Abstract
For the first time, the process of extraction fractionation of “hemicelluloses-free” birch wood in an ethanol medium into cellulose product and ethanol lignin is studied and optimized. The composition and structure of cellulose product and ethanol lignin obtained by extraction fractionation of “hemicellulose-free” birch wood and other products of their subsequent transformations are characterized by methods of infra-red spectroscopy, nuclear magnetic resonance, X-ray diffractometry, scanning electron microscopy, BET surface area analysis, high-performance liquid chromatography, gel permeation chromatography, gas chromatography, and chemical and elemental analysis. The possibility of producing enterosorbents from birch ethanol lignin that are more effective than the commercial enterosorbents “Polyphepan” based on hydrolyzed lignin is established. A new approach to the biorefinery of birch wood into xylose, levulinic acid and enterosorbents is proposed based on the integration of an optimized process of extraction fractionation of “hemicellulose-free” birch wood in ethanol medium, alkaline isolation of xylan and its hydrolysis to xylose, cellulose product conversion into levulinic acid and ethanol lignin processing to enterosorbents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wood is a renewable organic raw material, the processing of which into chemicals and biofuels does not affect the balance of CO2 in the atmosphere [1]. Woody biomass contains three main components: cellulose, hemicelluloses, and lignin. The conventional chemical technologies of wood processing use mainly cellulosic component and hemicellulose and lignin remain as a waste. With this in mind, the development of new biorefinery processes that ensure the conversion of all the main components of woody biomass into valuable products is an urgent task [2,3,4,5].
Complex processing of all components of the lignocellulose biomass is achieved via its catalytic oxidative [6] and reductive [7] fractionation. The composition of wood fractionation products depends on the chemical composition of the biomass, which can be significantly different for coniferous and deciduous trees [8].
Birch is one of the most widespread deciduous trees in Russia. A feature of the chemical composition of its biomass is a high (up to 30 wt.%) content of hemicelluloses consisting mainly of xylan [9]. Xylan is used to produce xylose, xylitol, organic acids, and other valuable products [10].
Xylan is isolated from plant biomass using different methods [11,12,13]. One of the most efficient methods for isolating xylan from plant materials is the alkaline extraction. An alkali can destroy hydrogen bonds between cellulose and hemicelluloses and ester bonds between hemicelluloses and lignin without damage of the xylan structure [14].
A promising method of fractionation of wood biomass into holocellulose and lignin is the high-temperature extraction with aqueous-organic and organic solvents (ethanol, methanol, ethylene glycol, glycerin, acetic and formic acids) [15].
The new directions in the processing of cellulose products are related to the one-pot catalytic synthesis of valuable monomeric compounds, e.g., levulinic acid (LA). The presence in LA of both carbonyl and carboxyl functional groups makes it a platform chemical for the synthesis of many high-demand products [16]. LA and its derivatives are widely used in pharmaceuticals, food industry, and organic synthesis, as plant growth regulators, preservation agents, stabilizers, fragrances, etc. The LA ester additives increase the stability of plastics and improve the characteristics of motor fuels [17]. The catalytic hydrogenation of LA is used to obtain γ-valerolactone (GVL), which serves as a fuel additive, a green solvent, and a raw material to produce new polymers [18].
In contrast to technical lignins, the organosolv lignins do not contain sulfur, have a relatively low molecular weight, and they are characterized by the narrower molecular weight distribution [19, 20]. The high content of hydroxyl, phenolic, and carboxyl groups in the organosolv lignins ensures their effective interaction with functional groups of different toxins [21]. Therefore, organosolv lignins can be used to produce effective enterosorbents.
The literature describes two approaches to the fractionation of birch wood biomass into valuable chemical products [22, 23]. Both of them involve the preliminary removal of hemicelluloses from birch wood in the form of xylan or xylose. Further, the “hemicellulose-free” wood is processed into vanillin, syringe aldehyde, and cellulose by catalytic oxidation with oxygen [22] or into methoxyphenols and microcrystalline cellulose by catalytic hydrogenation with hydrogen [23].
In this paper, for the first time, it is proposed to carry out the biorefinery of birch wood using an optimized method of extraction fractionation of “hemicellulose-free” wood in an ethanol medium into cellulose product and ethanol lignin. Integration of this fractionation process and the known methods of isolation and hydrolysis of xylan, conversion of cellulose product into LA makes it possible to process in a single technological cycle all the main components of woody biomass into xylose, levulinic acid, enterosorbents with a yield and quality comparable to the corresponding products, produced by less economical methods of separate processing of birch wood.
2 Experimental
2.1 Materials and reagents
The sawdust (fraction of 1.0‒2.0 mm) of birch (Betula pendula) harvested in the suburb of Krasnoyarsk city was used as a starting raw material. The cellulose content in the wood was determined by the Kürschner method. The lignin content was determined by hydrolysis of the sample with sulfuric acid (72 wt.%) at 20 °C for 2.5 h, with subsequent dilution of the reaction mixture with water and boiling for 1 h. The hemicellulose content was determined by hydrolysis of the wood sample with 2 wt.% of HCl at 100 °C for 3 h with the subsequent identification of monosaccharides gas chromatography. The extractives were identified by extracting wood with the alcohol‒benzene mixture (1:3) in a Soxhlet extractor for 8 h. Ash was identified gravimetrically by burning the wood sample in a muffle furnace at 550 °C for 3 h.
The contents of the main components in the birch wood were 47.3 wt.% of cellulose, 28.5 wt.% of hemicelluloses, 19.0 wt.% of lignin, 4.9 wt.% of extractives, and 0.3 wt.% of ash.
The reagents used were sodium hydroxide of reagent grade (GOST 4328–77, Russia), ethyl alcohol (95%, Russia), sulfuric acid of reagent grade (GOST 4204–77, Russia), and an Amberlyst-15 solid acid catalyst (dry, Acros Organics). The solutions were prepared using distilled water (GOST 6709–72). The monosaccharides used as chromatography standards were crystalline hydrate glucose (GOST 975–88); Panreac D-xylose 142,080.1208, D-mannose 373,195.1208, and D-sorbitol and Sigma-Aldrich LA (98%).
