Abstract
The direct synthesis of symmetrically substituted porphyrins: 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphyrin and 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrin – was proposed. The obtained compounds were identified by electron absorption, 1H NMR spectroscopy and mass spectrometry. The acid-base and coordination properties of 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphine and 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphine with respect to Zn2+ and Pd2+ ions in acetonitrile at 298–328 K were studied. The effect of substituents in the β-positions and meso-phenyl fragments of the macrocycle on the spectral and coordination properties of the analyzed compounds was revealed. β-Unsubstituted analogs studied earlier were used as objects of comparison.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Investigation of the electronic and steric effects influence of substituents on the porphyrin macrocycle properties is necessary to create the physicochemical basis for the preparation of new materials. Porphyrins substituted with a large number of phenyl fragments at the meso-positions of the macrocycle and with methoxy groups and halogens at the β-positions are the most accessible, well-studied, and successfully applied in practice [1–10]. Porphyrins solutions and their metal complexes in organic solvents are capable of changing their electron-optical properties when the porphyrin molecule when exposed to organic bases and acids in a wide pH range. The intracyclic macrocycle cavity can form a charge that provides a high chemical affinity of the molecule for cations and anions in solution. Chemical complexation of a macrocycle and a charged ion finds a strong spectrophotometric reply in the electron absorption spectra and is accompanied with a color reaction, which makes it possible to create receptors for the recognition ions of various natures. In this regard, a more detailed study of the acid-base and complexing properties of porphyrins and their analogs is necessary.
2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphyrin 1 [H2Br8(3,5-BrPh)P] and 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrin 2 [H2Br8(3,4,5-MeOPh)Р] were synthesized according to Scheme 1. With Adler’s method, 5,10,15,20-tetra(3,5-dibromophenyl) porphyrin [11] and 5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrin [12] were obtained, which were converted into cobalt complexes: 5,10,15,20-tetra(3,5-dibromophenyl) porphyrinate Co (II) 3 and a mixture of 5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrinates Co (II) and Co (III) (4). Then, based on optimized procedures [13–16], we synthesized 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphyrinate Co (II) 5 and 2,3,7,8,12,13,17,18-octabromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrinate Co (II) 6. The treatment of cobalt complexes 4, 6 with a mixture of perchloric and sulfuric acids leads to compounds 1 and 2. In the synthesis of porphyrin 1, bromine was used in addition to N-bromosuccinimide (NBS).
The acid-base and complexing properties of compounds 1 and 2 with respect to Zn2+ and Pd2+ ions were studied spectrophotometrically in acetonitrile at 298–328 K; previously studied β-unsubstituted analogs were used as objects of comparison. The substituent nature and its position in the macrocycle have a significant effect on the acidic properties of porphyrins.
Research of protonation and deprotonation of porphyrins 1 and 2 in the systems acetonitrile–HClO4 (A) and acetonitrile‒1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (B) [Eqs. (1)–(4)] has showed that the modification of the porphyrin macrocycle changes the acid-base properties of compounds 1 and 2 in compared with the β-unsubstituted analogs [H2(3,5-BrPh)P and H2(4-MeO-3-BrPh)P] [17].
Here Н2Р, HP‒, Р2‒, H3P+, H4P2+ are molecular, mono- and doubly deprotonated and protonated porphyrin forms.
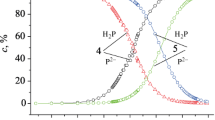
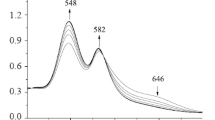
The electronic absorption spectra of compounds 1 and 2 in acetonitrile during titration with 0.01 M acetonitrile solutions of HClO4 and DBU are shown in Figs. 1–4. Analysis of absorption spectra showed that with an increasing concentration of HClO4 and DBU, the formation of two families of spectral curves is observed, each of which corresponds to its own set of isobestic points.
The presence of two families of isobestic points in the electronic absorption spectra is characteristic of stepwise protonation. But, the spectrophotometric titration curves constructed on the basis of the experimental data did not have pronounced steps, which does not deny stepwise ionization, but assumes close values of the protonation constants in each reaction [18]. The isobestic points and the nature of the change in the absorption spectra indicate that, as the concentrations of the two absorbing centers of the porphyrin molecule change, the ratio between the ionized forms during protonation and deprotonation of porphyrins is not violated. The effective extinction coefficients for all forms of porphyrins 1 and 2, participating in equilibria (1)–(4), were determined using the data on the absorption and total particle concentration of each porphyrin (Table 1). The total protonation and deprotonation constants for reactions (1)–(4) were calculated using Eqs. (5), (6).
