Abstract
Objective: It is worthy to note that the benzimidazole, thiazolidine-2,4-dione and 1,2,3-triazole pharmacophores have keen role in anticancer drug discovery. In order to overcome the limitations associated with reported antimitotic compounds, our group is first time combining these three pharmacophores and revealing their in vitro anticancer and anti-mitotic activities. Methods: As a part of our efforts on the development of novel anticancer agents, in this study, we concentrated on the synthesis of benzimidazole-thiazolidine-2,4-dione-1,2,3-triazoles (VIIa–VIIl) via piperidine catalyzed Knoevenagel condensation and Cu(I) catalyzed azide-alkyne cycloaddition reactions as key approaches. Then, in vitro anticancer and tubulin polymerization inhibition (VIIa–VIIl) were studied via IC50 values. Finally, docking interactions of three potent compounds (VIIg), (VIIh), and (VIIk) towards α,β-tubulin were studied using AutoDock tools. Results and Discussion: Three compounds namely (VIIg), (VIIh), and (VIIk) showed better results against A549, MCF-7, and HeLa human cancer cell lines than standard drug nocodazole. In addition, compounds (VIIg) and (VIIh) have shown greater inhibitory potency with IC50 values 0.62 and 0.31 μM respectively than standard Combretastatin A-4 (CA-4) against tubulin polymerization. Finally, in silico molecular docking studies for the compounds (VIIg), (VIIh), and (VIIk) with α,β-tubulin showed that they have great binding interactions with the target protein and to be specific the compound (VIIh) displayed highest binding energy, i.e. –9.23 kcal/mol. Conclusions: We propose that the remarkable in vitro anticancer activity of compounds (VIIg), (VIIh), and (VIIk), would be due to their tubulin polymerization inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Even though there has been a lot of progress in the search for cancer drugs, due to its lack of safety, selectivity, and efficiency etc. [1], targeted therapy which targets proteins selectively to prevent growth and spread of cancer cells is still necessary. As a result, targeting microtubules could be regarded as an excellent objective in the developing of anticancer drugs [2], since they are involved in the normalization of cellular functions such as motility, organelle transport, cell division and signal transduction maintenance etc [3, 4]. Some anticancer drugs like such as vinblastine, vincristine, and colchicine, have been shown to weaken structure and function of microtubules by inhibiting tubulin polymerization via their binding to vinca-binding site or colchicine of tubulin [5, 6]. As we know, for the past few years, antimitotic agents such as taxanes and vinca alkaloids have been utilized in clinical trials of varied cancer affected patients [2, 7]. Nonetheless, high toxicity, low solubility, and low oral bioavailability were made the above antimitotic agents less ideal in course of clinical trials for cancers [8]. As a result, chemists are searching for the development of new antimitotic compounds agents, to overwhelm the shortcomings mentioned above.
Several drugs with a wide range of therapeutic applications have been developed using the benzimidazole as a fundamental structural component [9–15]. The unique fused benzene and imidazole rings with two nitrogen atoms and an electron-rich aromatic ring, allow them to interact with a variety of biologically significant targets in a non-covalent manner led to give biologically activity [16, 17]. Interestingly, several anticancer drugs containing benzimidazoles were developed by numerous researchers worldwide and have proven effective in treating a diverse malignancies. In addition, a phase III clinical trial for Galeterone, another benzimidazole-based anticancer agent is currently underway [18–21].
Thiazolidine-2,4-dione(TZD) scaffold notable and fundamental frameworks used in drug discovery and development [22, 23]. A few analogues of TZD namely such as AZD1208, GSK1059615, and SMI-4a were currently undergoing clinical screening to treat tumor [24–30]. Interestingly, compounds with anticancer activities were also produced by modifying thiazolidine-2,4-dione at both N–3 and C–5 positions [31–35].
Conversely, the increased solubility and pharmacokinetic characteristics of 1,2,3-triazoles at the binding site play a major role in enhancing cytotoxicity against various cancer cells [36, 37]. Many 1,2,3-triazole based derivatives have a high dipole moment and can show dipole-dipole, π-stacking interactions and can form hydrogen bonding with biological targets easily [38]. Generally speaking, a variety of 1,2,3-triazole coupled heterocycles exhibited several anticancer activities [39–47].
In order to overcome the limitations associated with reported antimitotic compounds agents and encouraged by the previously developed benzimidazole [48–52], thiazolidine-2,4-dione [53], and 1,2,3-triazole based antitubulin agents, herein, we first time report the design and synthesis of benzimidazole-thiazolidine-2,4-dione1,2,3-triazoles as tubulin targeting anticancer agents.
RESULTS AND DISCUSSION
Chemistry
Synthesis of designed benzimidazole-1,2,3-triazolethiazolidine-2,4-dione hybrids (VIIa–VIIl) was presented in Scheme 1. First step involves the Knoevenagel condensation between commercially available 1-methyl1H-benzo[d]imidazole-5-carbaldehyde (I) and TZD (II) under piperidine catalysis in ethanol under reflux condition for 28 h gave 5-((1-methyl-1H-benzo[d]imidazol-5yl)methylene) thiazolidine-2,4-dione (III) [39]. This intermediate was then treated with 3-bromoprop-1-yne (IV) by means of K2CO3 in THF at 65°C for 13 h to give 5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)3-(prop-2-yn-1-yl)thiazolidine-2,4-dione (V). Finally, copper (I) catalyzed 1,3-dipolar cycloaddition between alkyne (V) and many aromatic azides (VIa–VIl) in THF at 40°C for 12 provided the anticipated compounds (VIIa–VIIl) in moderate to good yields.
