Synthesis of new asymmetric bis-spiropyrans of indoline series based on 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin was realized. Depending on the nature of the substituents in positions 1 and 5 of the indoline moiety the derived compounds may exist in solution in the spiro or merocyanine forms, or as a tautomeric mixture of these forms. UV irradiation of the cyclic forms leads to photocoloring associated with re-opening of one or both of the spiro moieties. The wide variability of spectral kinetic properties of bis-spiropyrans upon fluorescence of photoinduced forms allows one to consider them as molecular switches having absorptive and fluorescent signal functions with the possibility of practical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Photochromic spiropyrans are promising materials for optical recording media, molecular switches, and chemosensors.1 – 6 Their photoinitiated rearrangement includes reversible dissociation of Cspiro–O bond of the cyclic isomer and subsequent Z/E isomerization to the metastable merocyanine form. The latter can be converted to the original spiro form under irradiation with visible light. Previously, it was shown that spiropyrans containing fused benzopyranone moiety possess not only photochromic but also fluorescent properties.7 – 14
In this paper, we describe the synthesis as well as the results of the study on the structure and photochromism of coumarin series indoline bis-spiropyrans, the absorbance of cyclic forms of which can be expected to increase due to the enlargement of the spatial extent of the molecular system.2 , 4
Bis-spiropyrans 1a-e were synthesized by condensation of 3H-indolium perchlorates 2a-е with 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin (3)15 in the presence of triethylamine as the base in average yields (Scheme 1). In the ground state, the obtained compound may be either in the spirocyclic form 1S or in different merocyanine forms 1SM, 1MS, 1MM with one or two cycles opened as well as form the corresponding isomers upon irradiation (Scheme 2).
The structure of compounds 1а-е was established by 1H NMR spectroscopy in deuterated chloroform and confirmed by elemental analysis. The location and nature of the proton signals of the methyl groups at position 3 and N-methyl groups of the indoline fragments indicate that compounds 1а,b preferably assume the fully open merocyanine form 1MM. In the 1H NMR spectra of compounds 1а,b, twelve-proton singlet signals at 1.79 and 1.76 ppm, respectively, and six-proton singlet signals at 3.56 and 3.54 ppm, respectively, are observed. The diene bridge methine proton signals of these compounds appear as two doublets in 7.94-8.00- and 8.82-8.88-ppm ranges.
Compounds 1c,е exist as a tautomeric mixture of spiro and merocyanine forms. In the upfield region of 1H NMR spectra, along with signals at 1.12-1.20 and 2.70 ppm, corresponding to the two pairs of signals of magnetically nonequivalent protons of the geminal methyl and N-methyl groups (for compound 1c) of the indoline cycle in spirocyclic form, two signals at 1.77-1.81 and 3.52 ppm of the same groups in the merocyanine form are observed. Signals of the spirocyclic form protons H-3',11' appear as two doublets at 5.58-5.74 ppm. Furthermore, proton signals of the diene bridge of the merocyanine form appear as two doublets at 8.91 and 8.77 ppm. As determined by the relative signal intensity, the ratio of the spirocyclic form 1S to merocyanine form is 1:6 (for compound 1c) and 1:7 (for compound 1e). However, NMR spectroscopy data do not allow evaluating the specific contribution of tautomers 1SM, 1MS, 1MM in the steady state equilibrium.
Compound 1d exists predominantly in its spirocyclic form 1S; two pairs of signals of magnetically nonequivalent protons of the geminal methyl groups register at 1.16-1.34 ppm, two pairs of signals of diastereotopic protons H-3',11' of the pyran ring double bond appear as two doublets at 5.55 and 5.65 ppm, while signals of protons Н-4',12' appear as two doublets at 6.90 and 7.41 ppm.
A sufficiently sensitive method for studying the tautomeric equilibrium is electronic absorption spectroscopy, as spirocyclic forms must possess absorption in the UV region of the spectrum while merocyanine forms in the visible region.1 , 2 The presence of characteristic absorption bands in the spectra of bis-spiropyrans 1a-e in toluene (Table 1) indicates that in toluene solutions of these compounds an equilibrium exists between the cyclic 1S and merocyanine form 1SM, 1MS, 1MM isomers. However, according to the NMR spectral data, the equilibrium position is fundamentally dependent on the nature of the substituents on the indoline moiety of the molecule. Compound 1a, which does not have a substituent at position 5 of the indoline fragment, and compound 1b (R2 = Me) are characterized by intensive peaks at 473–561 nm corresponding to the absorption of merocyanine forms 1SM, 1MS, 1MM. In the case of compound 1d with electronwithdrawing 5-nitro group, on the contrary, the longwavelength absorption is almost completely absent indicating the prevalence of spiro form 1S. The remaining compounds exist as a tautomeric mixture of the spirocyclic and merocyanine forms.
