Abstract
The main components of the birch wood were fractionated into a microcrystalline cellulose, xylose and enterosorbents by an integration of heterogeneous catalytic processes of an acidic hydrolysis and a peroxide delignification of the wood biomass for the first time. The wood hemicelluloses were hydrolyzed to xylose at a temperature of 150°C in the presence of the Amberlyst® 15 solid acidic catalyst. Then, the lignocellulosic product of the wood hydrolysis was subjected to the peroxide delignification in the formic acid–water medium in the presence of the solid TiO2 catalyst with a formation of the microcrystalline cellulose (MCC) and the organic-soluble lignin. Yields of MCC and the organic-soluble lignin proved to be 64.5 and 11.5 wt % of a mass of the prehydrolyzed wood, respectively, under the determined optimal conditions (100°C, 7.2 wt % of Н2О2, 37.8 wt % of НСООН, LWR 15, and a duration of 4 h). The enterosorbents were prepared by a treatment of the organic-soluble lignin with 0.4% NaHCO3 or hot water. The sorption capacity of these enterosorbents was 97.7 and 236.7 mg/g according to methylene blue and gelatin, respectively. These values were significantly higher than those of the Polifepan commercial enterosorbent (44 and 115 mg/g, respectively). The products of the catalytic fractionation of the birch wood were characterized by physicochemical (FTIR, XRD, SEM, and GC) and chemical methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Interest in an application of renewable plant materials being alternative to oil and gas has quickened in the past few years [1]. Wood is a vast resource of the plant biomass. At present, the main direction of the chemical processing of wood is the cellulose preparation [2].
The present industrial technologies for cellulose production from wood are based on the use of sulfur-containing and chlorine-containing delignifying agents and result in the environmental pollution [2]. In addition, these technologies provide no utilization of other main components of wood, such as hemicelluloses and lignin.
The elaborated perspective methods for a preparation of cellulose from wood are based on the use of organic solvents and low-toxic delignifying agents. In particular, processes of organic-solvent delignification in a medium of acetic or formic acid are developed at a level of half-industrial installations [3, 4]. An application of catalysts increases an efficacy of the processes of the organic-solvent delignification and allows the use of water-organic solvents and mild conditions of a process. For example, the peroxide fractionation of the fir wood into cellulose and an organic-soluble lignin was proposed to perform in the mixture of formic acid and water in the presence of the TiO2 catalyst at a temperature of 100°С and atmospheric pressure [5]. The cellulose that contains approximately 2 wt % of the residual lignin and the low-molecular-weight organic-soluble lignin are prepared with high yields under the optimal conditions of the process. Distinct from the traditional technical lignins, the organic-soluble lignins contain no sulfur, have relatively low molecular mass, and a sufficiently low mass molecular distribution [6], resulting in their easier further conversion into valuable products.
At present, the creation of processes for complex processing of all basic components of the lignocellulose materials into the needed chemical products is an urgent problem. For this purpose, processes of a catalytic reducing and oxidizing fractionation of the wood biomass are used.
The reducing fractionation of the biomass is carried out at an increased pressure in the medium of hydrogen or a reducing agent (formic acid or alcohols) in the presence of applied metallic catalysts (Ru/C, Pd/C, Pt/C, Raney nickel, Ni/C, and Ni/Al2O3). The main products of such processes are the liquid hydrocarbons that contained phenolic monomers, dimers, and oligomers [7, 8].
The processes of the oxidative fractionation by a treatment with hydrogen peroxide or oxygen are performed in the aqueous-organic or aqueous media in the presence of such catalysts as mineral acids (H2SO4, HCl, or H3PO4) and inorganic salts and oxides (MnSO4, FeSO4, CoCl2, TiO2, ZnO, and others) [9–11].
The composition of products of the catalytic fractionation of wood is determined not only by the conditions of the process (nature of the catalyst, reaction medium, temperature, duration, etc.) but by the nature of the wood row material as well. Separate wood species can differ in the content of basic components and structure of lignin and hemicelluloses.
Previously, we designed the method for the extraction-catalytic fractionation of the larch-wood biomass with a production of dihydroquercetin, arabinogalactan, MCC, and organic-soluble lignin [12].