2.2 Extraction fractionation of birch wood biomass
The scheme of extraction fractionation of birch wood into xylan, cellulose, and ethanol lignin is given in Fig. 1.
Xylan was isolated from birch wood by the alkaline extraction as described in [24]. Birch wood sawdust was pretreated by a boiling water‒ethanol mixture (50:50) with a reflux condenser for 1 h at hydromodule 40 to remove fats and waxes. After the treatment, the sawdust was separated from the solution by filtration, washed with distilled water, and dried to the air-dry state at 60 °C for 24 h. Then, xylan was isolated by treatment of the deresined sawdust with a 4% aqueous solution of sodium hydroxide at room temperature at hydromodule 40 for 6 h under stirring. After that, the solution was separated from the precipitate by filtration and the precipitate was washed with distilled water until the neutral pH of washing water. The obtained lignocellulose product was dried at 60 °C for 24 h to the air-dry state.
The solution was neutralized with acetic acid and xylan was precipitated with 96% ethanol. The solution with the xylan precipitate was kept under cooling (+ 5 °C) for 24 h, then decanted, and kept again in ethanol under cooling for 24 h. Xylan was separated from the solution by centrifugation in an OHAUS Frontier 5816 centrifuge at 8000 rpm for 8 min, kept at a temperature of ‒18 °C for 24 h, and then dried in an Iney-6 freeze dryer to constant weight.
The fractionation of “hemicellulose-free” birch wood into soluble lignin and cellulose was performed in a ChemRe SYStem R-201 autoclave reactor (Republic of Korea). The lignocellulose sample (10 g) was added to 100 ml of 60% ethanol and the obtained mixture was loaded into a steel autoclave reactor with a volume of 300 ml. The autoclave was sealed, triply purged with argon, and heated to a specified temperature. After holding for a required time, the reactor was cooled to room temperature and the solid cellulose product was separated by filtration on a white ribbon filter and washed with 60% ethanol before discoloration of the washing solution [25]. The cellulose precipitate was dried at a temperature of 105 °C to a constant weight.
The filtrate was combined with a flushing water and cooled in a refrigerator to 13 °C. Ethanol lignin was precipitated from the solution by fivefold dilution with distilled water at + 4 °C. After aging in the refrigerator for 24 h, ethanol lignin was separated from the solution by centrifugation at 8000 rpm for 8 min, kept at a temperature of ‒18 °C for 24 h, and dried to a constant weight in the freeze dryer.
2.3 Conversion of cellulose product to levulinic acid
Levulinic acid was obtained by hydrolysis of cellulose product isolated from “hemicellulose-free” birch wood using a rotary steel autoclave (a rotation rate of 11 rpm) equipped with an internal fluoroplastic 35 ml tube. Cellulose (0.25 g) was placed in a tube, then 10 ml of 5% H2SO4 was added and the hydrolysis was carried out at temperatures of 180 and 200 °C for 2, 3, and 4 h. The obtained hydrolysate was analyzed for the LA content using a high-performance liquid chromatography (HPLC). Before performing the analysis, the hydrolysate was neutralized with sodium carbonate to pH 5 and extracted with ethyl acetate. The LA content in the ethyl acetate solution was calculated using the calibration curve.
2.4 Hydrolysis of xylan
Hydrolysis of xylan was carried out at temperatures of 110, 130, and 150 °C in a steel rotary autoclave (a rotation rate 11 rpm) equipped with an internal fluoroplastic 35 ml tube. The catalysts used were 0.1 M H2SO4 and an Amberlyst-15 solid acid. Xylan (0.075 g) was placed in a tube, then 15 ml of 0.1 M H2SO4 was added. The hydrolysis was carried out at temperatures 110 °C, 130 °C, and 150 °C with varying duration from 1 to 12 h. When using the solid catalyst, a mixture of xylan (0.075 g) and the catalyst (0.075 g) was placed in a tube, then 15 ml of distilled water was added. The hydrolysis temperature and time ranged within the same limits as in a case of 0.1 M H2SO4 catalyst.
After the specified time, the tube was cooled and the hydrolysate was filtered on a Büchner funnel with a paper filter under vacuum. The solid catalyst was washed with distilled water and dried in an oven at 105 °C and the xylose and furfural contents in the hydrolysate were analyzed.
2.5 Preparation of enterosorbents from ethanol lignin
Enterosorbents were obtained by treating ethanol lignin (10 g) with the 0.4% NaHCO3 solution at room temperature or with water at 95 °C for 30 min at hydromodule 20 [26]. After that, the solid precipitate was separated from the solution by filtration on a Büchner funnel with a blue ribbon paper filter and purified from low-molecular-weight substances using the MF-505–46 MFPI cellophane dialysis bags (US) with a pore size of 0.1 μm. The dialysis time was 8 h; water in the dialysis bag was periodically changed. Then, ethanol lignin was separated from the solution by centrifugation at 8000 rpm for 8 min, kept at a temperature of ‒18 °C for 24 h, and dried to a constant weight in a freeze dryer.
2.6 Biorefinery of birch wood into xylose, levulinic acid and enterosorbents
The scheme of biorefinery of birch wood into xylose, levulinic acid, and enterosorbents integrates the processes of xylan extraction isolation by birch wood treatment at 25 °C with 4 % NaOH of “hemicellulose-free” wood extraction fractionation with ethanol at 90 °C into cellulose product and ethanol lignin, xylan catalytic hydrolysis to xylose over solid Amberlyst-15 catalyst at 130 °C, cellulose product conversion to levulinic acid over of 5 % H2SO4 catalyst at 180 °C and ethanol lignin processing to enterosorbents (Fig. 2).
2.7 Analysis of the products
FTIR spectra were recorded using the Bruker Tensor 27 IR Fourier spectrometer (Germany) in the range of 4000‒400 cm‒1. The spectral data were processed using the OPUS spectroscopy software, version 5.0. To record FTIR spectra, the pressed tablets containing 4 mg of the sample in a potassium bromide matrix were prepared.