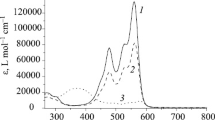
Merocyanine forms of bis-spiropyrans 1a-e exhibit a fluorescence maximum at 588–595 nm (Fig. 1, Table 1).
In addition to NMR spectroscopy and electronic absorption spectroscopy data, quantum-chemical calculations of relative stability, structural and spectral properties of bis-spiropyrans 1S and the corresponding merocyanine forms 1SM, 1MS, 1MM were carried out. Form 1S can exist as two diastereoisomers, the most stable of which (ΔE 6.6 kcal/mol) is depicted in Scheme 2. Only isomers thereof were considered in subsequent calculations. Structures of isomeric forms of compounds 1a,c,d have been optimized by density functional theory DFT methods using the hybrid functional PBE0 and the 6-31G** basis set. Solvent influence was accounted for within the framework of the polarized continuum model CPCM. The spectral characteristics of the studied isomers were determined on the basis of methods of non-stationary density functional theory (TD PBE0/6-31G**). The energetic characteristics of isomers of compounds 1a,c,d are shown in Table 2.
Data of relative stability of various isomers of merocyanine forms indicate that the completely open form 1MM for structures 1a,c can be formed in toluene solution, whereas form 1MS is energetically less favorable.
Excitation energy and the oscillator strength of the first four singlet transitions of the cyclic and merocyanine isomers of compounds 1a,с,d were calculated by TD PBE0/6-31G** method in toluene. Comparison of the calculated spectral characteristics with the experimental data allows one to attribute the absorption bands at 560– 570 and 470–480 nm to S0–S1 and S0–S3 transitions of 1SM isomers, respectively, and the absorption band at 525–537 nm to S0–S1 transitions of the completely open isomers 1MM.
Irradiation of solutions of compounds 1a-e with 365 nm wavelength light at 295 K leads to an increase of the intensity of the absorption bands of the merocyanine forms of the isomers, the shape and structure of which remains unchanged (Fig. 2). This suggests that the product of the thermal ring opening reaction and the photoreaction product are the same. After the cessation of UV irradiation, thermal recyclization reaction in solutions of compounds 1a-e is observed reverting the system to its original state (photobleaching also occurs when merocyanine forms are irradiated with light of a wavelength matching that of the absorption bands). Thermal relaxation time constant τt increases going from an N-benzyl substituent to N-alkyl substituent (with R2 = H) (Table 1).
The electron-withdrawing nitro group at position 5 of the indoline moiety (compound 1d) reduces the duration of thermal relaxation, the methyl group increases the lifetime of the colored form of compound 1b to 2430 s.
To conclude, photochromic indoline bis-spiropyrans based on 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin have been obtained, which depending on the nature of the substituents at positions 1 and 5 of the indoline moiety may exist in solution in the merocyanine or spiro forms or as a tautomeric mixture of these forms. UV irradiation of the cyclic isomers causes photocoloring associated with reversible opening of one or both of the spiro moieties.
Variability of spectral kinetic properties of bis-spiropyrans upon fluorescence of photoinduced forms allows one to consider them as molecular switches having absorptive and fluorescent signal functions with the possibility of practical application.