In Vitro Anticancer Activity
We have tested the newly prepared 1,2,3-trizoles for the anticancer activity against cancer cell lines such as A549, MCF-7, and HeLa using MTT assay and the nocodazole as reference drug. The results were displayed in Table 1 shown that the compounds (VIIg), (VIIh), and (VIIk) displayed superior activity against all the three cell lines than the nocodazole. In particular, (VIIh) has shown greatest activity among the three compounds mentioned above. In addition, structure-activity relationship (SAR) was established on the basis of IC50 data given in Table 1. First, with respect to compounds having electron releasing groups, (VIIh) having 3,5-dimethoxy group has displayed more activity and (VIIg) having 4-methoxy on it has exhibited next highest activity in that series. On other way the compounds having mono methyl and dimethyl substituents like (VIIb) and (VIIc) have exhibited less cytotoxicity than the reference. The compound (VIIa) having no substituent also displayed less activity than reference. In compounds with electron withdrawing groups, the compounds (VIIk) having 4-cyano substituent has shown superior activity than the nocodazole reference drug but rest of the compounds were exhibited less activity than the reference drug. Among the compounds having mono halogen substituents the order of anticancer activity was found to be greater for 4-Cl and least for 4-Br. Finally, the compound (VIIj) having two chlorine atoms at 3rd and 5th position of phenyl ring has exhibited greater activity than the compound (VIIe) having one chlorine atom at 4th position and compound (VIIl) having 4-nitro group has shown least activity in the series.
In Vitro Tubulin Polymerization Inhibitory Activity
The 12,3-triazole compounds (VIIg), (VIIh), and (VIIk) were further screened for tubulin polymerization inhibitory activity as per the method described earlier and CA-4 was taken as the standard [40]. The results were presented in Table 2 and as per the results the compounds (VIIg), (VIIh) were displayed greater inhibitory potency (IC50 equal to 0.62 and 0.31 μM, respectively) than standard CA-4. But the compound (VIIk) has shown less inhibitory potency with IC50 equal to 1.65 μM compared CA-4.
Molecular Docking Studies
We have studied docking interactions of three potent compounds (VIIg), (VIIh), and (VIIk) towards α,βtubulin [41] using AutoDock tools. The reference drug Combretastatin A-4 was also docked with α,β-tubulin. Among the three compounds, the compound (VIIh) has displayed highest binding energy (–9.23 kcal/mol) and inhibition constant (172.80 nano molar). In addition to this it has formed two hydrogen bonds with VAL250 and VAL353 residues with bond lengths 2.31 and 2.19 Å, respectively (Table 3, Figs. 1 and 2). The other two compounds (VIIg) and (VIIk) have exhibited almost similar binding energies –8.73 and –8.74 kcal/mol, respectively. The compound (VIIg) has formed two hydrogen bonds with ASN101 and VAL250 residues with bond lengths 1.94 and 2.80 Å, respectively. Similarly, it has formed π-π stacking with TYR224 residue. On the other hand the compound (VIIk) has formed one hydrogen bond with SER174 with bond lengths 1.83 Å. The standard drug Combretastatin A-4 has shown –6.76 kcal/mol, binding energy and 11.09 micromolar, inhibition constant. It has formed two hydrogen bonds with VAL353 and ILE355 residues with bond lengths 2.20 and 1.86 Å, respectively. Finally, we can say that three compounds have shown more binding interaction with target protein α,β-tubulin than Combretastatin A-4.
EXPERIMENTAL
General information. All the commercially accessible chemicals were utilized without being purified. Using Merck 60 F254 silica gel TLC plates, the purity of the entire compound was examined. Uncorrected melting points were determined using a hot stage melting point apparatus (Ernst Leitz Wetzlar, Germany). The Mercuryplus spectrometer, which operated at 400 and 100 MHz for 1H and 13C NMR, respectively, recorded the 1H and 13C NMR spectra. The chemical shifts were then compared to TMS. The Shimadzu QP5050A quadrupolebased mass spectrometer was used to obtain the ESI (electrospray ionization) mass spectra (ionising voltage of 70 eV). Elemental analyses were obtained with an Elemental Analyser Perkin–Elmer 240C apparatus.
Synthesis of 5-((1-methyl-1H-benzo[d]imidazol5-yl)methylene)thiazolidine-2,4-dione (III) [39]. In a 250 mL RBF, 1-methyl-1H-benzo[d]imidazole-5-carbaldehyde (I) (25 mmol), thiazolidine-2,4-dione (25 mmol) (II) and piperidine (2.5 mmol) in EtOH (40 mL) was stirred under reflux for 28 h. The reaction mixture was then allowed to get RT for overnight, the resulting solid was filtered, washed with cold EtOH and dried. Finally, the crude product was crystallized from EtOH.
5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)3-(prop-2-yn-1-yl)thiazolidine-2,4-dione (V). The compound (III) (15 mmol), K2CO3 (30 mmol) and 3-bromoprop-1-yne (IV) (21 mmol) were stirred in THF (40 mL) at 65°C for 13 h. The reaction mixture was then cooled to RT and filtered. The filtrate was extracted with ethyl acetate (2 × 50 mL) and excess of organic layer concentrated under rotary evaporator. Finally, the crude product was purified by 60–120 mesh size silica gel column chromatography employing (3 : 7) ethyl acetate/hexane as eluent to get 73% yield of colorless solid (V).