In this study, we used the birch wood as a starting material. Birch belongs to one of the basic species of trees which grow in the Russian Federation. The birch wood involves 25–30 wt % of hemicelluloses (mainly glucuronoxylans) [13].
We proposed a new approach to the heterogeneous catalytic fractionation of the birch-wood biomass into MCC, xylose, and enterosorbents. This approach was based on an integration of the stages of the hemicellulose hydrolysis in the presence of the Amberlyst 15 acidic catalyst and the peroxide delignification of the lignocellulose product of the hydrolysis in the medium of formic acid, water, and the TiO2 catalyst. The products of the wood fractionation were characterized by physicochemical and chemical methods.
EXPERIMENTAL
Sawdust of a middle-stem part of the birch wood was used as a feedstock. The Betula pendula birch was grown in surroundings of Krasnoyarsk (the fraction of 2–5 mm). The following chemical composition of the birch wood was determined by standard methods of the wood chemistry (wt %): 46.8% of cellulose, 21.75 of lignin, 27.3% of hemicellulose, 3.2% of extractive substances, and 0.6% of ash [14].
The air-dried birch wood (60 g) was subjected to a mechanical activation in an AGO-2 planetary-type mill for 30 min at a centrifugal acceleration before the hydrolysis. The centrifugal acceleration was made by milling bodies. The planetary-type mill was equipped with two drums that were simultaneously rotated around a central axis and around their own axes in the opposite direction. The wooden sawdust (5 g, the fraction of 2–5 mm) and the milling bodies (18 steel bolls with a diameter of 3–8 mm) were placed in the drums (a volume of each drum was 50 mL).
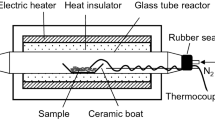
The activated birch wood was hydrolized at temperatures of 110, 130, 150, and 170°C in a rotating steel autoclave that was equipped with an interior fluoroplastic tube (35 mL). The autoclave was placed in a metallic air bath. The rotation speed of the autoclave was 11 rpm. The mixture of the wood and the solid catalyst was placed in the tube and flooded with the distilled water. The mass content of the wood in the water was 41 g/L. The ratio of the mass of the solid catalyst to the wood mass was 1 : 1.
On completion of the preset time, the reactor was cooled, and the nonhydrolyzed wood and the catalyst were filtered off in a Buchner funnel on a paper filter under vacuum, washed with water, and dried in a drying box at 103°C. After the hydrolysis, the mass of the solid catalyst was subtracted from the mass of the solid residue of the lignocellulosic product.
The Amberlyst® 15 (Acrosorganics) and KU-2-8 (Thermax Ltd.) commercial catalysts were used for the hydrolysis. The Amberlyst® 15 catalyst was a macroporous ion-exchange resin (the pore size was 0.45–0.60 mm) based on a copolymer of styrene and divinylbenzene. KU-2-8 was an ion-exchange resin based on a copolymer of styrene and divinylbenzene (the pore size was 0.315–1.25 mm), a water-insoluble matrix of a highly molecular and multifunctional polymer with acidic sulfogroups capable of an ion-exchange.
The choice of these ion-exchange resins was explained by their wide application to hydrolysis of different disaccharides and such polysaccharides as cellulose and hemicellulose [15, 16].
The catalytic peroxide delignification of the lignocellulosic product of the wood hydrolysis was performed in a glass reactor (250 cm3) that was equipped with a mechanical stirrer, a reflux condenser, and a thermometer according to the procedure [17]. Wood sawdust (10 g) was placed in the glass reactor, and the mixture of formic acid (especially pure, Ekos-1, Russia), hydrogen peroxide (especially pure, Ekos-1, Russia), distilled water, and the catalyst were added. The reaction mixture was intensively stirred (700 rpm) for 4 h at 100°C. The contents of hydrogen peroxide and formic acid in the reaction mixture were varied from 4 to 10 wt % of and from 30 to 50 wt %, respectively, at a constant hydromodulus of 15. On completion of the reaction, the solid product was separated from the reaction solution by vacuum filtration in a Buchner funnel, washed with distilled water, and dried at 105°C to constant weight. The boiled-off liquor was evaporated (the formic acid was removed). A fivefold excess of water was added to the vat residue, and lignin was precipitated.