Nuclear magnetic resonance (NMR) spectra were recorded using the Bruker AVANCE III 600 MHz spectrometer at 25 °C. The 2D NMR spectrum of xylan dissolved in DMSO-d6 was recorded on 128 slices for 32 scans with a relaxation delay of 15 s using a variant of the spectral editing technique (hsqcedgp). To obtain the 31P-NMR spectra, ethanol lignin (0.01 g) was dissolved in a mixture of deuterated DMF/pyridine solvents (1:1 v/v). The cyclohexanol was used as a standard. The lignin sample was phosphorylated with 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxophosphalane.
X-ray diffraction (XRD) patterns of the solid samples were obtained using the PANalytical X'Pert Pro diffractometer (CuKα radiation, λ = 0.154 nm). The patterns were recorded at the 2θ angles ranging from 10 to 50° with a step of 0.01° in a cell 2.5 cm in diameter.
The surface morphology of the solid samples was studied using the Hitachi TM-1000 scanning electron microscope (Japan) equipped with a SwiftED3000 energy dispersive analyzer (Oxford Instruments Analytical Ltd) at an accelerating voltage of 15 kV and a resolution of 30 μm.
The elemental composition of the samples was determined using the Vario El Cube ELEMENTAR analyzer (Germany).
Weight-average molecular weight Mw, number-average molecular weight Mn, and polydispersity (PD) of the ethanol lignin samples were determined by gel permeation chromatography (GPC) using the Agilent 1260 Infinity II Multi-Detector GPC/SEC system with triple detection (refractometer RI, viscometer VS, and light scattering LS). The separation was made on a PLgel Mixed-E column with tetrahydrofuran (THF) stabilized with 250 ppm butyl hydroxytoluene (BHT) as an eluent. The column was calibrated using the polydisperse polystyrene standards (Agilent, USA). The eluent flow rate was 1 ml/min and the injected sample volume was 100 μl. Before the analysis, the samples were dissolved in THF (5 mg/ml) and filtered through a 0.45-μm Millipore PES membrane filter. The data collection and processing were performed using the Agilent GPC/SEC MDS software.
The texture characteristics of the samples were determined from the nitrogen adsorption and desorption isotherms measured at ‒196 °C under relative pressures P/P0 from 0.005 to 0.995 using the Micromeritics ASAP 2020 adsorption automatic analyzer (USA). Before the measurements, the samples were degassed at a temperature of 80 °C for 24 h under vacuum. The porous structure of the samples was characterized by the Brunauer–Emmett–Teller (BET) specific surface area (SBET) determined from the absorption branch of the isotherm and by the total pore volume (Vtot) calculated from the sorbed nitrogen volume at a relative pressure of P/P0 ≥ 0.995.
The individual composition and content of monosaccharides were determined using the VARIAN-450 gas chromatograph with a flame ionization detector and with VF-624 ms capillary column with a length of 30 m and an inner diameter of 0.32 mm. The monosaccharides were preliminarily derivatized with the formation of trimethylsilyl derivatives using the procedure [27].
3 Results and discussion
Although the separate methods of birch wood processing to produce xylan, xylose, levulinic acid, and organosolv lignin are well known, the possibility of integrating these methods in a single technological cycle in order to utilize all the main components of wood biomass and to reduce the cost of target products is studied for the first time.
It can also be expected that the yield, composition, and structure of products obtained from wood using an integrated process may differ from those produced by separate methods.
3.1 Extraction fractionation of birch wood biomass into xylan and lignocellulose
Xylan is isolated from plant biomass using different methods [11,12,13]. Alkaline hydrolysis was reported to be advantageous over acid hydrolysis or autohydrolysis (steam explosion) to extract long chain hemicelluloses [28]. Alkaline hydrolysis at low temperature allows to produce the high molecular weight hemicelluloses. High temperatures (100–240 °C) and pressures under steam explosion conditions lead to the dissolution of hemicelluloses and partly of lignin [29]. Besides, at temperatures above 200 °C, cellulose decomposes to form cellooligosaccharides, glucose, 5-hydroxymethylfurfural, etc. [30].
The method employed in this work makes it possible to obtain pharmaceutical grade xylan with a high yield at a low temperature using an available and cheap reagent NaOH [24].
The yield and composition of xylanes isolated from various types of plant raw materials may vary. The yield of xylan extracted under the same conditions from Acacia sawdust [31] was 23.5 wt.%, from sugarcane bagasse [32] — 19.6 wt.%, and from eucalyptus [33] — 16.1 wt.%.
The yield and composition of the isolated xylans can also vary in the case of birch trees of different species.
In this study, the Betula pendula birch wood was fractionated into xylan and the lignocellulose product under the optimal extraction conditions established in [24]: the NaOH concentration 4 wt.%, the process temperature 100 °C, and the process time was 1 h. Using the alkaline extraction of Betula pendula wood and the subsequent precipitation with ethanol, the xylan was isolated with a yield of 23% on the weight of absolutely dry wood (82.4% on the wood hemicelluloses weight) and the cellulose product with a yield of 67.5% on the weight of absolutely dry wood. Under the same conditions, the yield of xylane from Betula pubescens wood was 19.6 wt.% [24].
The sample of xylan isolated from Betula pendula wood by the alkaline extraction was characterized by the elemental analysis, FTIR, 2D NMR, and XRD methods.
The FTIR spectrum of xylan (Fig. 3) is similar to the spectrum of commercial birch wood xylan [33]. The absorption band at 1044 cm−1 corresponds to the stretching vibrations of the C–C bond, at 1166 cm−1 ‒ to the stretching vibrations of the C – O – C bond between xylopyranose units, at 1579 and 1410 cm−1 ‒ to symmetric vibrations of the ‒COO group of glucuronic acid, at 3427 cm–1 ‒ to stretching vibrations of the xylan O–H groups involved in hydrogen bonds, at 2927 cm–1 ‒ to the C–H stretching vibrations. The absorption band at 895 cm−1 proves β-configuration of the glycosidic bond 1 → 4 between the xylopyranose units of the main chain of xylan.
The 2D NMR spectrum of birch wood xylan (Fig. 4) contains cross peaks characteristic of (1 → 4) -β-D- xylopyranose and it is similar to the spectrum presented in [33]. The presence of methyl group of 4-O-methyl-D- glucuronic acid is indicated by signals at 58.6/3.35 ppm.