Experimental
Electronic absorption spectra and kinetics of thermal recyclization reactions of the studied compounds were registered on an Agilent 8453 spectrophotometer at 293 K. Photolysis of solutions was carried out in a Newport system consisting of a 200-W mercury vapor lamp equipped with an interference light filter set. Fluorescence measurements were performed on a Cary Eclipse (Varian) fluorescence spectrophotometer. Spectrophotometric grade toluene (Aldrich) was used to prepare solutions. 1H NMR spectra were acquired on a Varian Unity-300 (300 MHz) spectrometer at 295 K, chemical shifts were assigned relative to the signals of residual solvent CDCl3 protons. IR spectra were recorded on a Varian Excalibur 3100 FT-IR spectrophotometer by the attenuated total reflectance technique using a ZnSe crystal. Mass spectra were recorded on a Shimadzu GCMS-QP2010SE GC-MS system with direct sample injection (70 eV ionization energy). Elemental analysis was performed on a KOVO CHNanalyzer. Melting points were determined in glass capillaries on a PTP(M) apparatus. Structures of isomeric forms of compounds 1a,c,d were optimized by the DFT method using the PBE0 hybrid functional and the 6-31G** basis set.16 The agreement of optimized structures with the minima was proven by normal vibrational frequency calculation data (force constant matrix). Solvent influence was calculated with the conductor-like polarized continuum model (CPCM) approach.17 Dielectric permittivity of media correspond to toluene (ε 2.379). Spectral characteristics of the studied isomers were determined on the basis of non-stationary density functional theory methods (TD PBE0/6-31G**).18 The systematic heightening of the singlet transition energies of the merocyanine isomers of compounds 1a,c,d were corrected according to formula E ex-corr= −0.0963 + 0.9321E ex. All calculations were done using the GAUSSIAN 03 software set.19
Synthesis of compounds 1a–e (General method). Triethylamine (2.63 g, 0.26 mmol) was added to a heated solution of 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin (3)15 (0.27 g, 1.1 mmol) and 3H-indolium perchlorate 2а–е 20 (2 mmol) in 2-propanol (20 ml). The reaction mixture was heated under reflux for 5 h, and the solvent evaporated. The residue was purified by column chromatography on Al2O3 (eluent chloroform), and the product recrystallized from 2-PrOH.
4-Methyl-6,8-bis[2-(1,3,3-trimethylindolin-2-ylidene)-ethylidene]-2 Н -chromene-2,5,7(6 Н ,8 Н )trione Footnote 1 (1а). Yield 0.30 g (54%), maroon powder, mp 230–232°С. IR spectrum, ν, cm−1: 1737, 1685, 1607, 1573, 1233. 1H NMR spectrum, δ, ppm (J, Hz): Form 1MM: 1.79 (12H, s, 4CH3); 2.71 (3H, s, СН3); 3.56 (6H, s, 2СН3); 5.84 (1H, s, CH); 6.99–7.45 (8H, m, H Ar); 8.00 (2H, d, J = 13.8) and 8.88 (2H, d, J = 14.1, 2CH=CH). Mass spectrum, m/z (I rel, %): 558 [M]+ (58), 543 (100), 528 (6). Found, %: C 77.64; Н 6.65; N 5.82. C36H34N2O4. Calculated, %: C 77.40; Н 6.13; N 5.01.
4-methyl-6,8-bis[2-(1,3,3,5-tetramethylindolin-2-ylidene)ethylidene]-2 Н -chromene-2,5,7(6 Н ,8 Н )trione (1b). Yield 0.31 g (48%), maroon powder, mp >300°С. IR spectrum, ν, cm−1: 1734, 1678, 1608, 1575, 1230. 1H NMR spectrum, δ, ppm (J, Hz): Form 1MM: 1.75 (12H, s, 4CH3); 2.37 (6Н, s, 2CH3); 2.68 (3H, s, СН3); 3.54 (6H, s, 2СН3); 5.80 (1H, s, CH); 6.92 (2H, d, J = 7.8, H Ar); 7.10 (2H, d, J = 7.8, H Ar); 7.12 (2Н, s, H Ar); 7.94 (2H, d, J = 14.6) and 8.82 (2H, d, J = 14.2, 2CH=CH). Mass spectrum, m/z (I rel, %): 586 [M]+ (51), 571 (100), 556 (5). Found, %: C 77.66; Н 7.05; N 4.52. C38H38N2O4. Calculated, %: C 77.79; Н 6.53; N 4.77.
5,5''-Dichloro-1,1'',3,3,3'',3'',8'-heptamethyl-6' Н -dispiro[indoline-2,2'-dipyrano[2,3- f :2',3'- h ]chromene-10',2''-indolin]-6'-one (1c). Yield 0.28 g (40%), maroon powder, mp >300°С. IR spectrum, ν, cm−1: 1744, 1676, 1606, 1574, 1237, 908. 1H NMR spectrum, δ, ppm (J, Hz): Form 1S: 1.12 (6H, s) and 1.17 (6H, s, 3,3,3'',3''-CH3); 2.70 (6H, s, 1,1''-CH3); 5.58 (1H, d, J = 10.6) and 5.67 (1H, d, J = 10.6, 3',11'-CH); 5.80 (1H, s, 7'-CH); 6.41 (1Н, d, J = 8.1, H Ar); 6.80 (1Н, d, J = 8.1, H Ar); 6.98–7.20 (5Н, m) and 7.67 (1H, d, J = 10.6, H Ar, 4',12'-CH). Form 1MM: 1.77 (12H, s, 4CH3); 2.73 (3Н, s, СН3); 3.52 (6Н, s, 2CH3); 5.86 (1H, s, CH); 6.75 (1Н, d, J = 8.1, H Ar); 6.84 (1Н, d, J = 8.1, H Ar); 6.80–7.03 (4Н, m, H Ar); 8.48 (2H, d, J = 13.5) and 8.78 (2H, d, J = 14.0, 2CH=CH). Mass spectrum, m/z (I rel, %): 627 [M]+ (50), 612 (100), 597 (5). Found, %: C 68.64; Н 5.55; N 4.92. C36H32Cl2N2O4. Calculated, %: C 68.90; Н 5.14; N 4.46.
