Abstract
A series of novel hybrids of indole-pyrimidine moieties were synthesized and evaluated for their in vitro anti-cancer, in vitro anti-bacteria and in vitro anti-fungal activities. The results showed that most of these compounds possessed significant cytotoxic potency against four cancer cell lines, HeLa, HEK 293T and MCF-7. The compounds 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine,4-(4-chlorophenyl)-6-(1-methyl-1H-indol-3-yl) pyrimidin-2-amine and4-(1H-indole-3-yl)-6-phenylpyrimidin-2-amine showed good activity against HEK 293T. The compounds 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine and 4-(4-chlorophenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine showed good to moderate activity against MCF-7, whereas compound 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine exhibit moderate activity against HaLa S3 cell line. The newly synthesized derivatives were also screened for their in vitro anti-bacterial activity against Bacillus subtilis, B. megatherium, B. pumilis, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter aerogenes, Streptococcus pyogenes, Staphylococcus aureus, Proteus vulgaris, Escherichia coli, using streptomycin as a standard drug. Among tested compound 4-(3-(benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine shows more potent activity compared to standard, where as the remaining analogues exhibited well to moderate activity compared to standard. Anti-fungal screening results suggest that the compound 4-(4-chlorophenyl)-6-(1H-indol-3-yl)pyrimidin-2-amineshowed potent activity against Dreschleria halides. The remaining compounds showed nearest activity against all the tested fungal strains compared to standard drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The various studies have shown that the impact of cancer is truly shocking in low- or middle-income countries because of fewer resources are available for diagnostics and therapeutic purpose [1]. In recent decades, microtubules have been important molecular targets for the development of anticancer drugs due to their crucial roles in the regulating cancer cell survival and progression. Including cellular signaling, motility, cell shape maintenance, secretion, inter-cellular transport and spindle formation during mitosis [2–5]. Inhibiting tubulin polymerization or interfering with microtubule disassembly ultimately leads to cell cycle arrest orapoptos is of cancer cells [6–8]. Hence, there are commonly two major categories of anti-tubulin agents. Heterocyclic compounds are well-known pharmaceutically active products, and this development of simple and efficient methods of synthesis of compounds incorporating heterocyclic rings has given a new dimension to drug discovery [9]. The indole moiety is probably the most widely spread nitrogen heterocycle in nature. It is an essential part of the amino acid tryptophan and the neurotransmitter serotonin, and the in dolescaf fold is also found in numerous naturally occurring plant based alkaloids. The potential medical pharmacological attention have made them extremely attractive and rewarding research target. The biological activities of indoles cover a wide spectrum, including anti-cancer [10, 11], antimicrobial [12], anti-inflammatory [13], anti-malarial [14], cytotoxic [15] and anti-tubercular [16] activities.
The pyrimidine ring system which has a distinguished account starting from its discovery phase as constituent of nucleic acids to their present-day use in chemotherapy [17]. The pyrimidine ring is found in vitamins like thiamine, riboflavin and folic acid [18]. One of the early anti metabolite 5-fluorouracil, a pyrimidine derivative, is used as an anti-neoplastic agent [19]. Over the years, pyrimidine ring systems have been widely explored for their diverse range of biological activities [20–25]. In addition, many pyrimidine analogues have been developed as chemotherapeutic agents [26], anti-leukemic drugs [27], and calcium-sensing receptor antagonists.
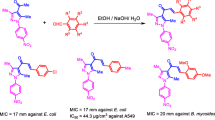
As reviewed from earlier literature reports, the indole and pyrimidine analogues to afford novel chemical entities with appreciable biological activity. Such compounds were found to be potent as anti-tumor [28, 29], antimicrobial [30, 31]and antioxidant [32] agents. Further, some derivatives of meridianins (I) and bis-indolo pyrimidine (II) (hyrtinadine-A)have show nexcellent inhibition against MCF-7 and HeLa cells [33, 34]. Heterocyclic’s possessing free amine group at position-2 of the ring has acquired a unique place in medicinal chemistry. In fact, 2-amino group is a common structural feature in some welle stablishedindolo-pyrimidine drugs [35]. With this in mind, the research has begun to study biologically active properties, especially anti-cancer and anti-bacterial by merging two units of pharmacophore, both an anti-cancer active N-methyl indole (Cediranib) scaffold and an anti-bacterial active 2,6-diamine pyrimidine derived from Iclaprim, which are designed and synthesized (Fig. 1). An aromatic substituent at the sixth position of the pyrimidine ring adapts the potency of anti-cancer and anti-bacterial activities. These results and as a part of our research work on the development of novel scaffolds with anti-cancer, anti-bacterial and anti-fungal activity.