As was shown in [34], xylan with a low content of uronic acid units and acetyl groups tend to form crystal structures. The XRD pattern of xylan isolated from birch wood by the alkaline extraction contains several small peaks indicative of a partially crystalline state of this biopolymer (Fig. 5).
The elemental composition of isolated xylan (Table 1) is similar to the composition of commercial xylan with a purity of > 99% described in [33].
The high oxygen content and O/C atomic ratio in isolated xylan as compared to that in commercial xylan are due to the presence of D-glucuronopyranose in the side chains of this biopolymer, which is consistent with FTIR and 2D NMR data (Figs. 3, 4).
3.2 Extraction fractionation of “hemicellulose-free” birch wood on cellulose product and ethanol lignin
Extraction of lignocellulose biomass with organic solvents is widely used for the isolation of organosolv lignins [35, 36]. The resulting organosolv lignins do not contain sulfur and have a higher reactivity compared to traditional technical lignins.
In the present study, the extraction fractionation of the “hemicellulose-free” birch wood (lignocellulose) into cellulose and soluble ethanol lignin was carried out in a water‒ethanol medium. The effect of temperature and time of the fractionation process on the yield of ethanol lignin and the yield and composition of the cellulose product was examined (Figs. 6, 7).
As the extraction process time varies from 3 to 5 h, the ethanol lignin yield increases from 9.9 to 13.7 wt.% at a temperature of 150 °C and from 15.8 to 16.8 wt.% at a temperature of 190 °C. The highest yield of ethanol lignin (17.1 wt.%) was observed at 210 °C and an extraction time of 3 h. Further increase in the time of extraction at 210 °C decreases the yield of ethanol lignin (Fig. 6).
The cellulose product with the highest cellulose content (93.3 wt.%) and low content of residual lignin (3.4 wt.%) was obtained at an extraction temperature of 190 °C and a time of 4 h. At an extraction temperature of 210 °C the highest yield of ethanol lignin was obtained, but the cellulose content in the cellulose product decreased to 92.0 wt.%.
To establish the optimal conditions of the process of lignocellulose extraction fractionation providing the high yields of both the cellulose product with a low residual lignin content and the soluble ethanol lignin, the process was mathematically optimized using the Statgraphics software package [37].
The factors chosen as independent parameters were extraction temperature X1 (°C) and extraction time X2 (h). The output parameters chosen for the optimization were cellulose yield Y1 (wt.%), ethanol lignin yield Y2 (wt.%), and residual lignin content Y3 in the cellulose product (wt.%). The constant parameters were hydromodule 10 and the ethanol:water ratio 60:40.
The optimization was made using the generalized parameter Wa calculated as in [37].
Data on the conditions and results of experiments for calculating the generalized optimization parameter are presented in Table 2. The yields of cellulose and lignin are both very important parameters, so the value of their weights δ is 1. The content lignin in the cellulose is the less important and the value of its weight δ is 0.5.
Analysis of variances showed that the factor X1 (temperature) contributes significantly to the generalized parameter of optimizations. This is indicated by the high values of dispersion ratios in lines A and AA. The influence of the source of variance on the output parameter is considered statistically significant if the P-Value level is less than 0.05. This condition is also satisfied for factors B and AB (Table 3).
After the mathematical processing, the following regression equation was obtained:
The regression equations can be used as a mathematical model for the extraction fractionation of lignocellulose with a water-ethanol mixture.
The predictive properties of the resulting equation are clearly demonstrated in Fig. 8, which compares the experimental values of the output parameter Wa (observed) with the values predicted by the model (predicted). The straight line corresponds to the calculated (predicted) values of Wa, the dots correspond to the experimental results. The rather high value of calculated coefficient of determination (R2 = 97.3%) indicates a good approximation quality of the regression equation.
Figure 9 shows the dependence of the output parameter Wa on the variable factors X1 and X2 in the form of a response surface.
The non-linear dependence of Wa on the process temperature (the optimum value of Wa is observed at a temperature of 190 °C) indicates that with a further increase in the temperature, the side reactions of depolymerization of both cellulose and lignin increase. It leads to a decrease in their yield. The calculated optimal parameters of the process of birch lignocellulose fractionation are the following: temperature 190 °C, time 5.2 h, hydromodule 10 and ethanol:water ratio 60:40. At these process conditions, the yields of cellulose product and ethanol lignin are 52.3 wt.% and 15.6 wt.%, respectively. The cellulose product contains 93.3 wt.% of cellulose.
3.3 Characterization of cellulose product and ethanol lignin isolated by ethanol from “hemicellulose-free” birch wood
In the present work, the composition and structure of cellulose product and ethanol lignin isolated from “hemicellulose-free” birch wood by ethanol at 190 °C were studied for the first time.
According to chemical analysis data, the isolated cellulose product contains 93.3 wt.% of cellulose, 4.2 wt.% of hemicellulose, and 3.4 wt.% of lignin.
The FTIR spectrum of the cellulose sample is characteristic of the structure of cellulose I [38]. The spectrum contains neither absorption bands in the range of 1700‒1740 cm‒1 corresponding to acetyl or uronic ether groups of hemicelluloses, nor absorption bands corresponding to phenylpropane structural units of lignin (1605‒1593, 1515‒1495, and 1470‒1460 cm‒1). That confirms the removal of these biopolymers during the extraction fractionation of birch wood (Fig. 10).
The XRD pattern of cellulose product (Fig. 11) contains maxima at 2θ angles of 15.2°, 16.2°, 22.5°, and 34.6° corresponding to the reflections from the 110, 101, 002, and 040 crystal planes characteristic of the structural modification of cellulose I [39]. The crystallinity index calculated from the formula presented in [40] is 0.68. The average cellulose crystallite size calculated using the Scherrer equation [40] is 2.3 nm.
The cellulose morphology was characterized by scanning electron microscopy (SEM). It can be seen in the electron microscopy image shown in Fig. 12 that cellulose consists of short loose fibers with numerous kinks and tears.
The presence of short defect fibers complicates the use of cellulose product isolated from birch wood by the extraction fractionation in the conventional processes of fibrous cellulose materials production. However, this cellulose can be chemically converted into valuable monomeric compounds: glucose, levulinic acid, 5-hydroxymethylfurfural, ethanol, and others.
