Abstract—
The holothurian Eupentacta fraudatrix is widely used for studying how regeneration works and as a source of biological active compounds. Data on the lipid content and composition of E. fraudatrix are limited to the description of fatty acid (FA) composition. The storage and structural lipids of E. fraudatrix gut have been analyzed by different chromatographic and mass spectrometry methods. Triacylglycerols, as storage lipids, are characterized by a high concentration of trophic FA markers. Among structural lipids, phosphatidylethanolamines and phosphatidylcholines contain a high concentration of molecular species with a simple ether linkage. Phosphatidylserines (PS) and phosphatidylinositols (PI) differ from other lipids with very-long-chain FA in composition. The FA 23:1n-9, which is characteristic of holothurians, has been detected in PS and PI. The study of marine organisms’ lipidome may contribute to understanding the biosynthetic pathways and distribution of trophic markers in lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Holothurians (Holothuroidea) are one of the most widespread classes of lower invertebrates. They play an important role in the ocean ecosystem, breaking down detritus and organic matter for bacteria and thus returning nutrients back to the oceans [1]. Holothurians are traditionally used for food in Southeast Asia.
Some sea cucumber species have a defense mechanism called evisceration: animals can eject their internal organs (such as intestines and respiratory tract) from their bodies when exposed to stress (for example, chemical stress or physical manipulation), while the lost organs can be quickly regenerated [2, 3]. Holothuria Eupentacta fraudatrix is one of the unique organisms used to study the regeneration process. Recently, an analysis of the dynamics of gene expression during intestinal regeneration in E. fraudatrix made it possible to identify a number of transcription factors that can participate in the transdifferentiation of coelomic epithelial cells into the enterocytes of holothurians [4].
Holothuria E. fraudatrix is known as a rich source of triterpene glycosides. A total of 37 glycosides were isolated and identified from the sea cucumber [5–7]. Most glycosides E. fraudatrix are characterized by the presence of a residue 3-O-methylxylose as the final link in the carbohydrate chain instead of 3-O-methylglucose found in most sea cucumber glycosides. This difference is considered a chemotaxonomic marker of the genus Eupentacta [7].
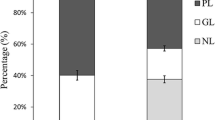
In numerous studies of sea cucumbers E. fraudatrix hardly take into account the composition of lipids, with the exception of the previously described composition of fatty acids (FA) in E. fraudatrix [8]. Lipids have important functions for the body. Neutral lipids (triacylglycerols (TG) and wax esters) serve as an energy reserve. Polar lipids (glycerophospholipids, sphingolipids, and glycolipids) are structural components of cell membranes involved in signaling. The composition of lipids and FAs has been described in sea cucumbers inhabiting the waters of Vietnam [8], Apostichopus japonicas [9], abyssal sea cucumbers [10] and deep-sea sea cucumbers of the Sea of Okhotsk [11].
Each class of lipids is a mixture of hundreds of molecular species of lipids, which includes various FAs. A quantitative description of the molecular composition of lipids is one of the problems solved by lipidomics. The increase in lipidome data contributes to the study of marine ecology and lipid biochemistry.
The purpose of this work is to determine the composition of molecular species in storage (triacylglycerols) and structural (phospholipids) lipids of the intestine in E. fraudatrix. The chemical structure and composition of phospholipid (PL) lipids were first identified using high performance liquid chromatography (HPLC) with high resolution tandem mass spectrometry (MS/MS). TG was analyzed by supercritical fluid chromatography using a low resolution mass spectrometer and light scattering detector.
RESULTS AND DISCUSSION
To determine the composition of FA and molecular species of lipids, we used adult sea cucumbers E. fraudatrix, collected in Peter the Great Bay, Sea of Japan. Lipids isolated from the intestines of five sea cucumbers were analyzed by liquid and gas-liquid chromatography with mass spectrometric, flame ionization and light scattering detection.
