Abstract
The slow evaporation of an acetone solution containing trans-[Ru(NO)Py4(OH)]2+ cations and hexafluorophosphate anions results in the crystallization of trans-[Ru(NO)Py4(OH)](PF6)2 ⋅ (CH3)2CO (I). The reactions of trans‑[Ru(NO)Py4(OH)]Cl2 ⋅ H2O with solutions of chloric or hydrochloric acid followed by the evaporation of the reaction solutions at ambient temperature afford trans-[Ru(NO)Py4(H2O)](ClO4)3 (II) or [H5O2]2[Ru(NO)Py4Cl]Cl4 (III), respectively. The obtained chloride complex III is unstable and at ambient temperature eliminates hydrogen chloride to transform into trans-[Ru(NO)Py4Cl]Cl2 ⋅ 4H2O (IV). The crystal structures of compounds I and III are determined by X-ray structure analysis (CIF files ССDC nos. 1421042 (I) and 1421041 (III)).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Interest of researchers in the nitrosoruthenium complexes is caused by two main factors. On the one hand, these compounds are biologically active and considered as low-toxicity regulators of the nitrogen(II) oxide concentration in biological systems [1, 2]. On the other hand, the nitrosoruthenium complexes are capable of forming two photoinduced long-lived metastable states (MS1 and MS2) differed in the coordination mode of the nitroso group to the central atom [3–5]. A phenomenon of this photoisomerization provides prospects for the synthesis of hybrid materials combining the photochromic properties with electrical conductance or magnetism [6, 7]. Among the presently known nitrosoruthenium complexes, the tetrapyridine complex [Ru(NO)-Py4Cl](PF6)2 ⋅ 0.5H2O has the record-breaking high degree of transition to the metastable states MS1 and MS2 (92 and 48%, respectively) [8, 9].
Several approaches to the synthesis of the tetrapyridinenitrosoruthenium complexes are presented in the literature. One of them [10] makes it possible to obtain the halide complexes [Ru(NO)Py4X]2+ (X = Cl, Br) and consists of the treatment of the corresponding trans-dinitro complex with hydrogen halide acid
Only compounds with \({\text{ClO}}_{4}^{ - }\) or \({\text{PF}}_{6}^{ - }\) anions in the external sphere can be isolated to the solid phase from aqueous solutions in high yields (70–85%). The hydroxo complexes [Ru(NO)Py4(OH)](ClO4)2 or [Ru(NO)Py4(OH)](PF6)2 can be obtained using chloric [11] or trifluoroacetic [12] acid in the synthesis.
Somewhat modified method for the synthesis of the nitrosotetrapyridine complexes is based on the nitrosylation of the dichlorotetrapyridine complexes with sodium nitrite in a hydrochloric acid medium [12]
Another approach to the synthesis of the tetrapyridine complex was described [13]. The trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O complex is obtained in a yield of ~70% by reflux of fac‑[Ru(NO)Py2Cl3] with an excess of an aqueous solution of pyridine.
In this work, we consider the transformations of the hydroxotetrapyridine complex [Ru(NO)Py4(OH)]2+ in an acidic medium, proposed the procedures for the synthesis of the chloro- and aquanitrosoruthenium complexes of the tetrapyridine series, and determined the crystal structures of trans-[Ru(NO)-Py4(OH)](PF6)2 ⋅ (CH3)2CO (I) and trans-[H5O2]2[Ru(NO)Py4Cl]Cl4 (III).
EXPERIMENTAL
The starting trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O compound was synthesized using a known procedure [13]. Other reagents and solvents were not lower than reagent grade and were used without additional purification.
Synthesis of complex I. A weighed sample of trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O (~0.5 g, 9.0 × 10−4 mol) was dissolved in a minimum amount of water (~10 mL), and a 10% excess of an aqueous solution of NH4PF6 was added. The precipitate formed was filtered off on a glass porous filter (40 pores), washed with ethanol and diethyl ether, and dried in an air flow. The yield of trans-Ru(NO)Py4(OH)](PF6)2 ⋅ H2O was ~95%. The compound was highly soluble in acetone from which crystals of the solvate of compound I suitable for X-ray structure analysis were obtained by slow evaporation.
