Abstract
Two transition-metal coordination polymers {[Zn2(L)2(HCOO)2]}n (I) and {[Cd2(L)3(HCOO)] ∙ 2H2O}n(II) (L = 1,3-bis(4-(methoxycarbonyl)benzyl)-1H-imidazol-3-ium anion) have been synthesized via solvo-thermal method. Both of the coordination polymers I and II were characterized by single-crystal X-ray diffraction (CIF files CCDC nos. 1834629 (I) and 1834630 (II)), FT-IR spectroscopy, elemental analysis and thermogravimetric analysis. The results shown compound I is a two dimensional sql layer structure containing interlocking structure between the left and right helix chains, and compound II is rare three dimensional six-fold interpenetrating dia topology framework. Luminescence investigations revealed that both of compound I and II emitted blue light.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The purpose of synthetic different coordination polymers is to pursue new function materials originating from metal centers, ligands and the space constructed by metal centers and ligands. Therefore, the design of ligands is particularly important for the construction and modification of coordination polymers [1–5]. An interesting ligand not only endows the coordination polymer with novel functions, but also brings plentiful post-modification reactions on ligand for bringing more novel and interesting functions [6, 7]. For example, NH2-substituted ligands were used to construct coordination polymers due to the Lewis base sites are easily modified by aldehydes, anhydride and so on [8–10]. However, in fact, the organic functional groups that can be modified in coordination polymers are relatively rare.
Imidazole-based zwitterionic ligands can be used as precursors of N-heterocyclic (NHC) ligands, and their complexes exhibit potential applications as green clean catalysts and luminescent materials [11]. Therefore, imidazole-based zwitterionic ligands are purposefully introduced into coordination polymers, making them as the precursors of NHC ligands, which can coordinate with various metal ions via post-modification [12]. While, the number of coordination polymers constructed by imidazolium-based zwitterionic ligands is much smaller than that of coordination polymers assembled by other ligands. Here, we have designed flexible imidazolium-based zwitterionic ligand (L = 1,3-bis(4-(methoxycarbonyl)benzyl)-1H-imidazol-3-ium anion) to synthesize two novel coordination polymers {[Zn2(L)2(HCOO)2]}n (I) and {[Cd2(L)3(HCOO)] ∙ 2H2O}n (II) under solvothermal conditions, respectively. Compound I is a two dimensional sql layer structure containing interlocking structure between the left and right helix chains, and compound II is rare three dimensional six-fold interpenetrating dia framework. Both of compound I and II emitted blue light via luminescence investigations.
EXPERIMENTAL
Materials and methods. All chemicals purchased were of reagent grade and used without further purification. 1H NMR spectra were recorded on a JEOL JNM-LA500FT instrument (500 MHz) in CDCl3 with TMS as the internal standard. The elemental analyses (C, H, and N) were carried out applying a Perkin-Elmer 240C elemental analyzer. The IR spectra in the 4000–400 cm–1 region were measured using a Nicolet IR-408 spectrometer and KBr pellets. Powder X-ray diffraction (PXRD) data were collected using a BRUKER D8 ADVANCE diffractometer using CuKα (λ = 1.542 Å). Thermogravimetric (TG) curve was recorded from room temperature to 800°C with the heating rate of 10°C/min on a Netzsch TG 209 instrument undernitrogen atmosphere. The Solid fluorescence emission and excitation spectra were measured on a Horiba Scientific fluorescence.
Synthesis of ester of H2L. Imidazole (0.136 g, 2.00 mmol) and K2CO3 (0.415 g, 3.00 mmol) were stirred for 15 min in 100 mL of MeCN. Then methyl 4-(bromomethyl)benzoate (0.916 g, 4.00 mmol) was added subsequently and stirred for 5 days. And then the mixture was filtered, and the MeCN in resulting solution was removed via the reduced pressure distillation. Finally, 150 mL H2O was added, then stirred the mixture for 30 min. The white solid was filtered, washed with Et2O (3 × 20 mL), and dried in suction [13]. White crystalline powder was obtained (0.219 g, 0.6 mmol, 30%).