Total lipids accounted for 4.6% of the wet weight of sea cucumber intestines. The content of TG and sterols was determined using supercritical fluid chromatography with a light scattering detector. The total amount of phospholipids and some classes of these lipids was determined by the amount of inorganic phosphorus during oxidation in HClO4 [12]. The content of the main classes of lipids in sea cucumbers is presented in Table 1. PL included six main classes: phosphatidylethanolamines (PE), phosphatidylcholines (PC), phosphatidylserines (PS), phosphatidylinositols (PI), lysophosphatidylcholines (LPC), and diphosphatidylglycerols (DPG).
Intestinal lipid content of E. fraudatrix was comparable to holothurians from the waters of Vietnam, studied earlier [8]. Intestines of E. fraudatrix contained lipids (TG, sterols, and PL), which constituted a significant part of the lipid extract (58.6%) of the sea cucumber intestine. PC, PE, PS, PI, lysophospholipids, and DPG have been found in widespread sea cucumber species such as Holothuria leucospilota, H. atra, Cucumaria frondosa, Isostichopus fuscus and etc. [8, 13]. Thus, the composition of polar lipids in E. fraudatrix (Table 1) was typical of sea cucumbers. The main class of phospholipids in E. fraudatrix was PC, the content of which was 60.8% of the total amount of PL. The predominance of PC in phospholipids was also reported for other sea cucumbers [8, 13].
The content and composition of fatty acids in the intestine E. fraudatrix was determined by gas-liquid chromatography with a flame ionization detector. The FA profile of sea cucumbers was distinguished by a high concentration of polyunsaturated fatty acids (PUFA), while the main PUFA was eicosapentaenoic acid (20:5n-3) (Table 2). n-3 PUFAs were predominant in intestinal lipids (39.9% of the total fatty acids), while the content of n-6 PUFA was only 1.9%. The content of FAs with an odd number of carbon atoms was 9.7%. FA 23:1n-9, a marker FA of holothurians, was found.
In acid methanolysis from phospholipids with an alkenyl group at position sn-1 long-chain aldehyde dimethyl acetals (DMA) are formed. Octadecanal dimethylacetal (18:0-DMA) was the only DMA, which accounted for 1.03% of the mixture of FAME and DMA obtained from the total lipids of sea cucumbers.
Intestinal FA composition of E. fraudatrix was similar to previously obtained data [8]. In our work, it was shown that the intestines of E. fraudatrix contained a higher concentration of 22:6n-3 (6.76% of the total amount of FA and DMA) in comparison with that in the whole organism of the previously studied E. fraudatrix (2.3% of the total amount of FA) [8]. The peculiarity of the intestinal FA composition we studied in E. fraudatrix showed there was a high level of FA with a branched structure and an odd number of carbon atoms, which is consistent with the previously obtained data for E. fraudatrix [8]. It is believed that branched FAs are synthesized by gram-positive and sulfate-reducing bacteria [14]. The intestines of the sea cucumber may contain a large number of these bacteria.
A high content of acids 16:4n-1 (3.51%) and 20:5n-3, as well as a ratio of 16:1n-7/16:0 exceeding 1, were found in the intestines of sea cucumbers. These parameters indicate a predominance of diatoms in the diet of E. fraudatrix [11]. It was previously shown that the sea cucumber C. frondosa also feeds mainly on diatoms during the period of their active flowering [15].
TG is the main form of energy storage in both terrestrial and marine animals [16]. Composition of TG intestinal lipids in E. fraudatrix is represented by 53 molecular species. Data on the main molecular species, arranged in order of increasing retention time, are given in Table 3. Basic TGs contained acyl fragments 20:5, 16:0, 14:0, 16:1, and 22:6.
