Abstract
A dinuclear Tb complex, Tb2(H2L)3(phen)2 (1), and two similar N-donor coordination complexes, Fe(phen)3·HL (2), Fe(bipy)3·HL·5H2O (3) (Na2H2L = 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt, phen = 1,10-phenanthroline and bipy = 2,2ʹ-bipyridine), have been synthesized and characterized by single crystal X-ray diffraction and elemental analyses. Complex 1 features a phenoxo-O bridged Tb dinuclear structure, in which the dinuclear Tb3+ ions reside in distorted double-capped triangular prism and dodecahedral coordination environments, respectively. Introduction of phen and bipy moieties contributes to formation of low dimensional dinuclear structure. Complexes 2 and 3 feature similar N-donor mononuclear structures, where HL3– anions remain uncoordinated with protonated phenol groups, respectively. Complex 1 exhibits the characteristic emission peaks of Tb3+ ion, and the luminescent properties of complexes 2 and 3 can be attributed to the intraligand transitions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, dinuclear and multinuclear coordination complexes received close attention because of their remarkable properties in catalysis, adsorption, molecular sieving, fluorescence, and single-molecule magnets [1–6]. Polyfunctional ligands are usually empolyed to generate dinuclear and multinuclear coordination complexes with various architectures in the bridging modes [7–9]. To date, a broad number of dinuclear and multinuclear discrete complexes based on bridging ligands have reported in [10–12]. Rational design and assembly still remain a challenge in constructing novel topologies of dinuclear complexes, targeting the structure–properties correlations [13–15]. Dinuclear structures depend on the geometries and functional groups of the organic ligands. A significant effort has been directed towards modification of organic ligands by different functional groups, such as carboxylate, phenol and sulfonate, and controlling self-assembly of the desired structures [16–18]. To our best knowledge, phenol oxygen atoms of organic ligands can efficiently bridge metal ions building stable dinuclear structures making it easy for the ligands containing the phenol groups to form dinuclear complexes with intricate structures.
Herein, we report the syntheses, characterization and properties of one dinuclear coordination complex with brdiging H2L2– ligands, Tb2(H2L)3(phen)2, and two similar N-donor coordination complexes, Fe(phen)3·HL and Fe(bipy)3·HL·5H2O. The TG and fluorescence properties of complexes 1–3 have been studied (Scheme 1).
RESULTS AND DISCUSSION
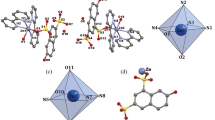
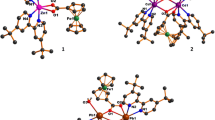
Crystal structure of Tb2(H2L)3(phen)2 (1). The complex 1 is characterized by dinuclear structure, and it crystallizes in the triclinic space group P-1 (Table 1). The asymmetric unit contains two Tb ions, three H2L2– ligands and two phen moieties (Fig. 1a). Crystallographically, there are two Tb sites with different coordination environments. The Tb1 ion is eight-coordinated with four phenol oxygen atoms originated from three H2L2– ligands, two sulfonate oxygen atoms from two H2L2– ligands as well as two nitrogen atoms of the bidentate chelating phen molecule, while Tb2 ion is eight-coordinate with five phenol oxygen atoms from three H2L2– ligands, one sulfonate oxygen atom as well as the bidentate chelating phen molecule. The Tb1 and Tb2 atoms reside in distorted double-capped triangular prism and dodecahedral coordination environments, respectively (Fig. 1b). The Tb–O distances are between 2.289(6)–2.432(6) Å, and the Tb–N distances are in the range of 2.506(8)– 2.556(6) Å (Table 2), that are comparable with those reported for Tb complexes earlier [19, 20]. The Tb1···Tb2 distance over three phenol oxygen atoms is 3.561 Å. The Tb1–O–Tb2 bond angles are 99.6(2)°, 96.4(2)°, and 97.9(2)° (Table 2). These values are comparable to those reported for Tb complexes [19, 20].
Three unique H2L2– ligands in complex 1 adopt one type of coordination mode (Fig. 2). Three H2L2– ligands bridge with two Tb ions via their phenol oxygen and sulfonate oxygen atoms. One phenol group monodentately coordinates with one Tb ion and the other bridges with two Tb ions. All phenol oxygen atoms of H2L2– ligands are 1H-protonated as required for the charge balance. One sulfonate group coordinates with one Tb ion, acting as a monodentate coordination type, whereas the other sulfonate remains non-coordinated and deprotonated. As presented in other references [21], hydrothermal reactions tend to give the 3D coordination polymers with diverse topological structures. When various auxiliary chelating ligands, such as phen and bipy, were used, various types of low dimensional assemblies including mononuclear or dinuclear complexes were formed [22]. The π–π interconnections of two phen moieties between adjacent dinuclear molecules lead to formation of a one-dimensional structure (Fig. 3).