Ethanol lignin isolated by the extraction fractionation of birch wood at 190 °C for 5 h is a light-brown fine powder with a density of 1.42 g/cm3. The molecular weight distribution (MWD) of birch ethanol lignin was established using GPC method (Fig. 13).
The ethanol lignin MWD curve contains a broad (main) peak and a small shoulder. The analysis of the MWD curve showed that most of the molecular weight of ethanol lignin does not exceed 5000 g/mol and the high molecular weight substances are almost absent.
The isolated birch ethanol lignin has a number-average molecular weight (Mn) of 750 g/mol, weight-average molecular weight (Mw) of 1625 Da and polydispersity index (PDI) of 2.17.
Some characteristics of the porous structure of birch ethanol lignin were determined by the BET analysis.
The nitrogen adsorption‒desorption isotherm observed for the ethanol lignin sample (Fig. 14) corresponds to the type-IV isotherm [41] which is typical for the meso-macroporous materials. The narrow hysteresis loop is indicative of a narrow pore size distribution in the ethanol lignin sample.
The BET specific surface area of the birch ethanol lignin sample is 42 m2/g, the total pore volume is 0.12 cm3/g, and the average pore diameter is 3.2 nm.
The FTIR spectrum of birch ethanol lignin (Fig. 15) includes absorption bands typical for the guaiacyl‒syringyl type of lignin [42]. A broad band with a maximum at 3457 cm‒1 corresponds to stretching vibrations of‒OH groups involved in the intermolecular hydrogen bonds. The absorption bands at 2936 and 2844 cm‒1 correspond to the asymmetric and symmetric stretching vibrations of C–H bonds in methyl groups. The band at 1710 cm‒1 corresponds to carboxyl groups. Skeletal vibrations of the aromatic ring are reflected in the absorption bands at 1593, 1512, 1461, and 1422 cm‒1 [43]. The absorption band at 1123 cm‒1 can be attributed to bending vibrations of the C–H bond in the aromatic ring and to stretching vibrations of the C–O bond in secondary alcohols. Stretching vibrations of the C–O bond in primary alcohols manifest themselves in the absorption band at 1034 cm‒1. The band at 834 cm‒1 corresponds to bending vibrations of C–H bonds in positions 2 and 6 of the syringyl ring.
Hydroxyl functional groups in ethanol lignin were identified by the 31P-NMR method (Fig. 16). The phosphorylating agent 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP) was used for derivatization. The cyclohexanol was used as an internal standard.
Table 4 gives the integration regions used to determine signals and the content of hydroxyl groups in ethanol lignin calculated as in [44, 45].
The data obtained show that the content of different hydroxyl groups in ethanol lignin increases in the sequence carboxylic ˂ aliphatic ˂ phenolic.
3.4 Extraction-catalytic fractionation of birch wood biomass into xylose, levulinic acid, and enterosorbents
We proposed to convert the products of birch wood extraction fractionation (xylan, cellulose product and ethanol lignin) into xylose, levulinic acid (LA), and enterosorbents using the known catalytic and extraction processes. The integration of the processes of extraction isolation of xylan, cellulose product, ethanol lignin and their subsequent conversion into xylose, LA, and enterosorbents ensures the complex processing (biorefinery) of birch wood according to the scheme shown in Fig. 2.
According to a proposed scheme birch wood biorefinery, the xylose is obtained by acid hydrolysis of xylan; LA — by the acidic transformation of cellulose product and enterosorbents — by treatment of ethanol lignin with a NaHCO3 solution or hot water.
3.5 Hydrolysis of birch xylan
Xylose is used as a substitute for citric acid, to produce xylitol, furfurol, trioxyglutaric acid, feed additives. Xylose is produced by acid-catalyzed hydrolysis of xylane [46,47,48].
Earlier, it was proposed to carry out the hydrolysis of arabinogalactan in the presence of acid catalysts 0.1 M H2SO4 and Amberlyst-15 [49]. Using a sulfuric acid catalyst, a high yield of monosaccharides was obtained at a temperature of 130 °C for 1.5 h. In the presence of a solid acid catalyst Amberlyst-15, the complete hydrolysis of arabinogalactan was observed at a temperature of 150 °C and a process time of 4 h.
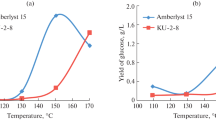
In this study, the activity of these catalysts was compared in the hydrolysis of xylan isolated from birch wood. To select the optimal of conditions xylan hydrolysis that ensure high yield of xylose the process temperature and time were varied (Fig. 17).
The catalytic hydrolysis of xylan in the presence of sulfuric acid catalyst gives a xylose yield 70 wt.% at a temperature of 110 °C and time of 4 h. (Fig. 17A).
Further increase in the hydrolysis temperature and time is impractical, since a part of xylose is converted to other products (Fig. 17).
In contrast to the sulfuric acid catalyst, the solid acid catalyst Amberlyst-15 is low active in the hydrolysis of xylan at 110 °C. However, at a hydrolysis temperature of 130 °C and a time of 10 h, the xylose yield in the presence of Amberlyst-15 catalyst reaches 62 wt.% (Fig. 18A). This yield is comparable to the yield of xylose observed at the hydrolysis of xylan at 110 °C for 4 h in the presence 0.1 M H2SO4 catalyst (70 wt.%). The hydrolysis of xylan in the presence of the Amberlyst-15 catalyst at a temperature of 150 °C, yields 60 wt.% of xylose after 12 h. Under these conditions, the furfural yield noticeably (up to 6 wt.%) increases (Fig. 18B).
Thus, the hydrolysis of xylan at a temperature of 130 °C in the presence of the solid acid catalyst Amberlyst-15 makes it possible to produce xylose with an yield of 62 wt.% with a slight formation of furfural.
It was found that the Amberlyst-15 catalyst retains its initial catalytic activity during 4 catalytic cycles. The results obtained indicate the potential possibility to replacing a toxic and corrosive sulfuric acid catalyst with a safe solid acid catalyst Amberlyst-15.