The commercial TiO2 (DuPont, United States) was used as a catalyst for the peroxide delignification. The quantity of the catalyst was 1% of the wood mass in all the experiments. The TiO2 catalyst consisted of rutile (92%) and anatase (8%) with an average particle size of approximately 10 μm and surface area of 3 m2/g (according to the BET method).
The enterosorbents were prepared by treatment of the organic-soluble lignin with 0.4% NaHCO3 for 15 min and with hot water (80°C) for 15 min. The enterosorbents were purified by dialysis in MF-505-46 MFPI cellophane dialysis bags (United States, pore size 0.1 μm) for 10 h at a one-hour interval of the water replacement. The adsorption capacity of the sorbents was determined with the use of methylene blue and gelatin [18].
IR spectra in the area of 4000–400 cm–1 were recorded on a Tensor 27 IR Fourier spectrometer (Bruker). The spectra were processed using the OPUS software (version 5.0). The samples for the absorption IR spectra were prepared as pressed tablets that contained 5 mg of the sample in the KBr matrix.
The X-ray picture of a cellulose sample was obtained with the use of a PANalyticalX’PertPro diffractometer with the CuKα irradiation (λ = 0.154 nm) at the 2θ angle interval from 10 to 50° with an increment of 0.01° in a cuvette 2.5 cm in diameter.
Morphology of the cellulose samples was characterized by scanning electron microscopy (SEM) on a SEM TM-1000HITACHI electron microscope (Japan) at a magnification of 10 000 times with a resolution of 35 nm.
The individual composition and content of monosaccharides in the hydrolysates were examined by a chromatography on a VARIAN-450 GC gas chromatograph with a flame-ionization detector on a VF- 624ms capillary column (a length of 30 m and a diameter of 0.32 mm). The chromatographic conditions were: helium gas carrier, injector temperature of 250°C, starting temperature of the column of 50°C (5 min), temperature rise to 180°C at 10°С/min; the time lag at 180°С was 37 min; the detector temperature was 280°C; and the duration of the chromatographic fractionation of monosaccharides was 55 min. The oligosaccharides were registered after 55 min of the chromatographic fractionation at a temperature increase to 250°С (10°C/min) and maintaining the column at this temperature for 30 min. The total time of the analysis was 92 min.
The hydrolysate sample was preliminarily subjected to a derivatization according to the procedure [19] with a formation of trimethylsilyl derivatives. A mixture of trimethylchlorosilane and hexamethyldisilazane in pyridine was used as silylating agent. Sorbitol was used as an internal standard. The obtained chromatogram involved the peaks of all monosaccharide tautomers in the hydrolysate sample. The peaks were identified on the basis of the known retention times tR of the tautomeric forms of monosaccharides for these chromatographic conditions. The ratio of the area of each characteristic monosaccharide peak to the area of the internal standard (Si/Sст) was calculated. The mass of every monosaccharide in the hydrolysate sample was found from these ratios using the calibration curve.
RESULTS AND DISCUSSION
Hydrolysis of Hemicelluloses of the Birch Wood Using the Solid Acidic Catalysts
The birch wood contained the maximum amount of hemicelluloses (up to 30 wt %) among the all-Russian wood species. The hemicelluloses of the birch wood mainly contained glucuronoxylans, which involve xylan (80%), glucan (10%), and uronic anhydride (6%) [20].
The conditions of the wood hydrolysis in which other wood components are minimally converted are necessary to find for a selective depolymerization of the hemicelluloses. A choice of catalysts is one of the basic directions of an improvement of the polysaccharide hydrolysis [21–23]. The use of the solid acidic catalysts has the following advantages: ecological safety and the absence of the corrosive activity and additional expenses on a neutralization of reaction solutions.
Activities of two solid acidic catalysts (Amberlyst®15 and KU-2-8) were compared at 110–170°C in order to choose the most effective catalyst for hydrolysis of the hemicelluloses of the birch wood.
It is known that an effective contact of a solid catalyst with a solid reagent is difficult to provide during the heterogeneous catalytic hydrolysis of plant raw materials [24]. The wood sawdust was subjected to grinding and the mechanical activation in an AGO-2 mill for an increase in the contact area. As a result of a fine mechanical grinding, the birch wood became more finely dispersed and more homogeneous. The average size of the wood particles was found to be decreased from 2–5 to 0.1–0.25 mm according to the scanning electron microscopy (SEM).