Crystal structures of Fe(phen)3·HL (2) and Fe(bipy)3·HL·5H2O (3). The structures of complexes 2 and 3 are similar, but the ligands containing N-donors. Therefore, the structure of complex 2 is discussed as a sample. Single crystal X-ray diffraction of complex 2 indicates that it crystallizes in the triclinic space group P-1 (Table 1). The asymmetric unit consists of one Fe ion, one HL3– anion and three phen species (Fig. 1c). The Fe1 is six-coordinate and demonstrates an octahedral coordination configuration (Fig. 1d) with the axial positions occupied by two nitrogen atoms (N2 and N3) and the equatorial positions occupied by four nitrogen atoms (N1, N4, N5, and N6). The Fe–N distances range from 1.966(4) to 1.983(4) Å for complex 2, while the N–Fe–N angles are between 82.65(16)° and 174.16(17)° (Table 2), that are consistent with those determined for the comparable structures [23, 24]. Anion HL3– uncoordinates with Fe ion, leaving one phenol group 1H-protonated for balancing charges.
Thermogravimetric analyses. TGA was performed by heating from 25 to 800°C with the rate of 10°C·min–1 under the atmosphere of nitrogen (Fig. 4). For complex 1, the first weight loss of 5.65% in the range of 25 to 111°C was attributed to the loss of five water molecules (calcd 6.07%). The weight remained constant up to 263°C at which the second weight loss of 24.9% between 263 and 498°C was attributed to the loss of two phen molecules (calcd 24.3%). The skeleton gradually disintegrated between 498 and 624°C. The residue remained stable up to 800°C and consisted of metal sulfate and metal oxide. For complexes 2 and 3, the initial weight losses of 4.11% and 9.88% were consistent with those of the calculated values (4.16 and 10.20%), corresponding to the loss of two and five water molecules in the temperature ranges of 25–279 and 25–185°C, respectively. In the temperature ranges of 279–367 and 185–337°C the weight losses of 30.57 and 30.86% could take place due to complete decomposition of HL3– anion (calcd 30.92 and 30.27%), respectively. The third weight loss steps indicated decomposition of the phen and bipy molecules in the temperature ranges of 367–800 and 337–800°C, respectively. The residues were the mixtures of metal sulfates and metal oxides, respectively [25].
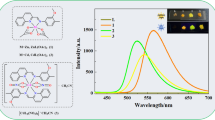
Luminescence properties. Emission spectra of the complexes 1–3 and free Na2H2L at room temperature in the solid state were recorded (Fig. 5). For the complex 1, the dominant peak at 545 nm was hypersensitive, giving green luminescence output, which corresponded to the 5D4→7F5 transition. Six weak emission peaks at 490, 583, 618, 647, 674, and 705 nm originated from 5D4→7F6, 5D4→7F4, 5D4→7F3, 5D4→7F2, 5D4→7F1, and 5D4→7F0 transitions, respectively [26]. No emission bands from the ligands were observed, indicating that the ligands efficiently transferred the excitation energy to the Tb ions. Emissions observed for complex 1 indicated that it might be potentially applicable as a material for diode devices [27]. Free Na2H2L exhibited the maximum fluorescent emission at 440 nm upon excitation at 315 nm, which was attributable to the intraligand π–π* and n–π* transitions [28]. Complex 2 exhibited photoluminescent emission with the maximum at 422 nm upon excitation at 315 nm. In the case of complex 3, a similar photoluminescence with the maximum emission at 430 nm upon excitation at 315 nm was recorded. Peak profiles recorded for complexes 2 and 3 were similar with that of free Na2H2L ligand. In addition, those were slightly shifted toward lower wavelengths in comparison with those of free Na2H2L ligand indicating that the emission bands of complexes 2 and 3 originated from the intraligand transitions.
EXPERIMENTAL
All chemicals and solvents used in the experiments were of analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd, and used without further purification. Elemental analyses were carried out after removing water molecules from the samples by heating on an Elementar Vario EL analyzer. Thermogravimentric analyses were carried out on a 1100SF thermal analyzer at the heating rate of 10°C/min under the atmosphere of nitrogen. Photoluminescence analyses were performed on a Perkin Elemer LS55 fluorescence spectrometer.
X-Ray single crystal data were collected for the complexes 1–3 on a Bruker Smart Apex II CCD diffractometer equipped with graphite monochromated MoKα radiation (λ = 0.71073 Å) at 296 and 298 K, respectively. After absorption correction, the structures were solved by the direct method and refined by a full-matrix least squares method on F2 using SHELXT 2014 and SHELXL 2018 programs [29, 30]. Hydrogen atoms were generated geometrically and treated by a mixture of independent and constrained refinements. The crystallographic dataFootnote 1 are summarized in Table 1, and the selected bond lengths and angles are presented in Table 2.