IR (KBr; ν, cm–1): 3145 w., 3085 m., 3041 m., 2976 m., 1719 v.s., 1614 m., 1550 m., 1437 m., 1289 v.s., 1188 s., 1151 s., 1107 s., 1023 m., 963 m., 873 s., 803 m., 737 s., 652 m., 618 m. 1H NMR (500 MHz; CDCl3, δ, ppm): 11.22 (s., NCHN); 8.09 (d., J = 8.0, 4Har); 7.56 (s., 2CH=); 7.12 (d., J = 8.0, 4Har); 5.68 (s., 2CH2); 3.94 (s., 2 MeO).
The synthesis routine of ester H2L is given below:

Synthesis of I. Ester of H2L (9.1 mg, 0.025 mmol) and Zn(NO3)2 ∙ 6H2O (11 mg, 0.039 mmol) were thoroughly dissolved in a mixed solvent of 2 mL water and 10 mL DMF and heated to 120°C for 96 h in a 23 mL Teflon-lined stainless-steel autoclave and then cooled to room temperature at a rate of 5°C/h. Colorless cubic crystal particles were obtained after several days. The yield was 90% (based on H2L).
For C40H32N4O12Zn2 | |||
Anal. calcd., % | C, 53.84 | H, 3.58 | N, 6.28 |
Found, % | C, 53.70 | H, 3.45 | N, 6.17 |
IR (KBr; ν, cm–1): 3093 w., 3042 w., 1616 v.s., 1557 m., 1374 v.s., 1154 s., 747 s., 623 m.
Synthesis of II. The mixture of ester of H2L (9.4 mg, 0.026 mmol) and Cd(NO3) ∙ 6H2O (11 mg, 0.039 mmol), 5 mL H2O and 5 mL DMF was heated to 100°C for 96 h in a 20 mL glass vial, and then cooled to room temperature, finally colorless needle crystals were afforded. The crystalline solid of II can be obtained in a range temperature from 85 to 160°C. The different reaction conditions are responsible for the sizes of the needle-shaped crystals. The yield was 80% (based on H2L).
For C29H25N3O8Cd | |||
Anal. calcd., % | C, 53.05 | H, 3.81 | N, 6.40 |
Found, % | C, 52.90 | H, 3.675 | N, 6.36 |
IR (KBr; ν, cm–1): 3123 m., 3094 m., 1597 v.s., 1544 v.s., 1398 v.s., 1513 s., 859 s., 747 s., 624 s.
X-ray crystallography. Diffraction data for complex I and II were recordedon a Bruker SMART Apex CCD diffractometer using graphite-monochromat MoKα radiation (0.71073 Å) at 293 K in the ω–2θ scan mode. The single crystal data of II was mounted on an Oxford diffractometer Super Nova TM at 120(2) K with a graphite-monochromatic MoKα radiation (λ = 0.71073 Å) using the ω–scan technique. Using Olex2 [14], the structure was solved by the ShelXS structure solution program and refined with the ShelXL refinement package using Least Squares minimization [15]. For II, the residual electron density maxima of about 5.6 e Å–3 around the Cd(II) with the distance of 0.85 Å can be accounted for series termination errors. Hydrogen atoms were riding on carbon atoms geometrically. The crystallographic data for X-ray diffraction analysis are listed in Table 1.
Supplementary material for structures has been deposited with the Cambridge Crystallographic Data Centre (CCDC nos. 1834629 (I) and 1834630 (II); deposit@ccdc.cam.ac.uk or http://www.ccdc.cam. ac.uk).