Hemicellulose depolymerization occurs in the course of the wood hydrolysis in mild conditions. The influence of the temperature of the birch-wood hydrolysis on a content of xylose (Fig. 1a) and glucose (Fig. 1b) in the hydrolysates was studied. The solid acidic catalysts had low activity at 110–130°C, and exhibited their maximum activity at different temperatures. Amberlyst®15 was maximally active at 150°C, whereas KU-2-8 was mostly effective at 170°C. The maximum xylose content (7.2 g/L) was achieved in the presence of the Amberlyst®15 catalyst at 150°C.
Such xylose content is comparable with that obtained during the birch-wood hydrolysis by 2% H2SO4 at 100°C and at atmospheric pressure for 5 h [25].
An increase of the process temperature to 170°C is accompanied by hydrolysis of the cellulose amorphous part, resulting in an enhancement in the glucose content to 1.5 g/L. Thus, the further increase in the hydrolysis temperature is not reasonable, because the yield of the target cellulose is decreased during the further delignification of the birch-wood lignocellulose.
The birch wood is barely hydrolyzed under the chosen conditions in the absence of a catalyst. A repetitive use of the Amberlyst®15 catalyst results in a decrease in its activity, whereas the activity of the KU-2-8 catalyst remains unchanged.
The Peroxide Delignification of the Lignocellulosic Product of the Birch-Wood Hydrolysis
The lignocellulosic product was prepared by a prehydrolysis of the birch wood in the presence of the Amberlyst®15 catalyst at 150°С for 1 h. The product had the following chemical composition: 61.2 wt % of cellulose, 27.3 wt % of lignin, and 9.5 wt % of the residual hemicellulose.
We performed a mathematical optimization of the peroxide delignification of the hemicellulose-free birch wood in order to find the conditions which would provide maximum yields of the qualitative cellulose and the organic-soluble lignin.
Previously, we published the results of an investigation of the delignification of different wood species in the medium of acetic and formic acids [5, 12, 26]. As followed from these investigations, lignin was almost completely removed from the wood after treatment at 100°С for 4 h at a hydromodulus of 15 and TiO2 content of 1 wt %. Therefore, these conditions were chosen for the peroxide delignification of the prehydrolyzed birch wood.
The influence of concentrations of hydrogen peroxide and formic acid on the yields of the cellulose product and the organic-soluble lignin was studied for an optimization of the peroxide catalytic delignification of the pre-hydrolyzed birch wood.
The generalized parameter of the optimization (Wa) was used for the optimization of the process. This paramenter involved the following output parameters: Y1, the yield of the cellulose product (wt %); Y2, the content of cellulose in the cellulose product (wt %); and Y3, the yield of the organic-soluble lignin. The weights of these parameters (δ) were distributed according to their comparative importance for an evaluation of the results of the process in general (Table 1). The values of the output parameters (see Table 1) were presented as the average value of three experiments.
The following variable factors were used: the Х1 concentration of НСООН (30–50 wt %) in the reaction mixture and the X2 concentration of Н2О2 in the reaction mixture (4–10 wt % ). The fixed parameters were temperature (100°С), the duration of the process (4 h), and the hydromodulus of the process (15).
The optimization was performed with the use of the complete factorial of type 32, where 2 was the quantity of the variable factors (the concentrations of НСООН and Н2О2) and 3 was levels of the factor variation (lower, basic, and upper) [27].
The value of the main optimization parameter was calculated from the equation:
where δj, the weights of the out parameters; dj, the private utility function, which was calculated from the equation:
where φ0(x), is the response of the yj output parameter at the x; \(y_{j}^{{( + )}}\) and \(y_{j}^{{( - )}}\), the best and the worse values of the output parameter within the limits of the examined area, respectively. The results of implementing the design matrix were given in Table 1.
The response surface of the generalized parameter of the optimization was obtained using the Statgraphics program [28] and presented in Fig. 2.
The following optimum conditions of the fractionation of the prehydrolyzed birch wood met the optimization conditions: Y1→ max, Y2→ max, and Y3→ max. They corresponded to the following values of the factors: Х1 and Х2 were 7.2 and 37.8 wt %, respectively. Under the optimum conditions of the peroxide delignification of the hemicellulose-free birch wood, the yields of cellulose and the soluble lignin achieved 64.8 and 11.5% of the mass of the lignocellulosic product, respectively.