Synthesis of Tb2(H2L)3(phen)2 (1). A mixture of Tb(NO3)3·6H2O(0.045 g, 0.1 mmol) with Na2H2L (0.031 g, 0.1 mmol), phen (0.018 g, 0.1 mmol) and deionized water (20 mL) was placed in a Teflon-lined stainless steel autoclave (25 mL) and stirred at room temperature for 1 h. Then the mixture was heated at 120°C for 36 h, followed by cooling down to room temperature. Yellow blocked crystals were collected by filtration. Yield 55% (based on Na2H2L). Calculated, %: C 34.02, H 1.69, N 3.78. C42H25N4O24S6Tb2. Found, %: C 34.17, H 1.92, N 3.82.
Synthesis of Fe(phen)3·HL (2). A mixture of Fe(NO3)3 (0.024 g, 0.1 mmol) with Na2H2L (0.031 g, 0.1 mmol) and phen (0.018 g, 0.1 mmol) in 20 mL of distilled water was stirred at room temperature for 1 h and then stored for 3 days till red blocked crystals were formed. Yield 60% (based on phen). Found, %: C 58.45, H 3.41, N 9.77. C42H28N6O8S2Fe. Calculated, %: C 58.33, H 3.24, N 9.72.
Synthesis of Fe(bipy)3·HL·5H2O (3). Complex 3 was synthesized by the method similar to that of complex 2. The bipy was used instead of phen. Yield 60% (based on phen). Found, %: C 33.75, H 1.97, N 9.62. C36H38N6O13S2Fe. Calculated, %: C 48.98, H 4.31, N 9.52.
CONCLUSIONS
In summary, we have reported the syntheses, crystal structures and characterization of three new complexes, namely Tb2(H2Tiron)3(phen)2, Fe(phen)3·HL, and Fe(bipy)3·HL·5H2O. The N2H2L is a multifunctional bridging ligand that can bind to two Tb ions to form a dinuclear complex 1. One-dimensional supramolecular structure derives from the π–π stacking interactions of the phen ligands in complex 1. Complexes 2 and 3 have similar N-donor mononuclear structures with uncoordinated HL3– anions balancing charges. Their low dimensional structural features depend on the mediation of auxiliary ligands such as phen and bipy. Furthermore, complex 1 shows characteristic emission peaks of Tb3+ ions corresponding to 5D4→7F5, 5D4→7F6, 5D4→7F4, 5D4→7F3, 5D4→7F2, 5D4→7F1, and 5D4→7F0 transitions. Complexes 2 and 3 exhibit the emission bands originated from the intraligand transitions.
Notes
Crystallographic data were deposited with the Cambridge Crystallographic Data Centre (CCDC 2043211, 2043209, 2043210), and can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/ or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax:(+44-1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk).
REFERENCES
Sougoule, A.-S., Mei, Z.-M., Xiao, X., Balde, C.-A., Samoura, S., Dolo, A., and Zhu, D.-S., J. Organomet. Chem., 2014, vol. 758, p. 19. https://doi.org/10.1016/j.jorganchem.2014.01.034
Dey, S., Sarkar, S., Mukherjee, T., Mondal, B., Zangrando, E., Sutter, J.-P., and Chattopadhyay, P., Inorg. Chim. Acta, 2011, vol. 376, p. 129. https://doi.org/10.1016/j.ica.2011.06.005
Alston, J.-R., Banks, D.-J., Mcneill, C.-X., Mitchell, J.-B., Popov, L.-D., Shcherbakov, I.-N., and Poler, J.-C., Phys. Chem. Chem. Phys., 2015, vol. 17, p. 29566. https://doi.org/10.1039/c5cp05419b
Guo, Y.-T., Ai, P.-F., Han, L.-Q, Chen, L., Li, B.-G., and Jie, S.-Y., J. Organomet. Chem., 2012, vol. 716, p. 222. https://doi.org/10.1016/j.jorganchem.2012.06.024
Suenaga, Y., Nakaguchi, Y., Fujishima, Y., Konaka, H., and Okuda, K., Inorg. Chem. Commun., 2011, vol. 14, p. 440. https://doi.org/10.1016/j.inoche.2010.12.023
Yang, H.-X, Guo, S.-P., Tao, J., and Lin, J.-X., Crystal Growth Des., 2009, vol. 9, p. 4735. https://doi.org/10.1021/cg9005983
Chartrand, D. and Hanan, G.-S., Chem. Commun., 2008, vol. 53, p. 727. https://doi.org/10.1039/b715205a