Synthesis of benzimidazole-thiazolidine-2,4-dione1,2,3-triazoles (VIIa–VIIn). A mixture of intermediate (V) (0.67 mmol), aryl azides (VIa–VIn) (1 mmol) and Cu(I) (0.067 mmol) in THF (10 mL) was stirred at 40°C for 12 h. The progress of reaction as analyzed by the TLC, the resulting mixture was extracted with ethyl acetate (2 × 20 mL), the excess of organic layer was concentrated under rotary evaporator. The resulting crude products were purified by 60–120 mesh size silica gel column chromatography using (1 : 1) ethyl acetate/hexane eluent.
In vitro anticancer activity. Individual wells of a 96-well tissue culture micro titer plates were inoculated with 100 µL of complete medium containing 1 × 104 cells. Then the plates were incubated at 37°C in a humidified 5% CO2 incubator for 18 h prior to the experiment. After medium removal, 100 µL of fresh medium containing the test compounds and nocodazole at different concentrations such as 0.5, 1, and 2 µM were added to each well and incubated at 37°C for 24 h. Then the medium was discarded and replaced with 10 µL MTT dye. Plates were incubated at 37°C for 2 h. The resulting formazan crystals were solubilized in 100 µL extraction buffer. The optical density (O.D) was read at 570 nm with micro plate reader (Multi-mode Varioskan Instrument-Themo Scientific). The percentage of DMSO in the medium never exceeded 0.25%.
In vitro tubulin polymerization inhibition. We have used a commercial kit (cat. #BK011P) and followed method stated in the kit. The tubulin reaction mixture containing 2.0 mM of MgCl2, 0.5 mM of EGTA, 80.0 mM of PIPES (pH = 6.9), 1 mM of GTP and 10.2% glycerol was prepared. Later, 5 μL of testing hybrids at indicated concentrations were added and mixture was pre-warmed to 37°C for 1 min. Then the reaction was initiated by adding 55 μL of tubulin solution. The fluorescence intensity was recorded at each 60 s for 90 min in a multifunction microplate reader. IC50 was calculated from area under curve.
Molecular docking studies. Molecular docking study was conducted using AutoDock 4.2. The protein was retrieved from Protein Data Bank, which has the PDB ID: 1SAO. After adding polar hydrogens, the ligand and water are taken out of protein and calculated gasteiger charges. After energy minimization, the ligands are drawn using chem draw 12 and saved as.mol files. They are then transformed using Discovery Studios into a PDB file. By selecting 60 points along three axes of coordinates and applying a grid spacing of 0375, a grid box is created. The DPF file was produced using Lamarckian GA (4.2) method. The GPF file was converted to a GLG file using the Cygwin interface and DPF file was converted to a DLG file from which the results were retrieved. The 2D and 3D images are being rendered using Schrodinger’s maestro v9.5 vizualizer interface.
5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (III). Colorless solid; Yield: 64%; mp: 163–165°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.73 (s, 3H, N–CH3), 7.35 (d, J = 8.2 Hz, 1H, Ar–H), 7.60 (d, J = 8.2 Hz, 1H, Ar–H), 7.68 (s, 1H, alkene-H), 7.95 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H) 12.92 (br s, 1H, thiazolidine-2,4-dione-NH).
5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)3-(prop-2-yn-1-yl)thiazolidine-2,4-dione (V). Colorless solid; Yield: 73%; mp: 167–169°C; 1H NMR (300 MHz, DMSO-d6), ppm: 2.32 (s, 1H, alkyne-1H), 3.72 (s, 3H, N–CH3), 4.46 (s, 2H, N–CH2), 7.34 (d, J = 8.2 Hz, 1H, Ar–H), 7.58 (d, J = 8.2 Hz, 1H, Ar–H), 7.69 (s, 1H, alkene–H), 7.95 (s, 1H, Ar–H), 8.03 (s, 1H, Ar–H).
(Z)-5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)-3-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-dione (VIIa). Colorless solid; Yield: 77%; mp: 201–203°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.74 (s, 3H, N–CH3), 4.82 (s, 2H, N–CH2), 7.35–7.43 (m, 4H, Ar–H), 7.60 (d, J = 8.2 Hz, 1H, Ar–H), 7.71 (s, 1H, alkene-H), 7.79–7.86 (m, 2H), 7.96 (s, 1H, Ar–H), 8.04 (s, 1H, Ar–H), 8.45 (s, 1H, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.7, 46.8, 106.9, 114.7, 120.7, 122.2, 124.1, 126.1, 127.6, 128.1, 129.1, 130.5, 132.9, 137.8, 141.3, 143.6, 144.8, 164.7, 170.1; ESIMS: m/z 417 [M + H]+; C21H16N6O2S; Calculated, %: C, 60.57; H, 3.87; N, 20.18; Found, %: C, 60.59; H, 3.85; N, 20.21.