The cellulose that was prepared under the optimum conditions of the peroxide delignification of the solid product of the hydrolysis of the birch wood contains 4.8 wt % of the residual hemicelluloses and 1.8 wt % of lignin.
The following characteristic absorption bands in the IR spectrum of the obtained cellulose (Fig. 3) correspond to: the valent vibrations of the hydrogen-bond –OH groups at 3364 сm–1; the valent vibrations of ‒CH groups at 2901 cm–1; the deformation vibrations of ‒CH groups at 1431 сm–1; the asymmetric vibrations of C–O–C at 1163 сm–1; the asymmetric vibrations of the glucose ring at 1110 сm–1; and the vibrations of the β-glycoside bonds between the glucose units in the cellulose at 897 сm–1 [29, 30].
The absorption band at 1727 сm–1 that corresponds to the valent vibrations of the С=О bond in the carbonyl groups of hemicelluloses [30] points to the presence of the residual hemicelluloses in the isolated cellulose. The absence of the absorption bands of phenylpropane fragments of lignin at 1605–1593, 1515–1495, and 1470–1460 сm–1 suggest the deep oxidative destruction of lignin under the conditions of the peroxide delignification in the mixture of formic acid and water in the presence of the TiO2 catalyst.
The XRD method (Fig. 4) demonstrated that the unit cell of the cellulose sample that was obtained from the birch wood was identical to the monoclinic unit cell of cellulose I [31]. An index of the cellulose crystallinity was calculated from the height ratio between the intensity of the crystalline peak (I002 – IAM) and the total intensity (I002) after a subtraction of the background signal that was measured without the cellulose [32] and proved to be 0.70.
The SEM image of the sample of the starting birch wood is presented in Fig. 5a. The wood microstructure is clearly observed in the image. Bordered pits are seen in walls of fibers of the libriform. The particle surface is heterogeneous. The cellulose sample (Fig. 5b) consists of microfibrils, and some of the fibrils are arranged in bunches. Their surface is smooth and homogeneous, possibly, as a result of the removal of extractive substances, lignin, and hemicelluloses.
The basic characteristics of the cellulose from the birch wood and the Vivapur 101 industrial microcrystalline cellulose are given in Table 2.
Thus, the possibility of isolation of the cellulose with a supramolecular structure and a functional composition inherent to the microcrystalline cellulose from the prehydrolyzed birch wood was demonstrated in this study.
Lignin that was prepared by the peroxide delignification of the prehydrolyzed birch wood under the optimum conditions was a light-brown fine-dispersed powder with a density of 1.23 g/сm3, an average molecular mass (Mw) of 1702 Da, and the polydispersity degree of 2.11. The SBET specific surface, the total pore volume, and the average pore diameter were 29.8 m2/g, 0.012 сm3/g, and 3.7 nm, respectively.
Previously, we demonstrated the possibility of a preparation of effective enterosorbents from the organic-soluble lignins [33]. The sorption capacity of the organic-soluble lignins was increased by their treatment with 0.4% NaHCO3 or hot water.
Substances of different molecular mass and chemical nature were used as markers for an investigation of the adsorption capacity of the prepared enterosorbents similarly to those described in the paper [18]. Iodine and methylene blue simulated a class of low-molecular-weight toxicants, whereas gelatin imitated the protein-binding activity for a sorption of pathological agents of the protein nature (microorganisms and their toxins, middle-mass molecules, and bioactive intestinal polypeptides of the endogenous origin) [18].
The results of the determination of the adsorption capacity of the samples of the sorbents that were prepared from the organic-soluble lignin of the birch wood are given in Table 3. The Polifepan therapeutic agent (ZAO Saintek, St. Petersburg) was used as a comparison substance.
All the enterosorbents from the organic-soluble birch lignin exhibit high sorption activity towards iodine, methylene blue, and gelatin. The sorption ability towards iodine, which characterized the microporous structure of the sorbent, is found to be weakly dependent on the method of lignin treatment and proves to be 38.3–42.8%. These values are comparable with that of the Polifepan sorbent (38.7%).