3.6 Acid conversion of birch cellulose product
Levulinic acid (LA) is a platform product for speciality chemical and fuels, which are widely used in pharmaceuticals, food industry and organic synthesis [50]. LA is produced by acid-catalyzed hydrolysis of cellulose [51,52,53]. For this purpose, dissolved mineral acids and solid acid catalysts can be applied. Since the dissolved acid catalysts are more active, then solid acid catalysts, we used 5% H2SO4 for hydrolysis of cellulose product isolated from “hemicellulose-free” birch wood. The low content of lignin and hemicelluloses in the cellulose product obtained by the extraction fractionation of “hemicellulose-free” birch wood makes it promising to use for production of LA. The effect of temperature and time of cellulose product hydrolysis with a 5% H2SO4 on the LA yield was examined (Fig. 19).
It was found that both the temperature and time of the acid conversion of cellulose product significantly affect the LA yield. The highest LA yield (31.0 wt.%) was observed at a process temperature of 180 °C and a time of 2 h (Fig. 19). With a further increase in temperature and time of the acid conversion of cellulose, the LA yield decreases due to the intensification of secondary reactions, in particular, the formation of solid residues of a humic nature. The yield of humic substances varies from 11.2 to 19.0% from the cellulose weight depending on the process conditions (Fig. 19).
The elemental composition of solid residues ranges within 65.11‒67.26% for C and 4.55‒4.66% for H. It is similar to the elemental composition of humic substances (63.1 of C and 4.2 of H) [54]. The solid residue of a humic nature can be used for preparation of enterosorbents.
3.7 Enterosorbents production from birch ethanol lignin
The hydrolysis lignin is traditionally used for production of commercial enterosorbents “Polyphepan”.
The absence of sulfur in birch ethanol lignin, the low ash content, and a high concentration of hydroxyl groups make this product promising for the production of enterosorbents. To remove unwanted impurities from ethanol lignin, two regimes of its treatment were used: with a 0.4% aqueous solution of NaHCO3 and with hot water.
The enterosorbent yield after treatment of ethanol lignin by NaHCO3 at 25 °C was 85.8 wt.% (9.8% from wood weight) and after treatment with hot water — 90.4 wt.% (10.3% from wood weight).
The adsorption capacity of enterosorbents was studied using substances of different molecular weight and chemical nature. Iodine and methylene blue represent a class of low-molecular-weight toxicants and gelatin — a class of high-molecular-weight substances with a protein-binding activity (microorganisms, toxins, proteins). A commercial enterosorbent Polyphepan (ZAO Saintek, St. Petersburg) produced from hydrolyzes lignin was chosen as a reference sample.
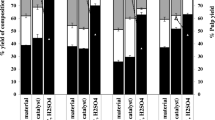
The characteristics of the studied enterosorbents and their sorption properties are given in Tables 5 and 6.
All enterosorbents demonstrate a high sorption capacity for marker substances. Their high sorption capacity for iodine (39.8–45.4%) indicates a microporous structure of these enterosorbents.
The enterosorbents produced from ethanol lignin surpass the commercial enterosorbent Polyphepan by a factor of 1.5‒2.5 in the capacity for methylene blue and by a factor of 1.5 in the capacity for gelatin. The high sorption capacity of enterosorbents from ethanol lignin is due to the high concentration of oxygen-containing functional groups capable of binding various molecules.
According to the pharmacological regulations, the content of water-soluble substances in enterosorbents should be no higher than 5%. The data given in Table 5 show that both enterosorbents produced from ethanol lignin meet the pharmacopoeia requirements for the content of water-soluble substances and ash.
4 Conclusions
It is first proposed to perform biorefinery of birch wood into xylose, levulinic acid and lignin enterosorbents by integrating the optimized process of extraction fractionation of “hemicellulose-free” birch wood with ethanol into cellulose product and ethanol lignin and the processes of alkaline isolation of xylan and its hydrolysis to xylose over solid catalyst Amberlyst-15 of cellulose product conversion to levulinic acid and of extraction processing of ethanol lignin to enterosorbents. The integration of these processes in a single technological cycle makes it possible to utilize all the main components of wood biomass and to reduce the cost of target products.
The products of birch wood biorefinery were characterized by methods of infrared spectroscopy, nuclear magnetic resonance, X-ray difractometry, scanning electron microscopy, BET surface area analysis, high performance liquid chromatography, gel permeation chromatography, gas chromatography, chemical and elemental analysis. It was established that the yields, composition and structure of target products obtained by integrating processing of birch wood are comparable to those produced by less economical methods of separate processing of birch wood. The products obtained by the extraction catalytic fractionation of birch wood biomass can find application in organic synthesis, medicine, veterinary, and other fields.