Molecular species of the same composition with different retention times obtained on a column with a reversed phase were found in the TG composition. For example, TG 22:6/16:0/16:1 had a shorter retention time (no. 19, 22.86 min) than a similar molecular species (no. 21, 24.64 min). Similar chromatographic behavior was observed for peaks 11 and 18, 24 and 27. Some of the molecular species of TGs had unusual retention times for their structure (the number of carbon atoms and the number of double bonds in FA residues). Thus, TG 20:5/18:1/16:0 (peak # 17) had a shorter retention time than TG 20:5/18:1/15:0 (peak # 19). Differences in the retention times of similar molecular species of TGs suggest that TGs contain branched FAs. Twenty-eight molecular TG species contained diatom markers (16:4n-1 and 16:1n-7). Moreover, the TG row (16:1/16:1/16:1, 16:1/16:2/16:1, 20:5/16:1/16:1 and 20:5/16:0/16:1) completely repeats the TG composition of diatoms [17]. We found that trophic marker FAs are present mainly in TG.
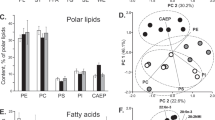
Structural lipids in the intestine of E. fraudatrix were represented mainly by sterols and phospholipids. Glycolipids were also found in sea cucumbers [9], but we did not determine the content and composition of these lipid classes. In the intestinal lipids of sea cucumbers, 63 molecular species of PE, PC, PS, PI, and LPC were identified. The main molecular species were 18:1alk/20:5 PE, 18:1alk/20:5 PC, 18:1/20:5 PC, 18:0/20:5 PC, 22:0/20:5 FS, 22:1/24:1 FS, 23:1/20:1 FS, 20:0/20:5 PI, 22:1/20:5 PI and 22:0/20: 5 PI (Fig. 1). The main FA in the composition of phospholipids of E. fraudatrix was acid 20:5n-3 (Fig. 1a). A high concentration of long-chain acyl fragments (up to 42 carbon atoms) is characteristic of PS (77.4% of the total PS) and PI (61.2% of the total PI).
(a) The content of the main molecular species (% of the phospholipid class) of phosphatidylethanolamines (PE), phosphatidylcholines (PC), phosphatidylserines (PS) and phosphatidylinositols (PI) in the intestine of Eupentacta fraudatrix. On the sn-1 and sn-2 axes, alkyl and acyl groups are located at positions sn-1 and sn-2, respectively, of the molecules of the corresponding class of phospholipids. Alkyl (X:Yalk) and acyl groups (X:Y) contain X carbon atoms and Y double bonds; (b) the distribution of alkyl/acyl and diacyl molecular species in each class of phospholipids; (c) the distribution of odd and even acyl fragments in each class of phospholipids.
Alkyl/acyl molecular species of PL were typical for PE (94.1% of the sum of all PE), PC (32.5% PC) and LPC (65.7% LPC) (Fig. 1b). The only alkyl/acyl molecular PI species with a concentration of less than 2% was 18:0alk/20:5 PI. PS did not contain alkyl/acyl molecular species.
A high content of alkyl/acyl molecular species was found in PE, PC, and LPC, which is consistent with the previously obtained data for sea cucumbers [13]. Molecular species with an alkyl bond in position sn-1 were found mainly in PCs of a number of sea cucumbers, while alkenyl bonds are characteristic of PE [13]. Ether lipids are the main structural components of cell membranes. A simple ether bond in phospholipids changes their physical properties and affects the dynamics of membranes [18]. No carbonyl oxygen in position sn-1 promotes the formation of stronger intermolecular hydrogen bonds between the head groups [19]. An alkenyl group of plasmalogens in position sn-1 promotes a denser packing of phospholipids in the membrane, which leads to a decrease in membrane fluidity and an increase in rigidity [18].
FAs with an odd number of carbon atoms were typical for FE (9.2% of the total FE), PS (24.6% of the total PS), and PI (16.5% of the total PI) (Fig. 1c). FA 19:1 was present in PE, long-chain acids 23:0 and 23:1 were found only in PS and PI.
The highest concentration of odd FAs was found in PS (Fig. 1c). The presence of residues 21:0 and 23:0 indicates a possible lengthening of FAs of bacterial origin (15:0, 17:0, and 19:0) in the tissues of marine organisms for further biosynthesis of their own lipids [20]. Acid 23:1n-9, characteristic of sea cucumbers, was found only in PS and PI. It is assumed that this FA is formed as a result of α-oxidation of 24:1n-9 [21]. Earlier marker for soft corals tetracosapolyenoic FAs (24:5n-6 and 24:6n-3) were also found mainly in PI and PS [22, 23]. The relationship of the biosynthetic pathways of PS and PI with marker FAs requires additional research.