However, the method of treating the organic-soluble lignin significantly affects the adsorption capacity of the examined sorbents towards the methylene blue and gelatin. This parameter characterizes the presence of mesopores. The lignin sample that was treated with 0.4% solution of NaHCO3 has the higher adsorption capacity towards these substances in comparison with the sorbent that was treated with hot water. In addition, both these samples exhibit much higher adsorption activity in comparison with the commercial Polifepan enterosorbent (Table 3).
The content of the water-soluble substances in enterosorbents must not be higher than 5% according to the pharmacological standards. The enterosorbent that was prepared by the treatment of the birch lignin with sodium bicarbonate complies with this requirement (see Table 4).
The enterosorbents from lignin of the birch wood are promising for an application in medicine and veterinary science.
The results demonstrated that the catalytic fractionation of the birch-wood biomass into the microcrystalline cellulose, xylose, and enterosobents should be used for the complex processing of the main components of birch wood (Fig. 6). Fractionation of the wood biomass was based on an integration of the heterogeneous catalytic processes of the hemicellulose hydrolysis with the formation of xylose and the peroxide delignification of the hemicellulose-free wood with the formation of microcrystalline cellulose and organic-soluble lignin. The Amberlyst®15 solid acidic catalyst was used for the hemicellulose hydrolysis. This catalyst exhibited the highest activity among all the examined catalysts and provided the high content of xylose in the hydrolysates (7.2 g/L) at 150°C. The microcrystalline cellulose and the organic-soluble lignin were prepared by the peroxide delignification of the lignocellulosic product of the birch wood hydrolysis in an aqueous medium of formic acid at 100°С in the presence of a TiO2 solid catalyst. The yields of the cellulose and the enterosorbents proved to be 64.5 and 11.5% of the mass of the prehydrolyzed wood, respectively. The treatment of the organic-soluble lignin with the 0.4%-solution of NaHCO3 or hot water gave the more effective enterosorbent in comparison with the commercial Polifepan enterosorbent that was prepared from the hydrolytic lignin.
CONCLUSIONS
We proposed the fractionation of the birch wood biomass into the microcrystalline cellulose, xylose, and enterosorbents by an integration of the hemicellulose hydrolysis and the peroxide delignification of the lignocellulosic product of the wood hydrolysis using heterogeneous catalysts. The effective solid catalysts were chosen for an intensification of these processes. The conditions of the hydrolysis of the hemicelluloses of the birch wood, the peroxide delignification of the lignocellulosic product of the wood hydrolysis, and the extraction of the organic-soluble lignin were optimized. The compositions and the structures of the solid and soluble products of the fractionation of birch wood were determined by physicochemical (FTIR, XRD, SEM, and GC) and chemical methods.
REFERENCES
Cherubini, F., The biorefinery concept: Using biomass instead of oil for producing energy and chemicals, Energy Convers. Manage., 2010, vol. 51, pp. 1412–1421. https://doi.org/10.1016/j.enconman.2010.01.015
Bajpai, P., Biorefinery in the Pulp and Paper Industry, Amsterdam: Elsevier, 2013. https://doi.org/10.1016/C2012-0-06724-5
Environmentally Friendly Technologies for the Pulp and Paper Industry, Young, R.A. and Akhtar, M., Eds., New York: Wiley, 1998.
Ferrer, A., Vega, A., Rodriguez, A., Ligero, P., and Jimenez, L., Milox fractionation of empty fruit bunches from Elaeis guineensis, Bioresour. Technol., 2011, vol. 102, pp. 9755–9762.