IR (ν, cm−1): 3580, 3395 ν(OH); 3127–2820 ν(CH); 1867 ν(NO); 1715 ν(CO); 1612, 1576, 1491, 1454, 1406, 1364 ν(Carom–Carom), ν(Carom–Narom); 1246, 1223, 1163, 1072, 1020 δ(CHpl); 974, 835 ν(Ru–OH); 756, 691, 652 δ(CHout-of-pl); 637, 556 ν(Ru–NNO), δ(Ru–NO); 459 ν(Ru–NPy).
Synthesis of trans-[Ru(NO)Py4(H2O)](ClO4)3 (II). Water (~20 mL) and concentrated HClO4 (~1 mL) were added to a weighed sample of trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O (~0.1 g, 1.8 × 10−4 mol). The obtained solution was evaporated at ambient temperature to a minimum volume (~2 mL). The precipitate formed was filtered off on a glass porous filter (40 pores) and washed with cooled ethanol (~1 mL) and with the same amount of diethyl ether. The yield of compound II was ~50%.
IR (ν, cm−1): 3450 ν(H2O); 3120–3050 ν(CH); 2800, 2400 ν(OH⋅⋅⋅O); 1934 ν(NO); 1650 δ(H2O); 1612, 1492, 1454, 1365 ν(Carom–Carom), ν(Carom–Narom); 1227, 1018 δ(CHpl); 1097 ν3(ClO4); 928 δ(Ru−OH2); 762, 696 δ(CHout-of-pl); 623 ν4(ClO4); 452 ν(Ru–NPy).
Synthesis of complex III. A weighed sample of trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O (~0.1 g, 1.8 × 10−4 mol) was dissolved in ~1 mL of a 6 М solution of HCl, and the mixture was left to evaporate slowly at ambient temperature to form slightly wet crystals of complex III suitable for X-ray structure analysis.
IR (ν, cm−1): 3450, 3380, 3236, 2500, 2100 ν(H5O2+); 3120–2800 ν(CH); 1921 ν(NO); 1729, 1641 ν(H5O2+); 1610, 1491, 1451, 1364 ν(Carom–Carom), ν(Carom–Narom); 1274, 1242, 1221, 1159, 1125, 1071, 1017 δ(CHpl); 880 δ(H5O2+); 765, 698, 651 δ(CHout-of-pl); 600 ν(Ru–NNO), δ(R–NO); 452 ν(R–NPy).
Synthesis of trans‑[Ru(NO)Py4Cl]Cl2 ⋅ 4H2O (IV). Hydrogen chloride was removed upon the complete drying of the crystals of compound II in air for several days at ambient temperature due to which compound V was formed in a quantitative yield.
IR (ν, cm−1): 3400 ν(H2O); 311–2800 ν(CH); 1921 ν(NO); 1641 δ(H2O); 1610, 1491, 1451, 1364 ν(Carom–Carom), ν(Carom–Narom); 1274, 1242, 1221, 1159, 1125, 1071, 1017 δ(CHpl); 765, 699, 651 δ(CHout-of-pl); 600 ν(R–NNO), δ(R–NO); 452 ν(R–NPy). The IR spectra of the samples in KBr pellets were recorded on a Scimitar FTS 2000 FTIR spectrometer in a range of 4000–375 cm–1.
The X-ray diffraction analysis of the powdered crystals was carried out on a Shimadzu XRD-7000 diffractometer (CuKα radiation, Ni filter, 2θ = 5°–60°). The samples were deposited as thin layers on the smooth side of a standard quartz cell.
The spectroscopic and diffraction data were processed using the OriginPro 7.5 program package [14].