Composition and content of the main classes of lipids in sea cucumbers E. fraudatrix does not differ significantly from lipids of other sea cucumber species [8, 13]. Molecular species of PE and PC contain mainly eicosapentaenoic acid (20:5n-3), which is consistent with the previously obtained data for six edible goluturia [13]. Long-chain FAs (>21 carbon atoms) with both even and odd numbers of carbon atoms were found mainly in PS and PI, which was confirmed earlier [13]. A feature of the intestinal lipids of sea cucumbers of E. fraudatrix is the high content of PUFA in the composition of PE and PC (97.2% of the total PE and 99.6% of the total PC).
Holothuria E. fraudatrix is used to study the pathways of regeneration [4] and as a source of biologically active compounds [7]. Intestinal storage and structural lipids of E. fraudatrix were first analyzed by various methods of chromatography and mass spectrometry. Previously, it was suggested that phospholipids are a relatively conserved part of the lipidome, much less influenced by food sources than nonpolar reserve lipid classes [24]. We have shown that fatty acids from food and bacteria living in the intestine are part of the reserve class of lipids, the triacylglycerols. PL biosynthesis included elongation of fatty acids from food. The study of the lipidome of marine organisms can help in the establishment of biosynthetic pathways and the distribution of trophic markers in lipids.
EXPERIMENTAL
Materials. Hexane, benzene, chloroform, methanol and 28% NH4OH solution analytical grade were used for lipid extraction and thin layer chromatography (TLC); for HPLC, hexane, 2-propanol, HCOOH, and triethylamine (Sigma-Aldrich, United States). Triacylglycerol and Phospholipid Standards were acquired from Avanti Polar Lipids Inc. (United States), tridecyl palmitate (analytical grade) from KNPO Diagnosticum (Russia).
Animals. Adults of sea cucumbers E. fraudatrix were collected in the Gulf of Peter the Great, Sea of Japan and contained in tanks with a volume of 3 m3 with running aerated seawater at 16°C for one week. Intestinal tissue was taken from five specimens (through an incision in the body wall of the sea cucumber). The intestines were rinsed with sterile seawater to remove food debris.
Lipid analysis. Extracts of total lipids were obtained according to the Folch method [25] with some modifications. The content of phosphorus-containing total lipids was measured on a UV-1800 spectrophotometer (Shimadzu, Japan), based on the amount of inorganic phosphorus during oxidation in HClO4 as previously published [12].
Fatty acid methyl esters (FAME) from intestinal lipids of E. fraudatrix were obtained in accordance with the method Carreau and Dubacq [26]. FAMEs were analyzed by gas chromatography on a GC-2010 chromatograph (Shimadzu, Japan) with a flame ionization detector and a capillary column 30 m × 0.25 mm (id) Supelcowax 10 (United States). The analysis was carried out under the following conditions: column temperature 205°C, injector and detector temperature 250°C. Helium was used as a carrier gas. FAME peaks were identified by comparing the retention times of individual fatty acid esters and equivalent chain lengths with standards (PUFA-3 mixture from Menhaden oil, Supelco, Bellefonte, United States). The FAME concentration (% of the total FA) was determined from the areas of the chromatographic peaks of the corresponding compounds, while the total area of the FAME peaks was taken as 100%, and the percentage concentration of individual FAs was calculated in relation to their total content.
The content and structure of molecular species of PL were determined on an LC-20A Prominence chromatograph (Shimadzu, Japan) with an LCMS-IT-TOF high-resolution tandem mass spectrometer (Shimadzu, Japan) according to the previously described conditions [23]. Determination of the content and identification of molecular species was carried out as described earlier [27, 28].
