydroxyphenyl)-4,4-difluoro-4-boron-3a,4a-diazaindacene in the composition of complex 3 (φf = 0.06, с = 6×10–7 M) undergoes quenching compared to the free molecule 2 (φf = 0.65, с = 12× 10–9 M) and 11-fold decreases. In the spectrum of triad 3, a band appears, λ = 577 nm (fluorescence of the porphyrin fragment, Fig. 4c). The data obtained suggest that the triad energy during the excitation of compound 2 partially relaxes with the emission of fluorescence (hνEmD) and is partially transferred to the porphyrin macrocycle, transferring it to the excited singlet state (Scheme 1).
When triad 3 is excited in DMF, λexc = 400 nm, only fluorescence spectra characteristic of Sn(IV) porphyrinate molecules are recorded. The quantum yield of compound 3 fluorescence increases by almost 2.5 times compared to compound 1 (Table 1). Judging by the data obtained, the mechanism shown in Scheme 2 is most likely implemented. The transition of compound 2 in the triad to its first excited state is followed by a rapid energy transfer and a transition to the porphyrin singlet excited state, which partially relaxes in the form of porphyrin fluorescence and partially undergoes an intercombination transition with the formation of the corresponding triplet excited state of porphyrin.
The fraction of energy that is emitted in the form of fluorescence followed by the intercombination transition (ISC) depends both on the solvent (polarity, viscosity, etc.) and on the triad structure. In the case of triad 3 in toluene almost all the energy of the second excited state undergoes an intercombination transition [34], whereas in in DMF solution of triad 3, presumably, most of this energy is emitted in the form of fluorescence of the porphyrin link. An increase in the fluorescence intensity of the porphyrin fragment in triad 3 compared to compound 1 provides a photoinduced energy transfer to SnP from donor 2.
The change in the acidity of the triad 3 solution in DMF affects the intensity of its fluorescence. At λexc = 400 nm in acidic media, compound 3 shows a lower fluorescence quantum yield than in neutral and alkaline media (Table 1). The quantum yield of the porphyrin fragment twice decreases when pH of the solution decreases to 0.7 (Table 1). For complex 3, on the contrary, an increase in fluorescence is observed in an alkaline meedium. A possible reason for the pH dependence of compound 3 fluorescence is probably the dependence of the fraction of deactivated excited state [D*–A–D*] energy on the acidity of the medium due to the partial energy transfer to the porphyrinate fragment. The greater the acidity and polarity of the medium, the less photoinduced energy transfer and the weaker the fluorescence of the SnP fragment, whereas the fluorescence of the BODIPY fragments is stronger.
During acid-base titration at λexc = 490 nm (Fig. 5), it was found that triad 3 is more sensitive to pH, especially in an acidic medium (compared with free compound 2). The fluorescence quantum yield of complex 3 in acidified DMF solution (pH 0.7), is more than twice higher than φf of this complex in pure DMF (Fig. 5a).
When pH changes in the range 5.2‒10.2, the quantum yield of compound 3 fluorescence (λexc = 490 nm) does not change so significantly (Fig. 5b, Table 1). A further increase in the pH to 11.2 leads to a sharp decrease in the fluorescence intensity of the compound 2 fragment as part of complex 3, and the low-intensity fluorescence of SnP fragment 1 (577 nm) does not undergo any changes during acid-base titration. A decrease in the fluorescence intensity of individual compound 2 occurs due to the formation of an anionic form that does not have fluorescent properties [10–12]. The decrease in φf values of triad 3 in a highly alkaline medium (λexc = 490 nm) is apparently due to its destruction with the formation of Sn porphyrinate 1 and the anionic form of compound 2.
According to the obtained spectral data, fluorescence of triad 5 in a slightly acidic medium first significantly increase (Fig. 6a), and with a further decrease in the pH of the solution from 4.2‒0.7 (Fig. 6c) its gradual attenuation is recorded (Table 1). A slight increase and then quenching of fluorescence at similar pH values of the solution were observed for compound 1. The quantum yield of triad 5 fluorescence at pH 0.7 increases 13-fold compared to φf in DMF (Table 1) despite the attenuation of fluorescence. The fluorescence of phenolphthalein does not exhibit pH-indicator sensitivity in acidic medium.
Phenolphthalein 4 demonstrates indicator properties due to the formation of various protolytic forms. It is particularly sensitive in alkaline aqueous solutions in the pH range 8.2‒12, forming a dianion. It was expected that its properties in the composition of triad 5 would be also presedsved.