1,1'',3,3,3'',3'',8'-Heptamethyl-5,5''-dinitro-6' Н -dispiro-[indoline-2,2'-dipyrano[2,3- f :2',3'- h ]chromene-10',2''-indolin]-6'-one (1d). Yield 0.29 g (40%), blue powder, mp >300°С. IR spectrum, ν, cm−1: 1741, 1685, 1606, 1578, 912. 1H NMR spectrum, δ, ppm (J, Hz): Form 1S: 1.16 (3H, s), 1.22 (3H, s), 1.31 (3H, s) and 1.34 (3H, s, 3,3,3'',3''-CH3); 1.86 (3Н, s, 8'-CH3); 2.78 (3H, s) and 2.88 (3H, s, 1,1''-CH3); 5.55 (1H, d, J = 10.5) and 5.65 (1H, d, J = 10.5, 3',11'-CH); 5.83 (1H, s, 7'-CH); 6.49 (1Н, d, J = 8.7, H Ar); 6.56 (1Н, d, J = 8.7, H Ar ); 6.90 (1H, d, J = 10.5) and 7.41 (1H, d, J = 10.5, 4',12'-CH); 7.93 (1H, s, H Ar); 7.96 (1H, s, H Ar); 8.15 (1Н, d, J = 8.4, H Ar); 8.20 (1Н, d, J = 8.4, H Ar). Mass spectrum, m/z (I rel, %): 648 [M]+ (66), 633 (100), 603 (5). Found, %: C 66.34; Н 5.05; N 8.82. C36H32N4O8. Calculated, %: C 66.66; Н 4.97; N 8.64.
1,1''-Dibenzyl-3,3,3'',3'',8'-pentamethyl-6' Н -dispiro[indoline-2,2'-dipyrano[2,3- f :2',3'- h ]chromene-10',2''-indolin]-6'-one (1е). Yield 0.43 g (55%), maroon powder, mp 280–282°С. IR spectrum, δ, cm−1: 1708, 1672, 1609, 1588, 1230, 924. 1H NMR spectrum, δ, ppm (J, Hz): Form 1S: 1.17 (6H, s) and 1.20 (6H, s, 3,3,3'',3''-СН3); 2.63 (3Н, s, 8'-CH3); 4.05 (4H, m, 2CH2); 5.64 (1H, d, J = 10.5) and 5.74 (1H, d, J = 10.5, 3',11'-CH); 5.87 (1H, s, 7'-CH); 6.95–7.37 (20H, m, H Ar, 4',12'-CH). Form 1MM: 1.81 (12H, s, 4СН3); 2.69 (3Н, s, CH3); 5.21 (4H, s, 2CH2); 5.84 (1H, s, CH); 7.28–7.47 (18H, m, H Ar); 8.42 (2H, d, J = 14.1) and 8.78 (2H, d, J = 14.1, 2CH=CH). Mass spectrum, m/z (I rel, %): 710 [M]+ (52), 619 (100), 552 (8). Found, %: C 80.83; Н 5.65; N 4.12. C48H42N2O4. Calculated, %: C 81.10; Н 5.96; N 3.94.
Notes
Here and further the name corresponds to the tautomeric form prevalent in CDCl3 solution.
References
Minkin, V. I. Russ. Chem. Rev. 2013, 82, 1. [Usp. Khim. 2013, 82, 1.]
Bertelson, R. C. In Organic Photochromic and Thermochromic Compounds, Crano, J. C.; Guglielmetti, R. J., Eds.; Plenum Press: New York, 1999, vol. 1, p. 11.
Minkin, V. I. Chem. Rev. 2004, 104, 2751.