Here Kb1,2—the total protonation constant for the first and second steps, Ind— the indicator ratio [H2P]/[H4P2+]. The pH–log сHClO4 dependence [19] was used to calculate the protonation constants.
Here K—the total protonation constant for the first and second steps, Ind—the indicator ratio [P2-]/[H2P], cDBU—titrant concentration DBU, mol/L. The error in the constants measuring did not exceed 3–5% (Table 1).
Analyzing the data in the Table 1, it can be concluded that the basicity values of compounds 1 and 2 in system A decrease by 3.5 and by one order of magnitude, respectively, compared to H2(3,5-BrPh)P, H2(4-MeO-3-BrPh)P. Additional bromination of compounds 1 and 2 leads to the manifestation of acidic properties of porphyrins 1 and 2 in system B, in contrast to compounds H2(3,5-BrPh)P, H2(4-MeO-3-BrPh)P.
The formation kinetics of zinc and palladium complexes of porphyrins 1 and 2 in acetonitrile was studied spectrophotometrically [20] in the systems Zn(OAc)2–acetonitrile (B) and Zn (OAc)2–DBU–acetonitrile (C), Pd(OAc)2–acetonitrile (D), and Pd(OAc)2–DBU–acetonitrile (E). The application of spectrophotometry is possible due to significant differences in the electronic absorption spectra of porphyrins and their complexes.
In an individual solvent (acetonitrile) the interaction of porphyrins 1 and 2 in systems В–F with metal acetates proceeds according to Eqs. (7), (8) [9].
Here H2P and P2‒—the molecular and doubly deprotonated forms of porphyrins 1 and 2, M2+—the zinc or palladium cation, OAc—the acetate ion, Solv—the solvent molecule, and n—the coordination number of the metal cation.
Clear isobestic points were observed in the spectra of the reacting systems, and reactions (7) and (8) conformed to the first order about porphyrin, which is also evidenced by the straight-line character of the dependences log (с0H2P/сH2P) on time τ (s). The straight-line dependence of log keff on log сMeOAc)2 (slope tangent 0.999‒1.100, correlation coefficient 0.999) also indicates the first order of formation of palladium and zinc complexes. The kinetic parameters of the formation of zinc and palladium complexes in acetonitrile are presented in Table 2.
The formation of zinc complexes of compounds 1 and 2 in acetonitrile proceeds by two ways [reactions (7) and (8)] in systems C, D. Palladium complexes of compounds 1 and 2 are formed only in system F according in reaction (8).
From a comparison of the kinetic parameters of coordination of Zn (II) and Pd (II) with porphyrins 1, 2, it follows that the introduction of substituents in β-positions of the porphyrin changes the coordination properties of the macrocycle, which is apparently associated with the electronic effect of the substituents and the ability of the tetrapyrrole macroring to deformation. The ligands activity in such systems is also determined by the structure of acid-base complexes formed in solutions, the ionizing ability of which depends on the degree of proton transfer from an acid molecule to a base (solvent) molecule. The nature of the metal-complexing agent significantly affects the value of the complexation rate constant. In system С [reaction (7)], the rate of formation of zinc complexes for β-substituted porphyrins 1 and 2 decreases by a factor of ~ 2 in comparison with the previously studied unsubstituted analogs (Table 2). In system D [reaction (8)], an increase in the rate of complexation by a factor of ~ 2.5 is observed in comparison with the reactions in system B. Previously, it was established that unsubstituted at the β-positions analogs of porphyrins 1 and 2 form palladium complexes in system E several orders of magnitude slower than the formation of their zinc complexes in system C [16]. Porphyrins 1 and 2 form palladium complexes only in system F in the presence of an organic base, which facilitates reaction (8) with lower energy costs due to the necessity for cleavage and solvation of the N‒H bond of the reaction center. In system E, the palladium complexes formation with porphyrins 1 and 2 under experimental conditions does not occur. Probably, the introduction of bromine atoms in β-positions leads to an increase in the acidic properties and to a decrease in the complexing properties of porphyrins 1 and 2 because of the inevitable energy costs during the interaction of particles with the same charge.