For Pht-SnP-Pht 5 in the region of alkaline pH values in DMF, an increase in fluorescence was recorded (Fig. 7), which is typical for individual compounds 1 and 4, however, triad 5 shows significantly greater sensitivity under comparable conditions. With an increase in the pH of the DMF solution to 8.3, the quantum yield of triad 5 fluorescence increases 24-fold. No further significant change in fluorescence was observed as the pH of the solution increased to 13.5 (Table 1), however, a bathochromic shift of 2 nm of the emission band was recorded when pH 9.3 was reached, but with a further increase in the pH of the solution, no band displacement occurred (Fig. 7).
The observed spectral changes seem to indicate the formation of the anionic form of phenolphthalein in complex 5. With an increase in the pH of the DMF alkaline solution, a slight increase in the quantum yield of fluorescence of individual phenolphthalein is observed. It can be assumed that the appearance of indicator dependence of Pht–SnP–Pht 5 triad is associated not only with the formation of the anionic form of phenolphthalein in triad 5, but, to a greater extent, with a change in the fraction of nonradiative energy with varying pH of the medium.
Thus, the fluorescent properties of Sn(IV) porphyrinates are significantly affected by the nature of axial substituents. Complex formation with molecules 2 leads to a significant increase in fluorescence. In addition, triad 3 acquires pH-indicator properties depending on the excitation wavelength. When porphyrin is excited in triad 3, indicator properties associated with fluorescence quenching appear in acidic medium, which are absent in individual compound 1. When triad 3 is excited at the absorption wavelength of compound 2 in a highly alkaline medium, a sharp quenching of fluorescence caused by the destruction of the triad is recorded, which makes it possible to use compound 3 as a pH indicator for an alkaline medium. In turn, triad 5, despite a rather low fluorescence in a neutral medium, demonstrated a strong increase in fluorescence throughout the pH range.
EXPERIMENTAL
Synthesis and spectral characteristics of dihydroxy-Sn(IV)2,3,7,8,12,13,17,18- octaethylporphyrinate (SnP) 1 are presented in [30].
Complex of Sn(IV)-2,3,7,8,12,13,17,18-octaethylporphyrinate (SnP, 1) with 1,3,5,7-tetramethyl-8-(4-hydroxyphenyl)-4,4-diphtor-4-bora-3a,4a-diazaindacene (2), triad BODIPY-SnP-BODIPY (3). Compound 1 (18.2 mg, 0.012 mmol) and 10.2 mg (0.030 mmol) of compound 2 were dissolved in 10 mLof N,N-dimethylformamide (DMF) and boiled for 3 h. The purification was carried out with the help of colon chromatography (adsorbent ‒ aluminum oxide, eluent—DMF). Yield 6 mg (38%). EAS (DMF), λmax (log ε), nm: 383 (3.97), 401 (5.35), 534 (3.74), and 571 (4.02). 1H NMR spectrum (DMSO-d6), δ, ppm: 1.34 t (6H, CH3), 1.56 t (6H, CH3), 2.06 t (24Н, β-СН3), 4.24 m (16Н, β-СН2), 6.45 d (4НmAr(L), J = 8.9 Hz), 6.85 d (4НоАr(L), J = 8.9 Hz), 6.88 s (4 Н, β-Н), 10.51 s (4Н, meso-H). Mass spectrum (MALDI-TOF) (m/z): 1330.5623.
Complex of Sn(IV)2,3,7,8,12,13,17,18-octaethylporphyrinate (I) with phenolphthalein (Pht, 4), triad Pht–SnP–Pht (5). Compound 1 (15.15 mg, 0.022 mmol) and 17.50 mg (0.055 mmol) of phenolphthalein 4 were dissolved in 5 mLof DMF and boiled for 30 min. The reaction mixture was cooled, and the reaction product was extracted with chloroform. The extract was evaporated, and the dry residue was washed with alcohol. Yield 16 mg (64%). EAS (DMF), λmax (log ε) nm: 405 (5.41), 536 (4.21), and 574 (4.17). 1H NMR spectrum (DMSO-d6), δ, ppm: 2.06 t (24Н, β-СН3), 4.24 m (16Н, β-СН2), 6.54 d (4Нm, PhOH), 6.59 d (4Нm, PhOH), 6.75 t (2Hо, PhOH), 7.07 d (2Но, PhOH), 7.18 d (2Но, PhOH), 7.54 t (2Hо, PhOH), 7.67 m (3H, Ph), 7.76 d (3H, Ph), 7.82 t (1Н, Ph), 7.90 d (1Н, OH), 10.51 s (4Н, meso-H). Mass Spectrum (MALDI-TOF) (m/z): 1286.4235.