RESULT AND DISCUSSION
The synthesis of indole-pyrimidine derivatives were synthesized by two main steps. Initially the synthesis of 1-methyl-1H-indole-3-carbaldehyde obtained by the reaction of with1H-indole-3-carbaldehyde and CH3I in CH3CN. The second step involves one-pot three components of reaction indole carbaldehyde, substituted acetophenone and guanidine hydrochloride in ethanol to give target indole-pyrimidines in good to high yields. The structure of title compounds and intermediate were established by FT-IR,1H-NMRand Mass spectroscopic analysis.1HNMR spectra of the title compounds (Va–k) showed proton of indole-substituted phenyl pyrimidine moiety chemical shift ranging from δ 7.11–8.65 ppm. The free amino proton of the pyrimidine nucleus appeared as a broad singlet in the range at δ 5.23–6.78 ppm. The N‑methyl protons of indole moiety appeared assinglet in range of δ 3.75–3.83 ppm.
In vitro the anti-cancer activities of the synthesized compounds (VIa–k) were evaluated against three different human cancer cell lines HeLa (cervical cancer), MCF-7(breast cancer) and HEK 293T (embryonic kidney) using doxorubicin as standard with the help of MTT assay [36] and the results are summarized Table 1. It was observed that majority of compounds exhibited good to moderate anticancer activity against HeLa (cervical cancer), MCF-7 (breast cancer), and HEK 293T (embryonic kidney) cancer cell lines. Among all compounds, (Vg) which contains methyl substituent showed good activity against HEK 293T with IC50 values ranging from 66.22 ± 4.26 μg/mL. The analogues (Vj), (Vk) and (Va) bearing 3-benzyloxy, 4-chloro substituent’s on aromatic nucleus have shown moderate activity against HEK 293T with IC50 values ranging from 67.89 ± 3.38, 67.42 ± 4.20 and 68.65 ± 3.61 μg/mL respectively. On the other hand (Vj) and (Vk) containing 3-benzyloxy, 4-chloro on aromatic nucleus showed good to moderate activity ranging from 39.03 ± 2.18 to 40.28 ± 3.59 against MCF-7. Whereas compound (Vj) exhibit moderate activity ranging of 43.22 ± 3.19 against HeLa with IC50 value.
In vitro anti-bacterial activity against en bacteria viz, E. coli, B. subtilis, B. megatherium, B. pumilis, P. vulgaris, P. mirabilis, S. aureus, K. pneumonia, E. aerogenes, S. pyogens. These results are summarized in Table 2. Streptomycin was taken as positive control. The test compounds and standard drugs are dissolved in DMSO of specific concentrations 600 and 900 µg/mL. Among the tested compounds (Vj) shows more potent activity which bearing 3-benzloxy and 3‑chloro substituent on aromatic nucleus (Vd) exhibit good activity,where as the compound (Vg) with methyl substituent on nitrogen of indole nucleus shows equi-potent activity against E. coli compared to the standard. The compound (Vc) and (Vi) shows good activity which having a 4-chloroand 4-trifluoromethyl substituent on aromatic nucleus compared to the standard against B. subtilis. The introducing of 2-fluoro-4-trifluoromethyl (Vg) and a methyl group (Vh) on aromatic nucleus more active than the standard drug. Whereas the compound (Va) shows nearest activity against B. pumilis. The compounds containing 3-chlorosubstituent (Vd) and 3-fluoro substituent (Ve) showed equipotent activity against K. pneumonia. Compounds bearing 4-choro substituent (Vc) and 2-fluoro-4-trifluoro methyl substituent’s (Vh) shows good activity against E. aerogens compared to standard drug.
In vitro anti-fungal activity against Candida albicans, Fusarium oxysporium, Dreschleria halides and Colletotrichum falcatum using itrazole as positive control and results were summarized in Table 3. The compounds (Vk), (Vd), (Vc) and (Vb) bearing 4-chloro, 3‑chloro,4-chloro and 3,4,5-trimethoxy substituent on benzene nucleus shows good activity against all tested fungal strains as compared to standard drug. Moderate to weak activity observed in the case of rest the compounds.
EXPERIMENTAL
All commercially available solvents and reagents were of analytical grade and used without further purification. Melting points were determined on a Gallenkamp melting point apparatus. 1H NMRexperiments were run at 300 MHz on a Bruker Avance NMR spectrometer using TMS as internal standard in DMSO-d6. Chemical shifts are quoted as δ ppm. The mass spectra were recorded on Jeol SX-102 spectrometer at 70 eV. Column chromatography was performed using silica gel (100–200 mesh size) purchased from Sigma-Aldrich and Thin Layer chromatography was carried out using aluminium sheet pre-coated with silica gel 60F254 purchased from Merck. IR spectra were obtained using Shimadzu IR-470 spectrometer. CHN analysis was carried out on a Carlo Erba EA 1108 automatic analyzer. Combustion analyzer was found to be within the limits of permissible limits.
General procedure for the synthesis of methyl-1H-indole-3-carbaldehyde (II): To a solution of 1H-indole-3-carbaldehyde (I) (435 mg, 3 mmol) in CH3CN (30 mL) was added Cs2CO3 (829 mg, 6 mmol), and the mixture was refluxed for 2 hours. To this solution, CH3I (3.3 mmol) was added, and the mixture was heated under reflux for further 1 hour. After the completion of the reaction, the solvent was evaporated under reduced pressure and water was added to the reaction mixture and extracted 3 times for ethyl acetate. The combined organic layers were then dried over Na2SO4 and concentrated under vacuum. The residue was purified by column chromatography with ethylacetate : hexane (1 : 9) to affordmethyl-1H-indole-3-carbaldehyde [38] (II).