Thus, chemical modification makes it possible to purposefully change the basic and complexing properties of macroheterocyclic ligands and the porphyrins reactivity.
EXPERIMENTAL
5,10,15,20-Tetra(3,5-dibromophenyl) porphyrin and 5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrin were synthesized according to published procedures [12, 21]. We used N-bromosuccinimide, cobalt acetate, sulfuric acid (Acros), aluminum oxide (Merck), chloroform, dichloromethane, hexane, dimethylformamide, perchloric acid, bromine (chemically pure).
Electronic absorption spectra were recorded on a Cary-100 (Varian) spectrophotometer. Mass spectra were recorded on a Maldi Tof Shimadzu Biotech Axima Confidence mass-spectrometer (matrix—dihydroxybenzoic acid). 1H NMR spectra were recorded on a Bruker AV III-500 device (internal standard—TMS). Elemental analysis was performed using Flash EA 1112 analyzer.
For investigation of the acid-base and coordination properties of porphyrins 1, 2, we used as solvent acetonitrile (Lab-Scan) of high purity (water fraction less than 0.03%), in which the starting compounds were in molecular form according to electronic absorption spectra.
Kinetic measurements (Cary 100 (Varian) spectrophotometer) were carried out in thermostated cuvettes with ground joint in the temperature range from 298 to 318 K (three replications at three different temperatures). The temperature variation did not exceed ±0.1°. Spectrophotometric titration of porphyrins 1 and 2 with perchloric acid solutions in acetonitrile was performed on a Cary 100 spectrophotometer. The experimental procedure (preparative part and processing of experimental data) is presented in detail in [22, 23].
5,10,15,20-Tetra(3,5-dibromophenyl) porphyrinate Со(II) (3). The mixture of 0.04 g (0.0322 mmol) tetra(3,5-dibromophenyl) porphyrin and 0.057 g (0.0322 mmol) Co(OAc)2 in 45 mL of DMF was refluxed for 10 min. The reaction mixture was cooled and spilled in water. The precipitate was filtered, washed with water, dried, and chromatographed on aluminum oxide, eluent— chloroform. Yield 0.031 g (0.0238 mmol, 74%). Electron absorption spectrum (DMF), λ, nm (log ɛ): 415 (5.33), 530 (4.24). 1Н NMR spectrum (CDCl3), δ, ppm: 15.76 br. s (8Н, pyroll), 13.05 br. s (8Но), 10.02 br. s (4Нп). Mass spectrum, m/z (Irel, %): 1304.3 (97) [M + Н]+. С44H20Br8CoN4. М 1302.9.
5,10,15,20-Tetra(3,4,5-methoxyphenyl) porphyrinates Со(II) and Со(III) (4). The mixture of 0.04 g (0.041 mmol) tetra(3,4,5-trimethoxyphenyl) porphyrin and 0.072 g (0.41 mmol) Cо(OAc)2 in 30 mL of DMF was refluxed for 15 s and treated as compound 3. Yield 0.036 g (0.0349 mmol, 85%). Electron absorption spectrum (DMF), λ, nm [Irel]: 419 [1.04], 433 [0.178], 535 [0.149]. Electron absorption spectrum (CHCl3), λ, nm [Irel]: 415 [1.31], 434 [1.26], 537 [0.24]. 1Н NMR spectrum (CDCl3), δ, ppm: Со(II)-porphyrin, 16.29 br. s (8Н, pyrrol), 12.72 br. s (8Но), 5.57 s (12Н, OCH3), 5.01 s (24H, OCH3); Со((III)-porphyrin, 9.15 s (8Н, pyrrol), 7.48 d (8Но, J 7.70 Hz), 4.20 s (12Н, OCH3), 3.99 (24Н, OCH3). Mass spectrum, m/z (Irel, %): 1032.08 (98) [M]+. С56Н52CoN4О12. М 1032.05.
2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphyrinate Со(II) (5). To the solution of 0.02 g (0.0154 mmol) of complex 3 in mixture of 8 mL chloroform and 2 mL DMF, 0.055 g (0.308 mmol) NBS was added, the mixture was kept at room temperature for 4 h. Bromine 0.04 mL (0.12 g) in 2 mL chloroform was added to the reaction mixture and was kept for 4 h. Bromine excess was neutralized with sodium tiosulfate solution. The reaction mixture was extensively washed with water, dried under Na2SO4, and evaporated. The residue was chromatographed on aluminum oxide, eluent—dichloromethane, and then chloroform. Yield 0.019 g (0.00982 mmol, 65%). Electron absorption spectrum (DMF), λ, nm (log ε): 457 (4.96), 566 (4.14). 1Н NMR spectrum (CDCl3), δ, ppm: 10.22 br. s (8Но), 8.13 br. s (4Нn). Found, %: С 27.09; Н 0.58; Br 65.53; N 2.78. С44H12Br16CoN4. Calculated, %: С 27.33; Н 0.63; Br 66.10; N 2.90. M 1934.01.