1H NMR spectra (operating frequency 500.17 MHz) were recorded using a Bruker Avance III 500 spectrometer. Mass spectra were obtained using a Shimadzu Biotech Amino Confidence Maldi mass spectrometer (Kratos Analytical Limited, UK). Electronic absorption spectra were taken on a Cary 300 spectrophotometer (Agilent, USA), the fluorescence spectra were taken on an RF 5301PC spectrofluorimeter (Shimadzu, Japan). The excitation wavelength λexc = 400 and 490 nm, the width of the excitation and emission slit is 5 nm.
The fluorescence quantum yield was calculated by formula (1).
Here φst is the quantum yield of the standard, Ix and Ist are the integral arfeas of the triad and standard in the fluorescence spectra, respectively, Ax and Ast are the optical densities of the triad and standard at the excitation wavelengths, respectively, nx and nst are the refractive indices for the triad and standard.
The acidity of the solution was controlled using a digital pH meter 98 108 for viscous liquids. The required pH values were obtained by adding aqueous KOH solutions to a dimethylformamide solution (1.82×10–7– 1.82 M) and HCl (1.2×10–3‒12 M).
REFERENCES
Radunz, S., Wedepohl, S., Röhr, M., Calderón, M., Tschiche, H.R., and Resch-Genger, U., J. Med. Chem., 2020, vol. 63, no. 4, p. 1699. https://doi.org/10.1021/acs.jmedchem.9b01873
Radunz, S., Kraus, W., Bischoff, F.A., Emmerling, F., Rune Tschiche, H., and Resch-Genger, U., J. Phys. Chem. A, 2020, vol. 124, no. 9, p. 1787. https://doi.org/10.1021/acs.jpca.9b11859
Radunz, S., Andresen, E., Würth, Ch., Koerdt, A., Rune Tschiche, H., and Resch-Genger, U., Anal. Chem., 2019, vol. 91, no. 12, p. 7756. https://doi.org/10.1021/acs.analchem.9b01174
Kadokawa, J., Suenaga, M., and Kaneko, Y., Chem. Lett., 2008, vol. 37, no. 12, p. 1232. https://doi.org/10.1246/cl.2008.1232
Kaur, P. and Singh, K., J. Mater. Chem. C, 2019, vol. 7, p. 11361. https://doi.org/10.1039/C9TC03719E
Salim, M.M., Owens, E.A., Gao, T., Lee, J.H., Hyun, H., Choi, H.S., and Henary, M., Analyst, 2014, vol. 139, p. 4862. https://doi.org/10.1039/C4AN01104J
Ulrich, G., Ziessel, R., and Harriman, A., Angew. Chem. Int. Ed., 2008, vol. 47, no. 7, p. 1184. https://doi.org/10.1002/anie.200702070
Li, X., Sun, S.S., Kim, I.J., and Son, Y.-A., Molec. Crys. Liquid Cryst., 2017, vol. 654, p. 131. https://doi.org/10.1080/15421406.2017.1358016
Boens, N., Leen, V., and Dehaen, W., Chem. Soc. Rev., 2012, vol. 41, p. 1130. https://doi.org/10.1039/C1CS15132K
Marfin, Y.S., Merkushev, D.A., and Usoltsev, S.D., J. Fluoresc., 2015, vol. 25, p. 1517. https://doi.org/10.1007/s10895-015-1643-9
Bañuelos, J., Arbeloa, F.L., Arbeloa, T., Salleres, S., Vilas, J.L., Amat-Guerri, F., Liras, M., and Arbeloa, I.L., J. Fluoresc., 2008, vol. 18, p. 899. https://doi.org/10.1007/s10895-008-0320-7
Gareis, Th., Huber, Ch., Wolfbeis, O.S., and Daub, J., Chem. Commun., 1997, p. 1717. https://doi.org/10.1039/A703536E
Su, D., Teoh, Ch.L., Gao, N., Xu, Q.-H., and Chang, Y.-T., Sensors, 2016, vol. 16, no. 9, p. 1397. https://doi.org/10.3390/s16091397
Zhu, H., Fan, J., Li, M., Cao, J., Wang, J., and Peng, X., Chem.–Eur. J., 2014, vol. 20, no. 16, p. 4691. https://doi.org/10.1002/chem.201304296
Vyšniauskas, A., López-Duarte, I., Duchemin, N., Vu, Th.-T., Wu, Y., Budynina, E.M., Volkova, Y.A., Cabrera, E.P., Ramírez-Ornelas, D.E., and Kuimova, M.K., Phys. Chem. Chem. Phys., 2017, vol. 19, p. 25252. https://doi.org/10.1039/C7CP03571C