Guglielmetti, R. In Photochromism: Molecules and Systems, Dürr, H.; Bouas-Laurent, H., Eds.; Elsevier: Amsterdam, 2003, p. 314.
Bercovic, J.; Krongauz, V.; Weiss, V. Chem. Rev. 2000, 100, 1741.
Minkin, V. I. In Molecular Switches, Feringa, B. L.; Browne, W. R., Eds.; Wiley: Weinheim, 2011, p. 37.
Traven, V. F.; Manaev, A. V.; Bochkov, A. Yu.; Chibisova, T. A.; Ivanov, I. V. Russ. Chem. Bull., Int. Ed. 2012, 61, 1342. [Izv. Akad. Nauk SSSR, Ser. Khim. 2012, 1327.]
Traven, V. F.; Miroshnikov, T. A.; Chibisova, T. A.; Barachevsky, V. A.; Venidiktova, O. V.; Strokach, Yu. P. Russ. Chem. Bull., Int. Ed. 2005, 54, 2417. [Izv. Akad. Nauk SSSR, Ser. Khim. 2005, 2342.]
Barachevsky, V. A.; Karpov, R. E.; Venidiktova, O. V.; Valova, T. M.; Strokach, Yu. P.; Miroshnikov, T. A.; Chibisova, T. A.; Traven, V. F. Russ. Chem. Bull., Int. Ed. 2005, 54, 2425. [Izv. Akad. Nauk, Ser. Khim. 2005, 2350.]
Dolotov, S. M.; Miroshnikov, T. A.; Chibisova, T. A.; Sin, S.-L.; Venidiktova, O. V.; Valova, T. M.; Dunaev, A. A.; Strokach, Yu. P.; Barachevsky, V. A.; Traven, V. F. Russ. Chem. Bull., Int. Ed. 2007, 56, 904. [Izv. Akad. Nauk, Ser. Khim. 2007, 870.]
Nikolaeva, O. G.; Tsukanov, A. V.; Shepelenko, E. N.; Lukyanov, B. S.; Metelitsa, A. V.; Kostyrina, O. Yu.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Int. J. Photoenergy 2009, ID 238615, doi: 10.1155/2009/238615.
Nikolaeva, O. G.; Gaeva, E. B.; Shepelenko, E. N.; Tsukanov, A. V.; Metelitsa, A. V.; Lukyanov, B. S.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Russ. J. Org. Chem. 2009, 45, 1091. [Zh. Org. Khim. 2009, 45, 1102.]
Nikolaeva, O. G.; Shepelenko, E. N.; Tsukanov, A. V.; Kozyrev, V. S.; Metelitsa, A. V.; Dubonosov, A. D.; Bren', V. A.; Minkin, V. I. Bull. SSC RAN 2010, 6(3), 12.
Nikolaeva, O. G.; Kostyrina, O. Yu.; Shepelenko, E. N.; Tsukanov, A. V.; Metelitsa, A. V.; Borodkin, G. S.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. Russ. J. Org. Chem. 2011, 47, 1370. [Zh. Org. Khim. 2011, 47, 1348.]
Minkin, V. I.; Dubonosov, A. D.; Bren, V. A.; Nikolaeva, O. G.; Tsukanov, A. V.; Burov, O. N.; Fedyanina, A. Yu. Russ. J. Org. Chem. 2013, 49, 374. [Zh. Org. Khim. 2013, 49, 387.]
Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158.
Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995.
Runge, E.; Gross, E. K. U. Phys. Rev. Lett. 1984, 52, 997.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R. Gaussian 03, Revision E.01, 2004.
Pottier, E.; Sergent, M.; Phan Tan Luu, R.; Guglielmetti, R. Bull. Soc. Chim. Fr. 1992, 101, 719.
This work was performed within the framework of implementing the Project part of State Assignment for scientific activity (project № 4.88.2014/K) of the Ministry of Education and Science of the Russian Federation. A. S. Cheprasov, A. V. Metelitsa, and I. V. Dorogan are grateful to the Russian Foundation for Basic Research for financial support (grant 13-03-00901).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(3), 229–223
Rights and permissions
About this article
Cite this article
Nikolaeva, O.G., Karlutova, O.Y., Cheprasov, A.S. et al. Synthesis of bis-spiropyrans based on 6,8-diformyl-5,7-dihydroxy-4-methylcoumarin and photochromic properties thereof. Chem Heterocycl Comp 51, 229–233 (2015). https://doi.org/10.1007/s10593-015-1689-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1689-2