Maeda, T., Furusho, Y., Shiro, M., and Takata, T., Chirality, 2010, vol. 18, p. 691. https://doi.org/10.1002/chir.20308
Renz, F., Hill, D., Kerep, P., Klein, M., MüllerSeipel, R., and Werner, F., Hyperfine Interact., 2006, vol. 168, p. 1051. https://doi.org/10.1007/s10751-006-9394-2
Xu, G.-H., Tang, B.-B., Hao, L., and Liu, G.-L., CrystEngComm, 2017, vol. 19, p. 781. https://doi.org/10.1039/C6CE02292H
Kim, J.-Y., Park, I.-H., Lee, J.-Y., Park, J.-M., and Lee, S.-S., Inorg. Chem., 2013, vol. 52, p. 10176. https://doi.org/10.1021/ic401648b
Mashima, K. and Nakamura, A., J. Organomet. Chem., 2002, vol. 663, p. 5. https://doi.org/10.1016/S0022-328X(02)01728-X
Koo, B.-K., Kim, J., and Lee, U., Inorg. Chim. Acta, 2010, vol. 363, p. 1760. https://doi.org/10.1016/j.ica.2010.02.032
Salehi, M. and Hasanzadeh, M., Inorg. Chim. Acta, 2015, vol. 426, p. 6. https://doi.org/10.1016/j.ica.2014.10.023
Wang, D.-Z., J. Molecular Struct., 2009, vol. 929, p. 128. https://doi.org/10.1016/j.molstruc.2009.04.013
Boghaei, D.-M., Gharagozlou, M., and Sayadi, M., J. Coord. Chem., 2007, vol. 60, p. 2283. https://doi.org/10.1080/00958970701261345
Chi, Y.-X., Niu, S.-Y., Wang, Z.-L., and Jin, J., Eur. J. Inorg. Chem., 2008, vol. 2008, p. 2336. https://doi.org/10.1002/ejic.200800106
Wang, D.-Z., J. Mol. Struct., 2009, vol. 929, p. 128. https://doi.org/10.1016/j.molstruc.2009.04.013
Aguilà, D., Barrios, L.-A., Luis, F., Repollés, A., and Aromí, G., Inorg. Chem., 2010, vol. 49, p. 6784. https://doi.org/10.1021/ic1008285
Yamaguchi, T., Sunatsuki, Y., Ishida, H., Kojima, M., Akashi, H., Re, N., Matsumoto, N., Pochaba, A., and Mroziński, J., Inorg. Chem., 2008, vol. 47, p. 5736. https://doi.org/10.1021/ic8000575
Chen, C., Ma, J.-F., Liu, B., Yang, J., and Liu, Y.-Y., Crystal Growth Des., 2011, vol. 11, p. 4491. https://doi.org/10.1021/cg2007167
Chu, Z.-H., Xie, Q.-F., and Chen, Y.-M., Chin. J. Inorg. Chem., 2013, vol. 29, p. 1893. https://doi.org/10.3969/j.issn.1001-4861.2013.00.222
Banse, F., Balland, V., Philouze, C., Riviere, E., and Girerd, J.-J., Inorg. Chim. Acta, 2003, vol. 353, p. 223. https://doi.org/10.1016/S0020-1693(03)00292-5
Mckeogh, I., Hill, J., Collins, E., McCabe, T., Powell, A., and Schmitt, W., New J. Chem., 2007, vol. 31, p. 1882. https://doi.org/10.1039/b711185a
Ying, S.-M. and Mao, J.-G., Eur. J. Inorg. Chem., 2004, vol. 2004, p. 1270. https://doi.org/10.1002/ejic.200300607
Wang, X.-J., Cen, Z.-M., Ni, Q.-L., and Jiang, X.-F., Crystal Growth Des., 2010, vol. 10, p. 2960. https://doi.org/10.1021/cg1000045
Kariaka, N., Litsis, O., Kolomzarov, Y., Gawryszewska, P., and Amirkhanov, V., Chem. J. Moldova, 2018, vol. 13, p. 54. https://doi.org/10.19261/cjm.2018.473
Li, S.-N., Zhai, Q.-G., Hu, M.-C., and Jiang, Y.-C., Z. Anorg. Allg. Chem., 2010, vol. 636, p. 1142. https://doi.org/10.1002/zaac.200900450
Sheldrick, G.M., SHELXT-2014, Program for X-ray Crystal Structure Solution, Göttingen: University of Göttingen, 2014.
Sheldrick, G.M., SHELXL-2018, Program for X-ray Crystal Structure Refinement, Göttingen: University of Göttingen, 2018.
Funding
Scientific Research Fund Project of Liaoning Provincial Education Department (no. L2019010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interests was declared by the authors.
Rights and permissions
About this article
Cite this article
Yin, P., Liu, C., Wang, Y. et al. Syntheses, Characterization, and Luminescence Properties of Three Coordination Complexes with Sulfonate-Phenol Anions and Trivalent Metal Ions. Russ J Gen Chem 91, 897–903 (2021). https://doi.org/10.1134/S1070363221050200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221050200