(Z)-5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)-3-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-dione (VIIb). Colorless solid; Yield: 75%; mp: 205–207°C; 1H NMR (300 MHz, DMSO-d6), ppm: 2.41 (s, 3H, Ar–CH3), 3.73 (s, 3H, N–CH3), 4.81 (s, 2H, N–CH2), 7.30–7.37 (m, 3H, Ar–H), 7.59 (d, J = 8.2 Hz, 1H, Ar–H), 7.72 (s, 1H, alkene-H), 7.83 (d, J = 7.9 Hz, 2H), 7.95 (s, 1H, Ar–H), 8.01 (s, 1H, Ar–H), 8.44 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 21.9, 37.6, 46.6, 106.9, 114.8, 120.6, 123.9, 124.8, 126.2, 127.5, 129.6, 130.4, 132.8, 136.6, 139.2, 141.2, 143.6, 144.9, 164.8, 170.3; ESI-MS: m/z 431 [M + H]+; C22H18N6O2S; Calculated, %: C, 61.38; H, 4.21; N, 19.52; Found, %: C, 61.40; H, 4.19; N, 19.55.
(Z)-3-((1-(3,5-Dimethylphenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((1-methyl-1H-benzo [d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIc). Light blue solid; Yield: 72%; mp: 209–211°C; 1H NMR (300 MHz, DMSO-d6), ppm: 2.43 (s, 6H, Ar–CH3), 3.74 (s, 3H, N–CH3), 4.82 (s, 2H, N–CH2), 7.11 (s, 1H, Ar–H), 7.35 (d, J = 8.2 Hz, 1H, Ar–H), 7.47 (s, 2H, Ar–H), 7.60 (d, J = 8.2 Hz, 1H, Ar–H), 7.71 (s, 1H, alkene-H), 7.96 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H), 8.46 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 22.2, 37.6, 46.9, 106.8, 114.9, 120.6, 124.2, 125.7, 126.2, 127.2, 127.8, 130.5, 132.7, 138.4, 140.3, 141.3, 143.7, 144.8, 164.7, 169.9; ESI-MS: m/z 445 [M + H]+; C23H20N6O2S; Calculated, %: C, 62.15; H, 4.54; N, 18.91; Found, %: C, 62.12; H, 4.56; N, 18.94.
(Z)-3-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIId). Light yellow solid; Yield: 80%; mp: 203–205°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.75 (s, 3H, N–CH3), 4.89 (s, 2H, N–CH2), 7.15 (d, J = 7.2 Hz, 2H, Ar–H), 7.36 (d, J = 8.2 Hz, 1H, Ar–H), 7.54–7.60 (m, 3H, Ar–H), 7.73 (s, 1H, alkene-H), 7.97 (s, 1H, Ar–H), 8.03 (s, 1H, Ar–H), 8.56 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.9, 46.9, 106.9, 114.8, 116.5, 120.7, 123.9, 124.5, 126.3, 127.7, 130.6, 132.8, 134.5, 141.6, 143.7, 144.8, 160.3, 164.9, 170.3; ESI-MS: m/z 435 [M + H]+; C21H15FN6O2S; Calculated, %: C, 58.06; H, 3.48; N, 19.34; Found, %: C, 58.08; H, 3.49; N, 19.31.
(Z)-3-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIe). Light yellow solid; Yield: 78%; mp 206–208°C; 1H NMR spectrum (300 MHz, DMSO-d6), ppm: 3.73 (s, 3H, N–CH3), 4.87 (s, 2H, N–CH2), 7.28 (d, J = 7.6 Hz, 2H, Ar–H), 7.36 (d, J = 8.2 Hz, 1H, Ar–H), 7.59 (d, J = 8.2 Hz, 1H, Ar–H), 7.67–7.73 (m, 3H, alkene-H, Ar–H), 7.95 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H), 8.53 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.6, 46.7, 106.9, 114.6, 120.6, 123.3, 124.2, 126.2, 127.6, 129.7, 130.5, 132.8, 134.4, 136.4, 141.4, 143.6, 144.7, 164.7, 170.2; ESI-MS: m/z 451 [M + H]+; C21H15ClN6O2S; Calculated, %: C, 55.94; H, 3.35; N, 18.64; Found, %: C, 55.97; H, 3.36; N, 18.62.
(Z)-3-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIf). Light orange solid; Yield: 78%; mp: 224–226°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.72 (s, 3H, N–CH3), 4.84 (s, 2H, N–CH2), 7.35–7.43 (m, 3H, Ar–H), 7.60 (d, J = 8.2 Hz, 1H, Ar–H), 7.70 (s, 1H, alkene-H), 7.81 (d, 2H, J = 8.0 Hz, Ar–H), 7.96 (s, 1H, Ar–H), 8.03 (s, 1H, Ar–H), 8.50 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.5, 46.8, 106.7, 114.7, 120.5, 121.1, 121.9, 123.9, 126.1, 127.7, 130.6, 132.1, 132.9, 135.9, 141.5, 143.7, 144.8, 164.6, 169.9; ESI-MS: m/z 495 [M + H]+; C21H15BrN6O2S; Calculated, %: C, 50.92; H, 3.05; N, 16.97; Found, %: C, 50.89; H, 3.07; N, 16.94.