RESULTS AND DISCUSSION
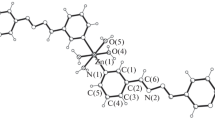
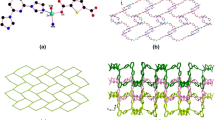
Single crystal X-ray diffraction analysis revealed that the asymmetric unit of I was composed of two crystallographic independence Zn atoms, two zwitterionic ligands and two formic anions which come from the decompose of DMF molecules (Fig. 1a). The Zn(1) atom is five-coordinate with two oxygen atoms (O(2), O(3)) from one bidentate chelation carboxylate group of zwitterionic ligands, two oxygen atoms (O(4), O(5)) of two formic anions, and one oxygen atom (O(1)) from one monodentate carboxylate group of zwitterionic ligand. The Zn(2) atom was located in the center of tetrahedron constructed by two oxygen atoms (O(7), O(8)) from two monodentate carboxylate groups of two zwitterionic ligands and two oxygen atoms (O(4), O(6)) from two formic anions. The Zn–O bond distances ranged from 1.950 to 2.487 Å. Two carboxyl groups of zwitterionic ligands employed the monodentate mode to link adjacent Zn(1) cations to form right-helix chain, while adjacent Zn(2) cations were linked by the mean of bidentate chelation of carboxyl groups on zwitterionic ligands to form left-helix chain. The left and right helix chain exhibited the same pitch of 14.90 Å. The zwitterionic ligands on the left and right chain presented “U”-type geometry, which can bring out interlocking structure between the adjacent left and right helix chains (Fig. 2a). Further, the adjacent Zn(1) and Zn(2) with distance of 3.480 Å on the adjacent helix chains were linked to two dimensional structure in the ab plane by two type of coordination mode 1η, 1η:μ2 and 2η:μ2 of formic anions. From the pure topological point of view, each Zn(1)–Zn(2) unit bonded by formic anions coordinated with four zwitterionic ligands, so the Zn dimeric unit and the ligands are simplified as 4-connected nodes and lines, respectively, the framework can be simplified as a two dimensional 4-connected sql-type layer structure (Fig. 2b).
Single crystal X-ray diffraction analysis revealed that in the asymmetry unit, there are one Cd cation, one and half zwitterionic ligands, and half formic anion originated form decompose of DMF molecule. Each Cd cation is surrounded by seven oxygen atoms consisted by carboxyl atoms (O(1), O(2), O(3), O(4) O(5), O(6)) from three carboxyl groups of zwitterionic ligand and formate oxygen atom (O(7)) (Fig. 1b). The Cd–O bond distance were from 2.267 to 2.438 Å, which are located in reasonable range in reported coordination compounds. All of carboxyl groups employed the bidentate chelation mode to coordinate with Cd centers, while the formic anion employed the 1,3-bridged mode to link with Cd nodes. Therefore, the Cd cations can be considered as 4-connected centers. Due to the ligands coordinated with Cd cations extend along the vertex of the tetrahedron rather than the plane, so three dimensional framework is formed (Fig. 3a). After analyzing the topology, this framework can be described as a uniform 4-connected dia net with the point symbol of 66 (Fig. 3c). There are very large potential voids in single dia framework, which can lead to the poor structural steady [16]. Therefore, mutual interpenetration of six independent equivalent frameworks generate a six-fold interpenetrating and close packing architecture for increasing the structural steady (Figs. 3b and 3d). In fact, dia-type interpenetration structures are very common in the reported interpenetrating frameworks, but the interpenetrating numbers are mainly concentrate between 2 and 5, the six-fold interpenetrating dia-type framework is scarce [17–20].
To investigate the purities of compounds I and II PXRD measurements at room temperature were studied. The results revealed that the PXRD patterns obtained from experiment measurements for I and II was in good agreement with the corresponding simulated pattern from the single-crystal diffraction data, respectively, indicating a good purity and homogeneity of the sample I and II.
The TG curves of I and II were presented in Fig. 4. TG of I shows almost no weight loss before the temperature of 380°C, and above that the structure began to decompose. For II, 2.72% weight loss happened before 210°C was in agreement with the corresponding calculated values of 2.75% due to the loss of one lattice water molecule. The frameworks of II was stable up to about 320°C. Thus, both of compounds I and II exhibited good thermal steady.
The luminescent properties of I and II were investigated in the solid state at room temperature (Fig. 5). The maximum adsorption peak occurred at 315 and 320 nm for I and II, respectively, which can be attributed to the π → π * transition of ligands [21]. For I, the maximum emission peak occurred at 440 nm upon excitation at 315 nm, II exhibited maximum emission at 475 nm excited by 320 nm. Both of I and II emitted blue light which may be mainly originated from ligand–metal charge transition (LMCT) of ligands [22].