2,3,7,8,12,13,17,18-Octobromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphinate Со(II) (6). To solution of 0.02 g (0.0194 mmol) of complex 4 in mixture 8 mL chloroform and 2 mL DMF, 0.069 g (0.388 mmol) NBS was added in three steps every 10 minutes. The reaction mixture of evaporated to minimum value, added 2 mL DMF, water, and solid NaCl. The residue was filtered, washed with water, dried, and chromatographed on aluminum oxide, eluent—chloroform. Yield 0.024 g (0.0144 mmol, 75%). Electron absorption spectrum (DMF), λ, nm (log ε): 457 (5.02), 572 (4.21). Electron absorption spectrum (CHCl3), λ, nm [Irel]: 448 [1.23], 566 [0.22]. 1Н NMR spectrum (CDCl3), δ, ppm: 10.05 br. s (8Но), 5.32 s (24Н, ОСН3), 4.18 s (12Н, ОСН3). Found, %: С 40.23; Н 2.54; Br 38.11; N 3.26. С56Н44Br8CoN4О12. Calculated, %: С 40.44; Н 2.67; Br 38.43; N 3.37. M 1663.15.
2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-tetra(3,5-dibromophenyl) porphyrin (1). To 0.025 g of cobalt complex 5 in 8 mL of chloroform, 3 mL of 58% perchloric and 2.5 mL of 96% of sulfuric acids were added. The obtained mixture was stirred at room temperature for 1 h. After reaction completion, organic layer was separated, washed with water, ammonia solution, and again with water, dried under Na2SO4, and evaporated to minimum value. The residue was chromatographed on aluminum oxide, eluent—chloroform. Yield 0.017 g (0.0106 mmol, 70%). Electron absorption spectrum (СH2Cl2), λ, nm (log ε): 469 (5.16), 569 (4.21), 624 (4.05), 682 (3.97), 727 (3.83). Found: С 27.65; Н 0.71; Br 67.58; N 2.86. С44H14Br16N4. Calculated: С 28.15; Н 0.75; Br 68.11; N 2.98. М 1877.1.
2,3,7,8,12,13,17,18-Octabromo-5,10,15,20-tetra(3,4,5-trimethoxyphenyl) porphyrin (2) was prepared analogously from 0.025 g of cobalt complex 6, 8 mL of chloroform, 3 mL of 58% perchloroc acid, 1.5 mL of 96% sulfuric acid; reaction time—1.5 h. Yield 0.017 g (0.0106 mmol, 70%). Electron absorption spectrum (СH2Cl2), λ, nm (log ε): 464 (5.08), 558 (4.12), 619 (3.94), 714 (3.75). Found, %: С 41.97; Н 2.78; Br 39.23; N 3.25. С56Н46Br8N4О12. Calculated, %: С 41.8; Н 2.89; Br 39.80; N 3.49. М 1606.24.
REFERENCES
Kim, J.B., Adler, A.D., Longo, F.R., and Dolphin, D., in The Porphyrins, Dolphin, D., Ed., New York: Academic Press, 1978, vol. 1, p. 88.
Syrbu, S.A., Ageeva, T.A., Semeikin, A.S., and Koifman, O.I., Russ. Chem. Bull., 2007, vol. 56, no. 4, p. 707. https://doi.org/10.1007/s11172-007-0108-y
Senge, M.O., Chem. Commun., 2006, no. 3, p. 243. https://doi.org/10.1039/B511389J
Campbell, W.M., Burrell, A.K., Officer, D.L., and Jolley, K.W., Coord. Chem. Rev., 2004, vol. 248, p. 1363. https://doi.org/10.1016/j.ccr.2004.01.007
Pukhovskaya, S.G., Guseva, L.Zh., Semeikin, A.S., and Golubchikov, O.A., Russ. J. Inorg. Chem., 2007, vol. 52, no. 2, p. 293. https://doi.org/10.1134/S0036023607020283
Pukhovskaya, S.G., Guseva, L.Zh., Semeikin, A.S., and Golubchikov, O.A., Kinet. Catal., 2007, vol. 48, no. 2, p. 190. https://doi.org/10.1134/S0023158407020024
Porfiriny: struktura, svoistva, sintez (Porphyrins: Structure, Properties, Synthesis), Enikolopyan, N.S., Ed., Moscow: Nauka, 1985, p. 205.