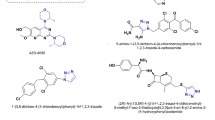
(Z)-3-((1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIg). Colorless solid; Yield: 69%; mp: 210–212°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.73 (s, 3H, N–CH3), 3.90 (s, 3H, Ar–OCH3), 4.82 (s, 2H, N–CH2), 6.97 (d, J = 7.4 Hz, 2H, Ar–H), 7.35 (d, J = 8.2 Hz, 1H, Ar–H), 7.56–7.63 (m, 3H, Ar–H), 7.71 (s, 1H, alkene-H), 7.95 (s, 1H, Ar–H), 8.04 (s, 1H, Ar–H), 8.43 (s, 1H, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.6, 46.6, 56.9, 106.8, 113.9, 114.6, 120.5, 123.7, 124.4, 126.3, 127.5, 130.5, 131.7, 132.8, 141.4, 143.7, 144.7, 158.3, 164.7, 170.1; ESI-MS: m/z 469 [M + Na]+; C22H18N6O3S; Calculated, %: C, 59.18; H, 4.06; N, 18.82; Found, %: C, 59.16; H, 4.09; N, 18.85.
(Z)-3-((1-(3,5-Dimethoxyphenyl)-1H-1,2,3-triazol4-yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIh). Colorless solid; Yield: 65%; mp: 215–217°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.74 (s, 3H, N–CH3), 3.87 (s, 6H, Ar–OCH3), 4.84 (s, 2H, N–CH2), 6.77 (s, 1H, Ar–H), 7.20 (s, 2H), 7.36 (d, J = 8.2 Hz, 1H, Ar–H), 7.59 (d, J = 8.2 Hz, 1H, Ar–H), 7.70 (s, 1H, alkene-H), 7.96 (s, 1H, Ar–H), 8.03 (s, 1H, Ar–H), 8.49 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.7, 46.7, 56.5, 97.7, 99.1, 106.8, 114.7, 120.6, 124.1, 126.1, 127.5, 130.5, 132.7, 140.6, 141.5, 143.7, 144.6, 160.1, 164.6, 170.2; ESI-MS: m/z 477 [M + H]+; C23H20N6O4S; Calculated, %: C, 57.97; H, 4.23; N, 17.64; Found, %: C, 57.95; H, 4.26; N, 17.68.
(Z)-3-((1-(4-Chloro-3,5-dimethoxyphenyl)-1H1,2,3-triazol-4-yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIi). Colorless solid; Yield: 71%; mp: 218–220°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.73 (s, 3H, N–CH3), 3.88 (s, 6H, Ar–OCH3), 4.83 (s, 2H, N–CH2), 6.79 (s, 2H, Ar–H), 7.34 (d, J = 8.2 Hz, 1H, Ar–H), 7.58 (d, J = 8.2 Hz, 1H, Ar–H), 7.71 (s, 1H, alkene-H), 7.97 (s, 1H, Ar–H), 8.04 (s, 1H, Ar–H), 8.51 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.6, 46.6, 56.8, 104.7, 106.7, 114.6, 118.7, 120.7, 124.3, 126.2, 127.4, 130.3, 132.7, 138.8, 141.5, 143.6, 144.7, 152.4, 164.8, 170.3; ESI-MS: m/z 511 [M + H]+; C23H19ClN6O4S; Calculated, %: C, 54.07; H, 3.75; N, 16.45; Found, %: C, 54.05; H, 3.76; N, 16.48.
(Z)-3-((1-(3,5-Dichlorophenyl)-1H-1,2,3-triazol-4yl)methyl)-5-((1-methyl-1H-benzo[d]imidazol-5-yl)methylene)thiazolidine-2,4-dione (VIIj). Light blue solid; Yield: 74%; mp: 211–213°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.72 (s, 3H, N–CH3), 4.85 (s, 2H, N–CH2), 7.35 (d, J = 8.2 Hz, 1H, Ar–H), 7.48 (s, 1H), 7.60 (d, J = 8.2 Hz, 1H, Ar–H), 7.72 (s, 1H, alkene-H), 7.77 (s, 2H), 7.96 (s, 1H, Ar–H), 8.04 (s, 1H, Ar–H), 8.54 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.6, 46.9, 106.9, 114.8, 118.9, 120.7, 124.4, 125.1, 126.2, 127.6, 130.6, 132.8, 136.2, 139.4, 141.6, 143.5, 144.8, 164.9, 170.4; ESI-MS: m/z 486 [M + H]+; C21H14Cl2N6O2S; Calculated, %: C, 51.97; H, 2.91; N, 17.32; Found, %: C, 51.99; H, 2.94; N, 17.36.
(Z)-4-(4-((5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)-2,4-dioxothiazolidin-3-yl)methyl)-1H1,2,3-triazol-1-yl)benzonitrile (VIIk). Light orange solid; Yield: 78%; mp: 208–210°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.74 (s, 3H, N–CH3), 4.86 (s, 2H, N–CH2), 7.34 (d, J = 8.2 Hz, 1H, Ar–H), 7.48 (d, J = 7.8 Hz, 2H), 7.59 (d, J = 8.2 Hz, 1H, Ar–H), 7.71 (s, 1H, alkene-H), 7.85 (d, J = 7.8 Hz, 2H), 7.95 (s, 1H, Ar–H), 8.02 (s, 1H, Ar–H), 8.55 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 37.8, 46.9, 107.1, 114.7, 117.4, 119.8, 120.6, 124.6, 125.2, 125.8, 126.3, 127.7, 130.4, 132.9, 139.9, 141.4, 143.6, 144.7, 164.7, 170.2; ESI-MS: m/z 442 [M + H]+; C22H15N7O2S; Calculated, %: C, 59.86; H, 3.42; N, 22.21; Found, %: C, 59.88; H, 3.40; N, 22.20.