Perronet, K., Bouyer, P., Westbrook, N., Soler, N., Fourmy, D., and Yoshizawa, S., J. Lumin., 2007, vol. 127, no. 1, p. 264. https://doi.org/10.1016/j.jlumin.2007.02.051
Nguyen, N.T., Verbelen, B., Leen, V., Waelkens, E., Dehaen, W., and Kruk, M., J. Lumin., 2016, vol. 179, p. 306. https://doi.org/10.1016/j.jlumin.2016.06.043
Ji, D., Zhao, R., Huang, Zh., and Xia, A., J. Lumin., 2007, vols. 122–123, p. 253. https://doi.org/10.1016/j.jlumin.2006.01.128
Stoll, L.K., Zgierski, M.Z., and Kozlowski, P.M., J. Phys. Chem. A, 2002, vol. 106, no. 1, p. 170. https://doi.org/10.1021/jp012416k
Ivashin, N.V., Opt. Spectrosc., 2021, vol. 129, p. 935. https://doi.org/10.1134/S0030400X21070092
Boguta, A. and Wróbel, D., J. Fluoresc., 2001, vol. 11, no. 2, p. 129. https://doi.org/10.1023/A:1016681502731:ehyfk
Ciftci, G.Y., Durmus, M., Senkuytu, E., and Kilic, A., Spectrochim. Acta A, 2009, vol. 74, no. 4, p. 881. https://doi.org/10.1016/j.saa.2009.08.028
Latterini, L., Elisei, F., Aloisi, G.G., Costantino, U., and Nocchetti, M., Phys. Chem. Chem. Phys., 2002, vol. 4, p. 2792. https://doi.org/10.1039/b201167k
Babu, B., Mack, J., and Nyokong, T., Dalton Trans., 2020, vol. 49, p. 15180. https://doi.org/10.1039/D0DT03296D
Babu, B., Mack, J., and Nyokong, T., New J. Chem., 2022, vol. 46, p. 5288. https://doi.org/10.1039/D2NJ00350C
Huang, H., Chauhan, S., Geng, J., Qin, Y., Watson, D.F., and Lovell, J.F., Biomacromolecules, 2017, vol. 18, no. 2, p. 562. https://doi.org/10.1021/acs.biomac.6b01715
Magaela, N.B., Balaji, R.M., Managa, B.M., Prinsloo, E., and Nyokong, T., Polyhedron, 2022, vol. 213, p. 115624. https://doi.org/10.1016/j.poly.2021.115624
Babu, B., Soy, R.C., Mack, J., and Nyokong, T., New J. Chem., 2020, vol. 44, p. 11006. https://doi.org/10.1039/D0NJ01564D
Ravikumarm, M., Raghavm, D., Rathinasamym, K., Kathiravanm,,, A., and Mothim, E.M., ACS Appl. Bio Mater., 2018, vol. 1, no. 5, p. 1705. https://doi.org/10.1021/acsabm.8b00507
Likhonina, A.E., Mamardashvili, G.M., and Mamardashvili, N.Z., J. Photochem. Photobio. A: Chem., 2022, vol. 424, p. 113650. https://doi.org/10.1016/j.jphotochem.2021.113650
Mamardashvili, G.M., Maltceva, O.V., Lazovskiya, D.A., Khodov, I.A., Borovkov, V., Mamardashvili, N.Zh., and Koifman, O.I., J. Molec. Liq., 2019, vol. 277, no. 1, p. 1047. https://doi.org/10.1016/j.molliq.2018.12.118
Lazovskiy, D.A., Mamardashvili, G.M., Khodov, I.A., and Mamardashvili, N.Z., J. Photochem. Photobio. A: Chem., 2020, vol. 402, p. 112832. https://doi.org/10.1016/j.jphotochem.2020.112832
Mamardashvili, G.M., Kaigorodova, E.Yu., Simonova, O.R., Lazovskiy, D.A., and Mamardashvili, N.Z., J. Molec. Liq., 2020, vol. 318, p. 113988. https://doi.org/10.1016/j.molliq.2020.113988
Lazarides, T., Kuhri, S., Charalambidis, G., Panda, M.K., Guldi, D.M., and Coutsolelos, A.G., Inorg. Chem., 2012, vol. 51, p. 4193. https://doi.org/10.1021/ic2026472
Funding
The work was supported by a grant of the Russian Science Foundation (project no. 22-23-00018) with the use of equipment from the Upper Volga Regional Center for Physical and Chemical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Likhonina, A.E., Bryksina, D.A. & Mamardashvili, N.Z. Fluorescent and Acid-Base Indicator Properties of Complexes Based on Sn(IV) Octaethylporphyrinate and Molecules of Dye: Phenolphthalein and 1,3,5,7-Tetramethyl-8-(4-hydroxyphenyl) (BODIPY). Russ J Gen Chem 92, 2786–2795 (2022). https://doi.org/10.1134/S1070363222120295
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222120295