Garyntseva, N.V., Sudakova, I.G., Chudina, A.I., Malyar, Yu.N., and Kuznetsov, B.N., Optimization of the process of abies wood peroxide delignification in the medium ‘formic acid-water’ in the presence of TiO2 catalyst, Zh. Sib. Fed. Univ., Khim., 2019, vol. 12, no. 4, pp. 522–535. https://doi.org/10.17516/1998-2836-0148
Kuznetsov, B.N., Malyar, Yu.N., Kuznetsova, S.A., Grishechko, L.I., Kazachenko, A.S., Levdansky, A.V., Pestunov, A.V., Boyandin, A.N., and Celzard, A., Isolation, study and application of organosolv lignins (review), J. Sib. Fed. Univ. Chem., 2016, vol. 9, no. 4, pp. 454–482. https://doi.org/10.17516/1998-2836-2016-9-4-454-482
Liu, X., Feng, Sh., Fang, Q., Jiang, Zh., and Hu, Ch., Reductive catalytic fractionation of lignin in birch sawdust to monophenolic compounds with high selectivity, Mol. Catal., 2020, vol. 495, p. 111164. https://doi.org/10.1016/j.mcat.2020.111164
Kazachenko, A.S., Baryshnikov, S.V., Chudina, A.I., Malyar, Yu.N., Sychev, V.V., Taran, O.P., D’yakovich, L., and Kuznetsov, B.N., Hydrogenation of abies wood and ethanol–lignin by molecular hydrogen in supercritical ethanol over bifunctional Ru/C catalyst, Khim. Rastit. Syr’ya, 2019, no. 2, pp. 15–26. https://doi.org/10.14258/jcprm.2019025108
Dussan, K., Girisuta, B., Haverty, D., Leahya, J.J., and Hayes, M.H.B., The effect of hydrogen peroxide concentration and solid loading on the fractionation of biomass in formic acid, Carbohydr. Polym., 2014, vol. 111, pp. 374–384. https://doi.org/10.1016/j.carbpol.2014.04.039
Ma, R., Xu, Y., and Zhang, X., Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production, ChemSusChem, 2015, vol. 8, pp. 24–51. https://doi.org/10.1002/cssc.201402503
Ramadoss, G. and Muthukumar, K., Influence of dual salt on the pretreatment of sugarcane bagasse with hydrogen peroxide for bioethanol production, Chem. Eng. J., 2015, vol. 260, pp. 178–187. https://doi.org/10.1016/j.cej.2014.08.006
Kuznetsov, B.N., Sudakova, I.G., Garyntseva, N.V., Levdansky, V.A., Ivanchenko, N.M., Pestunov, A.V., Djakovitch, L., and Pinel, C., Green biorefinery of larch wood biomass to obtain the bioactive compounds, functional polymers and nanoporous materials, Wood Sci. Technol., 2018, vol. 52, pp. 1377–1394. https://doi.org/10.1007/s00226-018-1029-7
Borrega, M., Nieminen, K., and Sixta, H., Effects of hot water extraction in a batch reactor on the delignification of birch wood, BioResources, 2011, vol. 6, no. 2, pp. 1890–1903.
Sjöström, E. and Alern, R., Analytical Methods of Wood Chemistry. Pulping and Papermaking, Berlin: Springer, 1999.
Vilcocq, L., Castilho, P., Carvalheiro, F., and Duarte, L., Hydrolysis of oligosaccharides over solid acid catalysts: A review, ChemSusChem, 2014, vol. 7, pp. 1010–1019. https://doi.org/10.1002/cssc.201300720
Hu, L., Lin, L., Wu, Z., Zhou, S., and Liu, S., Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts, Appl. Catal. B: Environ., 2015, vols. 174 175, pp. 225–243.
Sudakova, I.G., Garyntseva, N.V., Chudina, A.I., and Kuznetsov, B.N., Regularities of the process of peroxide delignification of pine wood in the presence of a sulfuric acid catalyst, Khim. Rastit. Syr’ya, 2018, no. 4, pp. 63–71. https://doi.org/10.14258/jcprm.2018044079
Reshetnikov, V.I., Evaluation of the adsorption capacity of enterosorbents and related medicinal preparations, Pharm. Chem. J., 2003, vol. 37, no. 5, pp. 246–251.
Ruiz-Matute, A.I., Hernandez-Hernandez, O., Rodriguez-Sanchez, S., Sanz, M.L., and Martinez-Castro, I., Derivatization of carbohydrates for GC and GC-MS analyses, J. Chromatogr., B, 2011, vol. 879, pp. 1226–1240. https://doi.org/10.1016/j.jchromb.2010.11.013
Testova, L., Vilonen, K.M., Pynnönen, H., and Tenkanen, M., Isolation of hemicelluloses from birch wood: Distribution of wood components and preliminary trials in dehydration of hemicelluloses, Lenzinger Ber., 2009, vol. 87, pp. 58–65.