(Z)-5-((1-Methyl-1H-benzo[d]imidazol-5-yl)methylene)-3-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4yl)methyl)thiazolidine-2,4-dione (VIIl). Light orange solid; Yield: 82%; mp: 213–215°C; 1H NMR (300 MHz, DMSO-d6), ppm: 3.74 (s, 3H, N–CH3), 4.89 (s, 2H, N–CH2), 7.36 (d, J = 8.2 Hz, 1H, Ar–H), 7.61 (d, J = 8.2 Hz, 1H, Ar–H), 7.73 (s, 1H, alkene-H), 7.86 (d, J = 7.1 Hz, 2H, Ar–H), 7.97 (s, 1H, Ar–H), 8.04 (s, 1H, Ar–H), 8.21 (d, J = 7.2 Hz, 2H, Ar–H), 8.63 (s, triazole ring-H); 13C NMR (75 MHz, DMSO-d6), ppm: 38.1, 46.9, 107.2, 114.8, 120.7, 123.4, 124.8, 126.3, 126.8, 127.7, 130.8, 132.9, 140.8, 141.6, 143.7, 144.8, 148.9, 165.1, 170.5; ESI-MS: m/z 484 [M + Na]+; C21H15N7O4S; Calculated, %: C, 54.66; H, 3.28; N, 21.25; Found, %: C, 54.68; H, 3.30; N, 21.23.
CONCLUSIONS
In summary, we reported the synthesis of some new benzimidazole-thiazolidine-2,4-dione-1,2,3-triazole conjugates (VIIa–VIIl). It involved some well-known reactions like Knoevenagel condensation and Cu(I) catalyzed azide-alkyne cycloaddition. All the synthesized compounds were tested for anticancer activity against three human cancer cell lines such as A549, MCF-7 and HeLa cell lines using MTT assay the compounds namely (VIIg), (VIIh), and (VIIk) were exhibited better results than standard nocodazole. Further on testing towards tubulin polymerization inhibition the compounds (VIIg), (VIIh) were shown greater inhibitory potency with IC50 equal to 0.62 and 0.31 μM, respectively than standard Combretastatin A-4 (CA-4). Finally, in silico molecular docking studies for the compounds (VIIg), (VIIh), and (VIIk) with α,β-tubulin shown that they have good binding interactions with the target protein.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Ayati, A., Emami, S., Asadipour, A., Shafiee, A., and Foroumadi, A., Eur. J. Med. Chem., 2015, vol. 97, pp. 699–718. https://doi.org/10.1016/j.ejmech.2015.04.015
Jordan, M.A. and Wilson, L., Nat. Rev. Cancer, 2004, vol. 4, pp. 253–265. https://doi.org/10.1038/nrc1317
Downing, K.H., Annu. Rev. Cell. Dev. Biol., 2000, vol. 16, pp. 89–111. https://doi.org/10.1146/annurev.cellbio.16.1.89
Mohamed, H.S., Amin, N.H., El-Saadi, M.T., and Abdel-Rahman, H.M., Bioorg. Chem., 2022, vol. 16, Article ID: 105687. https://doi.org/10.1016/j.bioorg.2022.105643
Sakchaisri, K., Kim, S.O., and Hwang, J., PLoS One, 2017, vol. 12, Article ID: 0173311.
Chen, H., Lin, Z., Arnst, K.E., Miller, D.D., and Li, W., Molecules, 2017, vol. 22, Article ID: 1281. https://doi.org/10.3390/molecules22081281
Wang, G., Li, C., He, L., Lei, K., Wang, F., Pu, Y., Yang, Z., Cao, D., Ma, L., Chen, J., Sang, Y., Liang, X., Xiang, M., Peng, A., Wei, Y., and Chen, L., Bioorg. Med. Chem., 2014, vol. 22, pp. 2060–2079. https://doi.org/10.1016/j.bmc.2014.02.028
Messaoudi, S., Treguier, B., Hamze, A., Provot, O., Peyrat, J.F., De Losada, J.R., Liu, J.M., Bignon, J., Bakala, J.W., Thoret, S., Dubois, J. Brion, J.D., and Alami, M., J. Med. Chem., 2009, vol. 52, pp. 4538–4542. https://doi.org/10.1021/jm900321u
Covarrubias, C.H., Vilchis-Reyes, M.A., Yeepez-Mulia, L., Sanchez-Diaz, R., Navarrete, V., Hernaandez, C.A., Castillo, R., and Hernaandez, L.F., Eur. J. Med. Chem., 2012, vol. 52, pp. 193–204. https://doi.org/10.1016/j.ejmech.2012.03.014
Lavrador-Erb, K., Ravula, S.B., Yu, J., ZamaniKord, S., Moree, W.J., Petroski, R.E., Wen, V., Malany, S., Hoare, S.R.J., Madan, A., Crowe, P.D., and Beaton, G., Bioorg. Med. Chem. Lett., 2010, vol. 20, pp. 2916–2919. https://doi.org/10.1016/j.bmcl.2010.03.027
Tahlan, S., Narasimhan, B., Lim, S.M., Ramasamy, K., Mani, V., and Shah, S.A.A., Mini-Rev. Med. Chem., 2019, vol. 19, pp. 1080–1092.