X-ray structure analyses of compounds I and III. The unit cell parameters were determined and a set of experimental intensities was obtained on an X8 APEX Bruker automated diffractometer (MoKα radiation, graphite monochromator, two-coordinate CCD detector). The structures were solved by a direct method and refined in the anisotropic (isotropic for H atoms) approximation. The positions of all hydrogen atoms were localized from the difference synthesis. All calculations were performed using the SHELX-97 program package [15]. The crystallographic characteristics and the main refinement parameters for compounds I and III are presented in Table 1. Selected interatomic distances for compounds I and III are given in Tables 2 and 3, respectively. The bond angles in compound III are listed in Table 3.
The data on the structures of compounds I and III were deposited with the Cambridge Crystallographic Data Centre (CIF files CCDC nos. 1421042 and 1421041, respectively; www.ccdc.cam.ac.uk/data_request/cif).
RESULTS AND DISCUSSION
The reaction of an aqueous solution of trans‑[Ru(NO)Py4(OH)]Cl2 ⋅ H2O with a minor excess of NH4PF6 results in the precipitation of trans-[Ru(NO)Py4(OH)](PF6)2 ⋅ H2O in an almost quantitative yield. The structure of this compound was described earlier [16]. The slow evaporation of a solution of the compound in acetone affords crystals of solvate I, whose structure was determined by X-ray structure analysis.
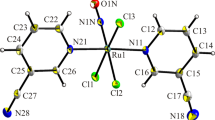
The geometries of trans-[Ru(NO)Py4(OH)]2+ in compound I and in similar hydrate are close, but the structure of compound I contains complex species of two types (A containing the central atom Ru(1) and В containing Ru(2), Fig. 1). The interatomic distances (Table 2) in these complexes differ insignificantly and are consistent, as a whole, with the published data [16].
The coordination polyhedron of each complex А and В is a distorted octahedron formed by four nitrogen atoms of the pyridine molecules in the equatorial plane, nitrogen atoms of the nitroso groups, and oxygen of the hydroxide ion. The planes of the Py rings are arranged according to the “propeller” type at dihedral angles of 60°–70° to the equatorial planes. The nonequivalent complexes А and В are packed in crystal in such a way that their N−Ru−O axes are oppositely directed and the planes of the Py rings are parallel in pairs (two pyridine molecules are coaxial). The planes of the pyridine rings in complexes А and В deviate insignificantly (0.5°–2°) from the corresponding Ru−N axes, except for the coaxial Py ligands for which this deviation reaches 9° and 7°, respectively.
The ONRu bond angles for complexes А and В are 174.2° and 177.0°, and NRuO are 179.1° and 179.6°, respectively. The NRuN bond angles at the Ru(1) and Ru(2) atoms deviate from ideal values (90°) by 0.1°–2.8° (for А) and 0.9°–3.5° (for В). These values agree with the published data for the known nitrosoruthenium complexes of similar structure [17–20].
As we expected, the reactions of the hydroxo complex trans‑[Ru(NO)(Py)4(OH)]2+ with solutions of strong acids are accompanied by the protonation of coordinated hydroxide ions
In the case chloric acid, the gradual evaporation of the reaction solution does not result in the coordination of the \({\text{ClO}}_{4}^{ - }\) ion to the Ru atom and the following aqua complex in the form of perchlorate salt is formed:
When hydrochloric acid is used, the slow evaporation of the reaction solution at ambient temperature affords yellow-orange crystals of compound III containing chloride ions in the internal sphere
It has previously been reported that the hydroxo complex trans-[Ru(NO)(Py)4(OH)]2+ quantitatively transforms into trans-[Ru(NO)Py4Cl]2+ on heating to 80°C [11]. However, as our experiments showed, it is preferential to synthesize this complex at ambient temperature, because the substitution of coordinated pyridine molecules can occur on heating [13, 21].