Berezin, D.B., Ivanova, Yu.B., and Sheinin, V.B., Russ. J. Phys. Chem. (A), 2007, vol. 81, no. 12, p. 1986. https://doi.org/10.1134/S003602440712014X
Uspekhi khimii porfirinov (Advances in Porphyrin Chemistry), Golubchikov, O.A., Ed., St. Petersburg: NII Khimii SPbGU, 2001, vol. 3, p. 47.
Koifman, O.I., Ageeva, T.A., and Syrbu, S.A., Macroheterocycles, 2020, vol. 13, no. 4, p. 183. https://doi.org/10.6060/mhc200814k
Adler, A.D., Longo, F.R., Kampas, F., and Kim, J., J. Inorg. Nucl. Chem., 1970, vol. 32, p. 2443. https://doi.org/10.1016/0022-1902(70)80535-8
Alberry, W.J., Bartlett, P.N., Jones, C.C., and Milgrom, L.R., J. Chem. Res. Synop., 1985, no. 12, p. 364.
Bhyrappa, P. and Krishnan, V., J. Inorg. Chem., 1991, vol. 30, no. 2, p. 239. https://doi.org/10.1021/ic00002a018
Hariprasad, G., Dahal, S., and Maiya, B.G., J. Chem. Soc. Dalton Trans., 1996, vol. 16, p. 3429. https://doi.org/10.1039/dt9960003429
Mamardashvili, N.G., Ivanova, Yu.B., and Chizhova, N.V., Macroheterocycles, 2019, vol. 12, no. 1, p. 22. https://doi.org/10.6060/mhc180900m
Ivanova, Y.B., Chizhova, N.V., and Mamardashvili, N.Z., Russ. J. Org. Chem., 2019, vol. 55, no. 10, p. 1554. https://doi.org/10.1134/S1070428019100142
Ivanova, Yu.B., Semeikin, A.S., Pukhovskaya, S.G., and Mamardashvili, N.G., Russ. J. Org. Chem., 2019, vol. 55, p. 1878. https://doi.org/10.1134/S107042801912011X
Bernshtein, I.Ya., Spektrofotometricheskii analiz v organicheskoi khimii (Spectrophotometric Analysis in Organic Chemistry), Leningrad: Khimiya, 1986, p. 202.
Pukhovskaya, S.G., Nam, D.T., Fien, C.D., Domanina, E.N., Ivanova, Y.B., and Semeikin, A.S., Russ. J. Phys. Chem. (A), 2017, vol. 91, no. 9, p. 1692. https://doi.org/10.7868/S0044453717090278
Berezin, B.D., Coordination Compounds of Porphyrins and Phthalocyanines, New York: Wiley, 1981, p. 286.
Semeikin, A.S., Koifman, O.I., and Berezin, B.D., Chem. Heterocycl. Compd., 1986, no. 6, p. 629. https://doi.org/10.1007/BF00575244
Ivanova, Yu.B., Churakhina, Yu.I., and Mamardashvili, N.Zh., Russ. J. Gen. Chem., 2008, vol. 78, p. 673. https://doi.org/10.1134/S1070363208040269
Ivanova, Yu.B. and Mamardashvili, N.Zh., J. Fluoresc., 2017, vol. 27, p. 303. https://doi.org/10.1007/s10895-016-1958-1
Funding
The study was performed using the equipment of “The upper Volga region centre of physico-chemical research.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 6, pp. 935–942 https://doi.org/10.31857/S0044460X2106010X.
Rights and permissions
About this article
Cite this article
Puchovskaya, S.G., Ivanova, Y.B., Chizhova, N.V. et al. Synthesis, Spectral, Acid-Basic, and Coordination Properties of Bromine- and Methoxy-Substituted Tetraphenylporphyrins. Russ J Gen Chem 91, 1050–1056 (2021). https://doi.org/10.1134/S1070363221060104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221060104