Shrivastava, N., Naim, M.J., Alam, M.J., Nawaz, F., Ahmed, S., and Alam, O., Arch. Pharm., 2017, vol. 350, Article ID: 201700040. https://doi.org/10.1002/ardp.201700040
Keri, R.S., Hiremathad, A., Budagumpi, S., and Nagaraj, B.M., Chem. Biol. Drug Des., 2015, vol. 86, pp. 19−65. https://doi.org/10.1111/cbdd.12462
Yadav, G. and Ganguly, S., Eur. J. Med. Chem., 2015, vol. 97, pp. 419−443. https://doi.org/10.1016/j.ejmech.2014.11.053
Gaba, M. and Mohan, C., Med. Chem. Res., 2016, vol. 25, pp. 173−210. https://doi.org/10.1007/s00044-015-1495-5
Gaba, M., Singh, S., and Mohan, C., Eur. J. Med. Chem., 2014, vol. 76, pp. 494−505. https://doi.org/10.1016/j.ejmech.2014.01.030
DeSimone, R., Currie, K., Mitchell, S., Darrow, J., and Pippin, D., 2004, vol. 7, pp. 473−493. https://doi.org/10.2174/1386207043328544
Tageja, N. and Nagi, J., Cancer Chemother. Pharmacol., 2010, vol. 66, pp. 413−423. https://doi.org/10.1007/s00280-010-1317-x
Cheson, B.D. and Rummel, M.J., J. Clin. Oncol., 2009, vol. 27, pp. 1492−1501. https://doi.org/10.1200/jco.2008.18.7252
Cheson, B.D., Brugger, W., Damaj, G., Dreyling, M., Kahl, B., Kimby, E., Ogura, M., Weidmann, E., Wendtner, C.M., and Zinzani, P.L., Leuk. Lymphoma, 2016, vol. 57, pp. 766–782. https://doi.org/10.3109/10428194.2015.1099647
Njar, V.C.O. and Brodie, A.M.H., J. Med. Chem., 2015, vol. 58, pp. 2077–2087. https://doi.org/10.1021/jm501239f
Welsch, M.E., Snyder, S.A., and Stockwell, B.R., Curr. Opin. Chem. Biol., 2010, vol. 14, pp. 347–361. https://doi.org/10.1016/j.cbpa.2010.02.018
Zhang, Q., Zhou, H., Zhai, S., and Yan, B., Curr. Pharm. Des., 2010, vol. 16, pp. 1826–1842. https://doi.org/10.2174/138161210791208983
Xie, J., Li, Q., Ding, X., and Gao, Y., Onco Target, 2017, vol. 8, pp. 50814–50823. https://doi.org/10.18632/oncotarget.15135
Liu, K., Rao, W., Parikh, H., Li, Q., Guo, T.L., Grant, S., Kellogg, G.E., and Zhang, S., Eur. J. Med. Chem., 2012, vol. 47, pp. 125–137. https://doi.org/10.1016/j.ejmech.2011.10.031
Keeton, E.K., McEachern, K., Dillman, K.S., Palakurthi, S., Cao, Y., Grondine, M.R., Kaur, S., Wang, S., Chen, Y., Wu, A., Shen, M., Gibbons, F.D., Lamb, M.L., Zheng, X., Stone, R.M., Deangelo, D.J., Platanias, L.C., Dakin, L.A., Chen, H., Lyne, P.D., and Huszar, D., Blood, 2014, vol. 123, pp. 905–913. https://doi.org/10.1016/j.ejmech.2011.10.031
Lin, Y.W., Beharry, Z.M., Hill, E.G., Song, J.H., Wang, W., Xia, Z., Zhang, Z., Aplan, P.D., Aster, J.C., Smith, C.D., and Kraft, A.S., Blood, 2010, vol. 115, pp. 824–833. https://doi.org/10.1182/blood-2009-07-233445
Asati, V., Mahapatra, D.K., and Bharti, S.K., Eur. J. Med. Chem., 2014, vol. 87, pp. 814–833. https://doi.org/10.1016/j.ejmech.2014.10.025
Gangadhar, K.H. and Benarjee, V.A., ChemistrySelect, 2022, vol. 7, Article ID: e202200270. https://doi.org/10.1002/slct.202200270
Venu, K., Saritha, B., and Sailaja, B.B.V., Tetrahedron, 2022, vol. 124, Article ID: 132991. https://doi.org/10.1016/j.tet.2022.132991
Aziz, N.A.A.M., George, R.F., Adl, K.E., and Mahmoud, W.R., Arch. Pharm., 2022, Article ID: e2200465. https://doi.org/10.1002/ardp.202200465
Ashwini, N., Satheesh Kumar, N., Narasimha Swamy, T., and Ravinder, M., Russ. J. Bioorg. Chem., 2023, vol. 49, pp. 976–987. https://doi.org/10.1134/S1068162023050047
Mallikarjuna, B., Satheesh Kumar, N., Praveen Kumar, K., Narsimha S., Ravinder, M., and Narasimha Swamy, T., ChemistrySelect, 2023, vol. 8, Article ID: e202204414. https://doi.org/10.1002/slct.202204414
Reddy, T.S., Kulhari, H., Reddy, V.G., Subba Rao, A.V., Bansal, V., Kamal, A., and Shukla, R., Org. Biomol. Chem., 2015, vol. 13, pp. 10136–10149. https://doi.org/10.1039/C5OB00842E
Stefely, J.A., Palchaudhuri, R., Miller, P.A., Peterson, R.J., Moraski, G.C., Hergenrother, P.J., and Miller, M.J., J. Med. Chem., 2010, vol. 53, pp. 3389–3395. https://doi.org/10.1021/jm1000979