References
Zhao X, Zhou H, Sikarwar VS, Zhao M, Park AHA, Fennell PS, Shen L, Fan LS (2017) Biomass-based chemical looping technologies: the good, the bad and the future. Energy Environ Sci 10(9):1885–1910. https://doi.org/10.1039/c6ee03718f
Den W, Sharma VK, Lee M, Nadadur G, Varma RS (2018) Lignocellulosic biomass transformations via greener oxidative pretreatment processes: access to energy and value-added chemicals. Front Chem 6:141. https://doi.org/10.3389/fchem.2018.00141
Banu JR, Preethi KS, Tyagi VК, Gunasekaran M, Karthikeyan OP, Kumar G (2021) Lignocellulosic biomass based biorefinery. Fuel 302(15):121086. https://doi.org/10.1016/j.fuel.2021.121086
Shen X, Sun R (2021) Recent advances in lignocellulose prior-fractionation for biomaterials, biochemicals, and bioenergy. Carbohyd Polym 261(1):117884. https://doi.org/10.1016/j.carbpol.2021.117884
Başakçılardan Kabakcı S, Tanış MH (2020) Pretreatment of lignocellulosic biomass at atmospheric conditions by using different organosolv liquors: a comparison of lignins. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00677-2
Kuznetsov BN, Sudakova IG, Garyntseva NV, Tarabanko VE, Yatsenkova OV, Djakovitch L, Rataboul F (2021) Processes of catalytic oxidation for the production of chemicals from softwood biomass. Catal Today 375:132–144. https://doi.org/10.1016/j.cattod.2020.05
Van den Bosch S, Schutyser W, Vanholme R, Driessen T, Koelewijn SF, Renders T, De Meester B, Huijgen WJJ, Dehaen T, Courtin CM, Lagrain B, Boerjan W, Sels BF (2015) Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ Sci 8(6):748–1763. https://doi.org/10.1039/c5ee00204d
Rowell RM (2012) Handbook of Wood Chemistry and Wood Composites (2nd ed.). CRC Press. https://doi.org/10.1201/b12487
Jin X, Hu Z, Wu S, Song T, Yue F, Xiang Z (2019) Promoting the material properties of Xylan-type hemicelluloses from the extraction step. Carbohyd Polym 215:235–245. https://doi.org/10.1016/j.carbpol.2019.03
Naidu DS, Hlangothi SP, John MJ (2018) Bio-based products from xylan: a review. Carbohyd Polym 179:28–41. https://doi.org/10.1016/j.carbpol.2017.09.0
Liu HM, Li YR, Wu M, Yin HS, Wang XD (2018) Two-step isolation of hemicelluloses from Chinese quince fruit: effect of hydrothermal treatment on structural features. Ind Crop Prod 111:615–624. https://doi.org/10.1016/j.indcrop.2017.11.035
Zhang W, Johnson AM, Barone JR, Renneckar S (2016) Reducing the heterogeneity of xylan through processing. Carbohyd Polym 150:250–258. https://doi.org/10.1016/j.carbpol.2016.05.013
Liu HM, Wang FY, Liu YL (2016) Hot-compressed water extraction of polysaccharides from soy hulls. Food Chem 202:104–109. https://doi.org/10.1016/j.foodchem.2016.01.129
Rashid R, Ejaz U, Ali FL, Hashmi LA, Bari A, Liu J, Wang L, Fu PC, Sohail M (2020) Combined pretreatment of sugarcane bagasse using alkali and ionic liquid to increase hemicellulose content and xylanase production. BMC Biotechnol 20. https://doi.org/10.1186/s12896-020-00657-4
Zhang K, Pei Z, Wang D (2016) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Biores Technol 199:21–33. https://doi.org/10.1016/j.biortech.2015.08
Malu TJ, Manikandan K, Cheralathan KK (2020) Chapter 6 - Levulinic acid—a potential keto acid for producing biofuels and chemicals. In Biomass, Biofuels, Biochemicals. Saravanamurugan S (ed) Elsevier, pp 171–197
Taran OP, Gromov NV, Parmon VN (2018) Chapter 2 - Catalytic Processes and Catalyst Development in Biorefining. In Sustainable Catalysis for Biorefineries. Frusteri F, Aranda D, Bonura G (ed) Royal Society of Chemistry, pp 25–64. https://doi.org/10.1039/9781788013567-00025
Zhang D, Zhao YP, Fan X, Liu ZQ, Wang RY, Wei XY (2018) Catalytic hydrogenation of levulinic acid into gamma-valerolactone over Ni/HZSM-5 catalysts. Catal Surv Asia 22(3):129–135. https://doi.org/10.1007/s10563-018-9246-5
Zhang K, Pei Z, Wang D (2016) Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour Technol 199:21–33. https://doi.org/10.1016/j.biortech.2015.08.102
Andreo-Martínez P, Ortiz-Martínez VM, García-Martínez N, Hernández-Fernández FJ, Pérez de los Ríos A, Quesada-Medina J (2021) A simple fractionation method and GPC analysis of organosolv extracts obtained from lignocellulosic materials. Biomass Conv Bioref 11:1807–1821. https://doi.org/10.1007/s13399-019-00593-0
Markelov DA, Nitsak OV, Gerashchenko II (2008) Comparative study of adsorption activity medical sorbents. Pharm Chem J 42(7):30–33
Kuznetsov BN, Sudakova IG, Garyntseva NV, Tarabanko VE, Chesnokov NV, Djakovitch L, Rataboul F (2020) Kinetic studies and optimization of heterogeneous catalytic oxidation processes for the green biorefinery of wood. Top Catal 63:229–242
Kuznetsov BN, Baryshnikov SV, Miroshnikova AV, Kazachenko AS, Malyar YN, Skripnikov AM, Taran OP (2021) Fractionation of birch wood by integrating alkaline-acid treatments and hydrogenation in ethanol over a bifunctional ruthenium catalyst. Catalysts 11:1362
Torgashov VI, Solovieva LV, Zubets OV, Kaputsky FN (2014) Obtaining xylan of pharmaceutical quality from birch wood. BSU Bulletin. Series 2: Chemistry. Biology Geography 1:21–26
Quesada-Medina J, López-Cremades FJ, Olivares-Carrillo P (2010) Organosolv extraction of lignin from hydrolyzed almond shells and application of the d-value theory. Biores Technol 101:8252–8260. https://doi.org/10.1016/j.biortech.2010.06
Garyntseva NV, Sudakova IG, Kuznetsov BN (2011) Properties of enterosorbents obtained from acetic acid lignins of abies, aspen and birch wood. J Sib Fed Univ Chem 2(4):121–126
Ruiz-Matute AI, Hernández-Hernández O, Rodríguez-Sánchez S, Sanz ML, Martínez-Castro I (2011) Derivatization of carbohydrates for GC and GC–MS analyses. J Chromatogr B 879:1226–1240
Panthapulakkal S, Kirk D, Sain M (2015) Alkaline extraction of xylan from wood using microwave and conventional heating. J Appl Polymer Sci 132 (4). https://doi.org/10.1002/app.41330