According to the data of IR spectroscopy, X-ray diffraction analysis, and gravimetry, on storage in air compound III loses hydrogen chloride molecules, which was used for the preparation of chloro complex IV, whose structure has previously been described [9]
The IR spectra of all compounds obtained exhibit intense bands at 1850–1920 cm−1 corresponding to the ν(NO) stretching vibrations. These values are in the range characteristic of the most part of nitrosoruthenium complexes containing the diamagnetic Ru(II) center and linearly coordinated NO+ species [22, 23]. The IR spectra of compounds I–IV also contain bands characteristic of coordinated pyridine molecules [24, 25]: narrow medium- and low-intensity ν(CH) bands at 3100–3000 cm−1, narrow medium- and high-intensity ν(Carom−Carom) and ν(Carom−Narom) bands at 1600–1350 cm−1, and medium- and high-intensity δ(CHpl) and δ(CHout-of-pl) bands at 1240–970 and 760–630 cm−1, respectively. In addition, the spectra of compounds I and II contain the medium- and low-intensity bands caused by the presence of the hydroxide ion (for I) or water molecules (for II) in the internal sphere.
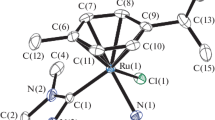
The structure of compound III was determined by X-ray structure analysis. Its crystal structure is built of the centrosymmetric complex cations trans-[Ru-(NO)Py4Cl]2+, anions Cl−, and cations H5O2+. The structure of the complex cation with the numeration of atoms and thermal vibration ellipsoids is shown in Fig. 2.
The square environment in the equatorial plane of the complex cation is formed by four nitrogen atoms of the pyridine molecules. The octahedral coordination mode of the ruthenium atom is supplemented by the nitroso group and chloride ion located in the trans-position to each other. The Ru−NPy, Ru−Cl, Ru−NNO, and N−O distances in this complex are characteristic of other known nitrosoruthenium complexes [9, 13, 26, 27]. The deviation of the bond angles at the ruthenium atom from 90° does not exceed 2.3°.
The structure of compound III contains layers parallel to the yz plane (Fig. 3). The interlayer space is occupied by H5O2+ and Cl−. The O⋅⋅⋅O distance in the H5O2+ cation is ~2.45 Å. The outer-sphere chloride ions form three short contacts with the π‑systems of two pyridine molecules (Cl⋅⋅⋅Py 3.6–3.7 Å). The Cl⋅⋅⋅O distances between the H5O2+ particles and chloride ions are 2.93 and 3.06 Å, and the shortest Ru⋅⋅⋅Ru distance is 7.7 Å.
To conclude, the slow evaporation of an acetone solution containing trans-[Ru(NO)Py4(OH)]2+ cations and hexafluorophosphate anions gave crystals of trans-[Ru(NO)Py4(OH)](PF6)2 ⋅ (CH3)2CO. The reactions of the hydroxotetrapyridine complex trans-[Ru(NO)Py4(OH)]Cl2 ⋅ H2O with solutions of strong acids at ambient temperature afford the aqua or acido complexes depending on the nature of the acid. In the case of using chloric acid, we isolated the aqua complex trans‑[Ru(NO)Py4(H2O)](ClO4)3, and the chloride complex [H5O2]2[Ru(NO)Py4Cl]Cl4 was isolated from a solution of hydrochloric acid. When this complex is kept is air at ambient temperature, the hydrogen chloride molecules are removed and trans-[Ru(NO)Py4Cl]Cl2 ⋅ 4H2O is formed.
REFERENCES
Silva, J.J., Osakabe, A.L., Pavanelli, W.R., et al., Br. J. Pharmacol., 2007, vol. 152, p. 112.
Tfouni, E., Truzzi, D.R., Tavares, A., et al., Nitric Oxide, 2012, vol. 26, p. 38.
Woike, T., Kirchner, W., Shetter, G., et al., Opt. Commun., 1994, vol. 106, p. 6.
Coppens, P., Novozhilova, I., and Kovalevsky, A., Chem. Rev., 2002, vol. 102, p. 861.
Schaniel, D., Woike, T., and Delley, B., Phys. Chem. Chem. Phys., 2005, vol. 7, p. 1164.