Bonandi, E., Christodoulou, M.S., Fumagalli, G., Perdicchia, D., Rastelli, G., and Passarella, D., Drug Discov. Today, 2017, vol. 17, pp. 1572–1581. https://doi.org/10.1016/j.drudis.2017.05.014
Mahboob Alam, Md., Arch Pharm., 2022, vol. 355, Article ID: 2100158. https://doi.org/10.1002/ardp.202100158
Xu, Z., Zhao, S.J., and Liu, Y., Eur. J. Med. Chem., 2019, vol. 183, Article ID: 111700. https://doi.org/10.1016/j.ejmech.2019.111700
Manoj Kumar, N., Satheesh Kumar, N., Narasimha Swamy, T., Ravinder, M., Murali Krishna, T., and Narsimha, S., J. Mol. Struct., 2022, vol. 1250, Article ID: 131722. https://doi.org/10.1016/j.molstruc.2021.131722
Huang, L., Liu, M., Man, S., Ma, D., Feng, D.Z., Guan, Q., Zuo, D., Wu, Y., Zhang, W., and Bao, K., Eur. J. Med. Chem., 2020, vol. 186, Article ID: 111846. https://doi.org/10.1016/j.ejmech.2019.111846
Mohammed, H.H.H., Ebeid, K., Abourehab, Md.A.S., Wafa, E.I., Alhaj-Suliman, S.O., Salem, A.K., Ghosh, P., Rahma, G.E.D.A.A., Hayallah, A.M., and Abbas, S.H., J. Enzyme Inhib. Med. Chem., 2022, vol. 37, pp. 1346–1363.
Odlo, K., Hentzen, J., Chabert, J.F.D., Ducki, S., Gani, O.A.B.S.M., and Sylte, I., Bioorg. Med. Chem., 2008, vol. 16, pp. 4829–4838. https://doi.org/10.1080/14756366.2022.2072308
Solum, E.J., Vik, A., and Hansen, T.V., Steroids, 2014, vol. 87, pp. 46–53. https://doi.org/10.1016/j.steroids.2014.05.020
Qi, Z.Y., Hao, S.Y., Bian, H.L., Hui, L., and Chen, S.W., Bioorg. Chem., 2020, vol. 94, Article ID: 103392. https://doi.org/10.1016/j.bioorg.2019.103392
Fu, D.J., Li, P., Wu, B.W., Zhao, Ch.B., and Zang, S.Y., Eur. J. Med. Chem., 2019, vol. 165, pp. 309–322. https://doi.org/10.1016/j.ejmech.2019.01.033
Goud, N.S., Kumar, P., and Bharath, R.D., Heterocycles, 2020, pp. 1–202. https://doi.org/10.5772/intechopen.90758
Kamal, A., Srinivasa Reddy, T., Vishnuvardhan, M.V.P.S., Nimbarte, V.D., Subba Rao, A.V., Srinivasulu, V., and Shankaraiah, N., Bioorg. Med. Chem., 2015, vol. 23, pp. 4608–4623. https://doi.org/10.1016/j.bmc.2015.05.060
Kamal, A., Kashi Reddy, M., Shaik, T.B., Rajender Srikanth, Y.V.V., Santhosh Reddy, V., Bharath Kumar, G., and Kalivendi, S.V., Eur. J. Med. Chem., 2012, vol. 50, pp. 9–17. https://doi.org/10.1016/j.ejmech.2012.01.004
Perin, N., Hok, L., Bec, A., Persoons, L., Vanstreels, E.D., Vianello, R., and Hranjec, M., Eur. J. Med. Chem., 2021, vol. 211, Article ID: 113003. https://doi.org/10.1016/j.ejmech.2020.113003
Ravikumar Reddy, S., Satheesh Kumar, N., Rajkumar, N., Narsimha, S., Muqeed, Md., Ravinder, M., and Narasimha Swamy, T., J. Mol. Struct., 2022, vol. 1268, Article ID: 133692. https://doi.org/10.1016/j.molstruc.2022.133692
Elzahhar, P.A., Alaaeddine, R., Ibrahim, T.M., Nassra, R., Ismail, A., Chua, B.S.K., Frkic, R.L., Bruning, J.B., Wallner, N., Knape, T., Von Knethen, A., Labib, H., El-Yazbi, A.F., and Belal, A.S.F., Eur. J. Med. Chem., 2019, vol. 167, pp. 562–582. https://doi.org/10.1016/j.ejmech.2019.02.034
Hamel, E., Cell Biochem. Biophys., 2003, vol. 38, pp. 1–21. https://doi.org/10.1385/CBB:38:1:1
Ravelli, R., Gigant, B., and Curmi, P., Nature, 2004, vol. 428, pp. 198–202. https://doi.org/10.1038/nature02393
Author information
Authors and Affiliations
Contributions
All authors made an equal contribution to the writing of this article.
Corresponding author
Ethics declarations
This article does not contain any studies involving patients or animals as test objects.
Informed consent was not required for this article. No conflicts of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Karthik, B., Ramakrishna, B., Kumar, B.A. et al. Design and Synthesis of Some New Benzimidazole-1,2,3-triazole-thiazolidine-2,4-dione Conjugates as Tubulin Polymerization Inhibitors. Russ J Bioorg Chem 50, 1434–1445 (2024). https://doi.org/10.1134/S1068162024040307
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024040307