The composition and amount of TGs in total lipids were determined using supercritical fluid chromatography (Nexera UC (Shimadzu, Japan)) with an ELSD LT II light scattering detector (Shimadzu, Japan) and a low-resolution mass spectrometer LCMS-8060 (Shimadzu, Japan) in the chemical ionization mode at atmospheric pressure and registration of positive ions [23]. For the quantitative determination of TG, an internal standard 16:0/16:0/18:1 TG (Avanti Polar Lipids Inc., United States) was used.
Data are presented as mean values ± standard deviation, fivefold replication. The data were processed using MS Excel.
CONCLUSIONS
For the first time, molecular species of storage and structural lipids of the intestine of E. fraudatrix were identified by gas–liquid and liquid chromatography using mass spectrometric, flame ionization and light-scattering detectors. It has been shown that fatty acids coming from food and bacteria living in the intestine are part of the reserve class of lipids, the triacylglycerols. After elongation, dietary fatty acids are used for the biosynthesis of phospholipids. The study of the lipidome of marine organisms can help to establish biosynthetic pathways and the distribution of marker fatty acids in lipids.
REFERENCES
Du, H.X., Bao, Z.M., Hou, R., Wang, S., Su, H.L., Yan, J.J., Tian, M.L., Li, Y., Wei, W., Lu, W., Hu, X.L., Wang, S., and Hu, J.J., PLoS One, 2012, vol. 7, p. 10. https://doi.org/10.1371/journal.pone.0033311
Dolmatov, I.Y. and Ginanova, T.T., Cell Tissue Res., 2009, vol. 336, pp. 41–58. https://doi.org/10.1007/s00441-009-0761-6
Mashanov, V.S., Dolmatov, I.Y., and Heinzeller, T., Biol. Bull., 2005, vol. 209, pp. 184–193. https://doi.org/10.2307/3593108
Boyko, A.V., Girich, A.S., Tkacheva, E.S., and Dolmatov, I.Y., Sci. Rep., 2020, vol. 10, p. 11. https://doi.org/10.1038/s41598-020-58470-0
Kalinin, V.I., Avilov, S.A., Kalinovskii, A.I., and Stonik, V.A., Chem. Nat. Compd., 1992, vol. 28, pp. 635–636. https://doi.org/10.1007/BF00630455
Silchenko, A.S., Kalinovsky, A.I., Avilov, S.A., Andryjaschenko, P.V., Dmitrenok, P.S., Martyyas, E.A., and Kalinin, V.I., Nat. Prod. Commun., 2012, vol. 7, pp. 1157–1162.
Silchenko, A.S., Kalinovsky, A.I., Avilov, S.A., Popov, R.S., Kalinin, V.I., Andrijaschenko, P.V., Dmitrenok, P.S., and Yurchenko, E.A., Nat. Prod. Commun., 2018, vol. 13, pp. 137–140.
Svetashev, V.I., Levin, V.S., Lam, C.N., and Nga, D.T., Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1991, vol. 98, pp. 489–494. https://doi.org/10.1016/0305-0491(91)90242-6
Lou, Q.M., Wang, Y.M., Liu, X.F., and Xue, C.H., J. Food Biochem., 2012, vol. 36, pp. 317–321. https://doi.org/10.1111/j.1745-4514.2011.00544.x
Drazen, J.C., Phleger, C.F., Guest, M.A., and Nichols, P.D., Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2008, vol. 151, pp. 79–87. https://doi.org/10.1016/j.cbpb.2008.05.013
Kharlamenko, V.I., Stepanov, V.G., Borisovets, E.E., Kiyashko, S.I., and Svetashev, V.I., Russ. J. Mar. Biol., 2015, vol. 41, pp. 448–455. https://doi.org/10.1134/s106307401506005x
Vaskovsky, V.E., Kostetsky, E.Y., and Vasendin, I.M., J. Chromatogr., 1975, vol. 114, pp. 129–141.