In conclusion, two Zn(II) and Cd(II) coordination polymers have been successfully assembled from flexible imidazole-based zwitterionic ligands under solvothermal conditions in this work. The results present an intriguing sql layer structure for I containing interlocking structure between the adjacent left and right helix chains and dia topology with rare six-fold interpenetrating nets for II. Moreover, structure stabilities and luminescent were also investigated. This work will further enrich the synthesis of imidazolezwitterionic ligands-based coordination polymers, which can be used as the precursors of NHC ligands to build novel NHC catalysts and luminescent materials.
REFERENCES
Stock, N. and Biswas, S., Chem. Rev., 2012, vol. 112, p. 933.
Chen, Y., Wang, D.K., Deng, X.Y., et al., Catal. Sci. Technol., 2017, vol. 7, p. 4893.
Wang, K., Xia, B., Wang, Q.L., et al., Dalton Trans., 2017, vol. 46, p. 1042.
Suresh, P., Babu, C.N., Sampath, N., et al., Dalton Trans., 2015, vol. 44, p. 7338.
Sun, Y.N., Xiong, G., Ding, F., et al., Inorg. Chem. Commun., 2015, vol. 62, p. 103.
Timur, I., Subhadip, G., Li, Z.Y., et al., Acc. Chem. Res., 2017, vol. 50, p. 805.
Karmakar, A., Paul, A., Rubio, G.M.D.M., et al., Eur. J. Inorg. Chem., 2016, vol. 36, p. 5557.
Kim, A. and Seong, H., CrystEngComm, 2016, vol. 18, p. 3524.
Li, L.J., Tang, S.F., Wang, C., et al., Chem. Commun., 2014, vol. 50, p. 2304.
Robinson, W., Flaig, T.M., Osborn, P., et al., J. Am. Chem. Soc., 2017, vol. 139, p. 12125.
Paladugu, S., Soumya, R., Chatla, N.B., et al., Dalton Trans., 2013, vol. 42, p. 10838.
Chizoba, I., Ezugwuab, N., Alam, K., and Mekh-man, Y.F., Coord. Chem. Rev., 2015, vol. 307, p. 188.
Siddappa, P., Karolin, D., Anthony, D., et al., Helv. Chim. Acta, 2010, vol. 93, p. 2347.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Cryst., 2009, vol. 42, p. 339.
Sheldrick, G.M., SHELXS-97, Program for Crystal Structure Determination, Göttingen: Univ. of Göttingen, 1997.
Lee, H.J., Kwon, H., Sim, J., et al., CrystEngComm, 2017, vol. 19, p. 1528.
Tan, Y.X., Si, Y.N., Wang, W.J., and Yuan, D.Q., J. Mater. Chem., A, 2017, vol. 5, p. 23276.
Pickwick, B.L., Pochodylo, A.L., and LaDuca, R.L., Inorg. Chim. Acta, 2017, vol. 466, p. 618.
Zhang, Y., Wang, L., and Zeng, M.H., Inorg. Chem. Commun., 2017, vol. 83, p. 123.
Shi, Z.Z., Qing, L., and Zheng, H.G., Dalton Trans., 2017, vol. 46, p. 4589.
Vasylevskyi, S.I., Regeta, K., Ruggi, A., et al., Dalton Trans., 2018, vol. 47, p. 596.
Jia, L.M., Tong, J., and Yu, S.Y., J. Photochem. Photobiol., A., 2018, vol. 355, p. 84.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of China (nos. 21671139 and 21501122), the Distinguished Professor Project of Liaoning province (2013204) and Doctoral Scientific Research Foundation of Liaoning Province (201601193 and 201601201).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, P., Xiong, G., He, Y.K. et al. Novel Zn and Cd Coordination Polymers Assembled from Imidazole-based Zwitterionic Ligands: Synthesis, Crystal Structures, and Luminescence Properties. Russ J Coord Chem 45, 741–747 (2019). https://doi.org/10.1134/S1070328419100087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419100087