Lian Z, Wang, Y, Luo J, Lai C, Yong Q, Yu S (2020) An integrated process to produce prebiotic xylooligosaccharides by autohydrolysis, nanofiltration and endo-xylanase from alkali-extracted xylan. Bioresource Technol: 123685. https://doi.org/10.1016/j.biortech.2020.12
Qaseem MF, Shaheen H, Wu A-M (2021) Cell wall hemicellulose for sustainable industrial utilization. Renew Sustain Energy Rev 144:110996. https://doi.org/10.1016/j.rser.2021.110996
Sharma K, Khaire KC, Thakur A, Moholkar VS, Goyal A (2020) Acacia Xylan as a substitute for commercially available Xylan and its application in the production of Xylooligosaccharides. ACS Omega 5(23):13729–13738. https://doi.org/10.1021/acsomega.0c00896
Bian J, Peng F, Peng X-P, Xu F, Sun R-C, Kennedy JF (2012) Isolation of hemicelluloses from sugarcane bagasse at different temperatures: structure and properties. Carbohyd Polym 88(2):638–645. https://doi.org/10.1016/j.carbpol.2012.01
Corradini FAS, Baldez TO, Milessi TSS, Tardioli PW, Ferreira AG, Campos Giordano GR, de de L C Giordano R (2018) Eucalyptus xylan: an in-house-produced substrate for xylanase evaluation to substitute birch wood xylan. Carbohyd Polym 197:167–173. https://doi.org/10.1016/j.carbpol.2018.05.088
Gabrielii I, Gatenholm P, Glasser WG, Jain RK, Kenne L (2000) Separation, characterization and hydrogel-formation of hemicellulose from aspen wood. Carbohyd Polym 43(4):367–374. https://doi.org/10.1016/s0144-8617(00)001
Johansson A, Aaltonen O, Ylinen P (1987) Organosolv pulping – methods and pulp properties. Biomass 13(1):45–65
Zhang Z, Harrison MD, Rackemann DW, Doherty WOS, O´Hara IM, (2016) Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem 18:201660–202381
Pen RZ (2003) Planning an experiment in Statgraphics. - Krasnoyarsk: SibSTU-Claretianum
Fan M, Dai D, Huang B (2012). Fourier transform infrared spectroscopy for natural fbres. In: Salih S (ed) Fourier transform—material analysis. In Tech, Rejeka https://doi.org/10.5772/35482
Nam S, French AD, Condon BD, Concha M (2016) Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohyd Polym 135:1–9. https://doi.org/10.1016/j.carbpol.2015.08.0
Park S, Baker JO, Himmel ME, Parilla PA, Jonson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulose performance. Biotechnol Biofuels 3:10. https://doi.org/10.1186/1754-6834-3-10
Mikova NM, Levdanskiy VA, Skwortsova GP, Zhizhaev AM, Lutoshkin MA, Chesnokov NV, Kuznetsov BN (2021) Structure and properties of organic xerogels derived from tannins and ethanol lignins of the Siberian fir. Biomass Conv Bioref 11:1565–1573. https://doi.org/10.1007/s13399-019-00561-8
Shi Z, Xu G, Deng J, Dong M, Murugadoss V, Liu Ch, Shao Q, Wu S, Guo Z (2019) Structural characterization of lignin from D sinicus by FTIR and NMR techniques. Green Chem Lett Rev 12(3):235–243. https://doi.org/10.1080/17518253.2019.1627428
Roeges NPG (1995) A guide to the complete interpretation of infrared specter of organic structures. Wiley New York, 340p
Pu Y, Cao S, Ragauskas AJ (2011) Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ Sci 4(9):3154. https://doi.org/10.1039/c1ee01201k
Fiţigãu IF, Peter F, Boeriu CG (2013) Structural analysis of lignin from different sources Internation. J Chem Mol Nuclear Mater Metall Eng 7(4):167–172
Mäki-Arvela P, Salmi T, Holmbom B, Willför S, Murzin DYu (2011) Synthesis of sugars by hydrolysis of hemicelluloses — a review. Chem Rev 111(9):5638–5666
Hilpmann G, Bechera N, Pahner F-A, Kusema B, Mäki-Arvela P, Lange R, Murzin DYu, Salmi T (2016) Acid hydrolysis of xylan. Catal Today 259:376–380
Carà PD, Pagliaro M, Elmekawy A, Brown DR, Verschuren P, Shiju NR, Rothenberg G (2013) Hemicellulose hydrolysis catalysed by solid acids. Catal Sci Technol 3(8):2057–2061
Yatsenkova OV, Skripnikov AM, Kozlova SA, Kuznetsov BN (2018) Kinetic study and optimization of hydrolysis of larch arabinogalactan in the presence of dissolved and solid acid catalysts. J Sib Fed Univ Chem 2:167–183. https://doi.org/10.17516/1998-2836-0066
Yan K, Jarvis C, Gu J, Yan Y (2015) Production and catalytic transformation of levulinic acid: a platform for speciality chemicals and fuels. Renew Sustain Energy Rev 51:986–997. https://doi.org/10.1016/j.rser.2015.07.021
Hu X, Ming C, Sun K, Shao Y, Zhang Z, Zhang S, Xiang J (2019) Conversion of cellulose to levulinic acid/ester over acid catalyst: impacts of dispersion of hydrogen ions on polymerisation reactions. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.9
Han Y, Ye L, Gu X, Zhu P, Lu X (2019) Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using γ-valerolactone as solvent. Ind Crops Prod 127:88–93. https://doi.org/10.1016/j.indcrop.2018.10
Badgujar KC, Wilson LD, Bhanage BM (2019) Recent advances for sustainable production of levulinic acid in ionic liquids from biomass: Current scenario, opportunities and challenges. Renew Sustain Energy Rev 102:266–284. https://doi.org/10.1016/j.rser.2018.12.00
Girisuta B, Janssen LPBM, Heeres HJ (2006) A kinetic study on the decomposition of 5- hydroxymethylfurfural into levulinic acid. Green Chem 8:701–709
Acknowledgements
This study was carried out with the use of the equipment of Krasnoyarsk Regional Center of Research Equipment of the Federal Research Centre “Krasnoyarsk Science Centre SB RAS”.
Funding
The reported study was supported by the Russian Science Foundation, Grant No. 21–13-00250 https://rscf.ru/project/21-13-00250/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing of interests
The authors declare no competing of interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuznetsov, B.N., Sudakova, I.G., Chudina, A.I. et al. Fractionation of birch wood biomass into valuable chemicals by the extraction and catalytic processes. Biomass Conv. Bioref. 14, 2341–2355 (2024). https://doi.org/10.1007/s13399-022-02498-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02498-x