Wang, X.C., Cong, P.X., Chen, Q.S., Li, Z.J., Xu, J., and Xue, C.H., J. Food Compos. Anal., 2020, vol. 94, p. 9. https://doi.org/10.1016/j.jfca.2020.103626
de Carvalho, C. and Caramujo, M.J., Molecules, 2018, vol. 23, p. 36. https://doi.org/10.3390/molecules23102583
Hamel, J.F. and Mercier, A., Can. J. Zool. Rev. Can. Zool., 1998, vol. 76, pp. 1194–1198. https://doi.org/10.1139/cjz-76-6-1194
Parrish, C., Abrajano, T., Budge, S., Helleur, R., Hudson, E., Pulchan, K., and Ramos, C., in Lipid and Phenolic Biomarkers in Marine Ecosystems: Analysis and Applications, Wangersky, P., Ed., Berlin: Springer-Verlag, 2000, pp. 193–223.
Li, S., Xu, J.L., Chen, J., Chen, J.J., Zhou, C.X., and Yan, X.J., J. Appl. Phycol., 2014, vol. 26, pp. 1389–1398. https://doi.org/10.1007/s10811-013-0159-4
Dean, J.M. and Lodhi, I.J., Protein Cell, 2018, vol. 9, pp. 196–206. https://doi.org/10.1007/s13238-017-0423-5
Lohner, K., Chem. Phys. Lipids, 1996, vol. 81, pp. 167–184. https://doi.org/10.1016/0009-3084(96)02580-7
Bosh, T.V. and Long, P.Q., Russ. J. Mar. Biol., 2017, vol. 43, pp. 471–478. https://doi.org/10.1134/s1063074017060049
Kaneniwa, M., Itabashi, Y., Endo, S., and Takagi, T., Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1986, vol. 84, pp. 451–455. https://doi.org/10.1016/0305-0491(86)90105-7
Imbs, A.B., Dang, L.P.T., Rybin, V.G., Nguyen, N.T., and Pham, L.Q., Biochem. Anal. Biochem., 2015, vol. 4, p. 205. https://doi.org/10.4172/2161-1009.1000205
Sikorskaya, T.V. and Imbs, A.B., Russ. J. Bioorg. Chem., 2018, vol. 44, pp. 712–723. https://doi.org/10.1134/s1068162019010151
Chen, H.K., Song, S.N., Wang, L.H., Mayfield, A.B., Chen, Y.J., Chen, W.N.U., and Chen, C.S., PLoS One, 2015, vol. 10, art. ID e0132519. https://doi.org/10.1371/journal.pone.0132519
Folch, J., Lees, M., and Sloane-Stanley, G.A., J. Biol. Chem., 1957, vol. 226, pp. 497–509.
Carreau, J.P. and Dubacq, J.P., J. Chromatogr., 1978, vol. 151, pp. 384–390. https://doi.org/10.1016/s0021-9673(00)88356-9
Imbs, A.B., Dang, L.P.T., and Nguyen, K.B., PLoS One, 2019, vol. 14, p. 22. https://doi.org/10.1371/journal.pone.0215759
Imbs, A.B., Dang, L.P.T., Rybin, V.G., and Svetashev, V.I., Lipids, 2015, vol. 50, pp. P. 575–589. https://doi.org/10.1007/s11745-015-4021-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any research involving humans and animals as research objects.
Conflict of Interest
The authors declare they have no conflict of interest.
Additional information
Abbreviations: DMA, dimethyl acetals; DPG, diphosphatidylglycerol; FA, fatty acids; LPC, lysophosphatidylcholine; MUFA, monounsaturated fatty acids; FAME, fatty acid methyl esters; SFA, saturated fatty acids; PUFA, polyunsaturated fatty acids; TG, triacylglycerols; PI, phosphatidylinositol; PL, phospholipids; PC, phosphatidylcholine; PE, phosphatidylethanolamine.
Corresponding author: phone: +7 (423) 231-09-05.
Rights and permissions
About this article
Cite this article
Ermolenko, E.V., Sikorskaya, T.V. & Dolmatov, I.Y. Distribution of Fatty Acids in Storage and Structural Lipids of the Holothurian Eupentacta fraudatrix. Russ J Bioorg Chem 48, 353–359 (2022). https://doi.org/10.1134/S106816202202008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202202008X