Degirmenci, V., Uner, D., Cinlar, B., et al., Sulfated zirconia modified SBA-15 catalysts for cellobiose hydrolysis, Catal. Lett., 2011, vol. 141, pp. 33–42. https://doi.org/10.1007/s10562-010-0466-1
Wu, C., Bing, L., Li, S., Yu, D., and Wang, D., Effect of coagulating agents on lignin and oligosaccharide contents in pre-hydrolysis liquor obtained in the production of dissolving pulp from poplar residual slabs, BioResources, 2016, vol. 11, no. 1, pp. 87–94. https://doi.org/10.15376/biores.11.1.87-94
Nakajima, K., Okamura, M., Kondo, et al., Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst, Chem. Mater., 2009, vol. 21, pp. 186–193. https://doi.org/10.1021/cm801441c
Kuznetsov, B.N., Yatsenkova, O.V., Chudina, A.I., Skripnikov, A.M., Kozlova, S.A., Garyntseva, N.V., and Chesnokov, N.V., Influence of mechanical and chemical activation of microcrystalline cellulose on its structure and reaction ability in hydrolysis over solid acid catalyst SBA-15, Zh. Sib. Fed. Univ., Khim., 2014, vol. 7, no. 1, pp. 122–133.
Yatsenkova, O.V., Chudina, A.I., Skripnikov, A.M., Chesnokov, N.V., and Kuznetsov, B.N., The influence of sulfuric acid catalyst concentration on hydrolysis of birch wood hemicelluloses, Zh. Sib. Fed. Univ., Khim., 2015, vol. 8, no. 2, pp. 211–221. https://doi.org/10.17516/1998-2836-2015-8-2-211-221
Kuznetsov, B.N., Sudakova, I.G., Garyntseva, N.V., Tarabanko, V.E., Yatsenkova, O.V., Djakovitch, L., and Rataboul, F., Processes of catalytic oxidation for the production of chemicals from softwood biomass, Catal. Today, 2021, vol. 375, pp. 132–144. https://doi.org/10.1016/j.cattod.2020.05.044
NIST/SEMATECH e-Handbook of Statistical Methods. http://www.itl.nist.gov/div898/handbook/. https://doi.org/10.18434/M32189
Pen, R.Z., Planirovanie eksperimenta v Statgraphics (Planning an Experiment in Statgraphics), 2nd ed., Krasnoyarsk, 2012.
Adel, A.M., Abd El-Wahab, Z.H., Ibrahim, A.A., and Al-Shemy, M.T., Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: Physicochemical properties, Carbohydr. Polymers, 2001, vol. 83, no. 2, pp. 676–687. https://doi.org/10.1016/j.carbpol.2010.08.039
Fan, M., Dai, D., and Huang, B., Fourier transform infrared spectroscopy for natural fibres, in Proceedings of the International Conference on Innovative Technologies (IN-TECH 2012), Rijeka, Croatia, 2012, Salih, S., Ed., pp. 45–68.
Nishiyama, Y., Langan, P., and Chanzy, H., Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction, J. Am. Chem. Soc., 2002, vol. 124, pp. 9074–9082. https://doi.org/10.1021/ja0257319
Park, S., Baker, J.O., Himmel, M.E., Parilla, P.A., and Jonson, D.K., Cellulose crystallinity index: Measurement techniques and their impact on integrating cellulose performance, Biotechnol. Biofuels, 2010, vol. 3, p. 10. https://doi.org/10.1186/1754-6834-3-10
Garyntseva, N.V., Sudakova, I.G., and Kuznetsov, B.N., Properties of enterosorbents obtained from acetic acid lignins of abies, aspen and birch wood, Zh. Sib. Fed. Univ., Khim., 2011, vol. 4, no. 2, pp. 121–126.
Funding
This study was supported by the Russian Scientific Foundation, project no. 21-13-00250, https://rscf.ru/project/21-13-00250/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by L. Onoprienko
Rights and permissions
About this article
Cite this article
Kuznetsov, B.N., Garyntseva, N.V., Sudakova, I.G. et al. Heterogeneous Catalytic Fractionation of Birch-Wood Biomass into a Microcrystalline Cellulose, Xylose and Enterosorbents. Russ J Bioorg Chem 48, 1476–1485 (2022). https://doi.org/10.1134/S1068162022070160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022070160