Kushch, L.A., Golhen, S., Cador, O., et al., J. Cluster Sci., 2006, vol. 17, p. 303.
Schaniel, D., Woike, T., Kusch, L., and Yagubskii, E., Chem. Phys., 2007, vol. 340, p. 211.
Schaniel, D., Cormary, B., Malfant, I., et al., Phys. Chem. Chem. Phys., 2007, vol. 9, p. 3717.
Cormary, B., Ladeira, S., Jacob, K., et al., Inorg. Chem., 2012, vol. 51, p. 7492.
Bottomley, F. and Mukaida, M., J. Chem. Soc., Dalton Trans., 1982, no. 10, p. 1933.
Coe, B.J., Meyer, T.J., and White, P.S., Inorg. Chem., 1995, vol. 34, no. 3, p. 593.
Calandreli, I., Oliveira, F.S., Liang, G., et al., Inorg. Chem. Commun., 2009, vol. 12, no. 7, p. 591.
Makhinya, A.N., Il’yin, M.A., Yamaletdinov, R.D., et al., Russ. J. Coord. Chem., 2016, vol. 42, p. 768. doi 10.1134/S1070328416120046
Origin Pro 7.5. SR0. V. 7.5714 B(714). Northampton: OriginLab Corporation, 2003.
Sheldrick, G.M., SHELX-97. Release97-1. Programs for the Refinement of Crystal Structures, Göttingen: Univ. of Göttingen, 1997.
Nishimura, H., Matsuzawa, H., Togano, T., et al., J. Chem. Soc., Dalton Trans., 1990, no. 1, p. 137.
Kostin, G.A., Borodin, A.O., Mikhailov, A.A., et al., Eur. J. Inorg. Chem., 2015, vol. 29, p. 4905.
Il’yin, M.A., Emel’yanov, V.A., Belyaev, A.V., et al., Russ. J. Inorg. Chem., 2008, vol. 53, no. 7, p. 1070.
Makhinya, A.N., Il’in, M.A., Baidina, I.A., et al., Russ. J. Struct. Chem., 2014, vol. 55, no. 4, p. 682.
Il’in, M.A., Makhinya, A.N., Baidina, I.A., and Tkachev, S.V., Inorg. Chim. Acta, 2014, no. 413, p. 90.
Makhinya, A.N., Il’in, M.A., Yamaletdinov, R.D., and Baidina, I.A., New J. Chem., 2016, vol. 40, p. 10267.
Mercer, E.E., McAlister, W.A., and Durig, J.R., Inorg. Chem., 1966, vol. 5, no. 11, p. 1881.
Rose, M.J. and Mascharak, P.K., Coord. Chem. Rev., 2008, no. 252, p. 2093.
Borges, S.S.S., Davanzo, C.U., Castellano, E.E., et al., Inorg. Chem., 1998, vol. 37, p. 2670.
Gomes, M.G., Davanzo, C.U., Silva, S.C., et al., Dalton. Trans, 1998, p. 601.
Il’in, M.A., Emel’yanov, V.A., and Baidina, I.A., Russ. J. Struct. Chem., 2008, vol. 49, no. 6, p. 1090.
Ookubo, K., Morioka, Y., Tomizawa, H., and Miki, E., J. Mol. Struct., 1996, vol. 379, p. 241.
ACKNOWLEDGMENTS
The authors are grateful to N.I. Alferova for recording IR spectra and to N.P. Korotkevich for the detection of powder diffraction patterns.
This work was supported by the Complex program for basic research of the Siberian Branch of the Russian Academy of Sciences, project no. II.1.16.1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Makhinya, A.N., Il’in, M.A., Korol’kov, I.V. et al. Some Transformations of trans-Tetrapyridine Complexes of Nitrosoruthenium: Crystal Structures of [Ru(NO)Py4(OH)](PF6)2 ⋅ (CH3)2CO and [H5O2]2[Ru(NO)Py4Cl]Cl4. Russ J Coord Chem 44, 613–618 (2018). https://doi.org/10.1134/S1070328418100093
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418100093