506 mg in 75% yield; mp: 196–198°C; 1H NMR (300 MHz, DMSO-d6): δ3.03 (s, 3H), 6.44–6.50 (m, 1H), 6.53 (d, 1H, J = 7.0 Hz), 6.61–6.66 (m, 1H), 7.13 (s, 1H), 7.36 (dd, 1H, J = 6.8, 1.5 Hz), 8.99 (s, 1H); MS (ESI, m/z): 160 [M + H]+.
General procedure for the synthesis of compounds (Va–k) (Scheme 1): The mixture of methyl-1H-indole-3-carbaldehyde/1H-indole-2-carbaldehyde (II) (200 mg, 1.37 mmol), acetophenone (IV) (0.15 mL, 1.37 mmol), guanidine hydrochloride (III) (263 mg, 2.75 mmol) and NaOH (2 mL) in ethanol was allowed to at reflux for 3 hours. After completing the reaction, the reaction mixture was poured into water, and then washed with water thoroughly. The product was filtered, dried and recrystallized from ethanol to afford pure compounds, similar procedure was applied for the rest of compounds.
4-(1H-indol-3-yl)-6-phenylpyrimidin-2-amine (Va): 201 mg in 51% yield: mp: 234–236°C; (FT-IR) (KBr, cm–1): 3544, 1641, 1221, 1175, 1085, 976; 1H NMR (300 MHz, DMSO-d6): δ6.78 (s, 2H, –NH2), 7.42 (s, 1H, pyrim-H),7.45 (d, 1H, J = 7.28 Hz, indole-H), 7.52 (d, 1H, J = 7.34 Hz, indole-H), 7.63 (t, 1H, indole-H), 7.67 (t, 1H, indole-H), 7.69–7.72 (m, 3H, Ar–H), 7.82(s, 1H, indole-H) 8.10 (d, 2H, J = 8.12 Hz), 9.42 (bs, 1H, indole-NH); MS (ESI, m/z): 287 [M + H]+; Anal. calcd. for C18H14N4: C, 75.50; H, 4.93; N, 19.57. Found: C, 75.48; H, 4.91; N, 19.55.
4-(1H-Indol-3-yl)-6-(3,4,5-trimethoxyphenyl)-pyrimidin-2-amine (Vb): 216 mg in 42% yield; mp: 214–216°C; (FT-IR) (KBr, cm–1): 3523, 1617, 1210, 1103, 1009, 910; 1H NMR (300 MHz, DMSO-d6): δ 3.78 (s, 3H, –OCH3), 3.81 (s, 3H, –OCH3), 3.84 (s, 3H, –OCH3), 6.72 (s, 2H, –NH2), 6.92 (s, 1H, –Ar–H), 6.96 (s, 1H, –Ar–H), 7.20 (s, 1H, pyrim-H),7.35 (d, 1H, J = 6.78 Hz, indole-H), 7.45–7.49 (m, 2H, indole-H), 7.60 (d, 1H, J = 6.81 Hz, indole-H), 7.90 (s, 1H, indole-H), 9.83 (bs, 1H, indole-NH); MS (ESI, m/z): 377 [M + H]+; Anal. calcd. for C21H20N4O3: C, 67.01; H, 5.36; N, 14.88. Found: C, 66.98; H, 5.34; N, 14.85.
4-(4-Chlorophenyl)-6-(1H-indol-3-yl)pyrimidin-2-amine (Vc): 230 mg in 52% yield; mp: 221–223°C; (FT-IR) (KBr, cm–1): 3576, 1645, 1242, 1134, 1023, 961; 1H NMR (300 MHz, DMSO-d6): δ 6.65 (s, 2H, ‒NH2), 7.27 (t, 1H, indole-H ), 7.32 (t, 1H,indole-H ), 7.44 (d, 1H, J = 8.36 Hz, indole-H), 7.52 (d, 1H, J = 8.36 Hz, indole-H), 7.63 (s, 1H, pyrim-H ), 7.72 (d, 2H, J = 8.36 Hz, Ar–H); 7.78 (d, 2H, J = 8.36 Hz, Ar–H), 7.98 (s, 1H, indole-H), 9.98 (bs, 1H, indole-NH); MS (ESI, m/z): 321 [M + H]+; Anal. calcd. for C18H13N4Cl: C, 67.40; H, 4.08; N, 17.47. Found: C, 67.39; H, 4.00; N, 17.45.
4-(3-Chlorophenyl)-6-(1H-indol-3-yl) pyrimidin-2-amine (Vd): 241 mg in 55% yield; mp: 194–196°C; (FT-IR) (KBr, cm–1): 3558, 1631, 1257, 1187, 1045, 947; 1H NMR (300 MHz, DMSO-d6): δ 6.76 (s, 2H, ‒NH2), 7.37–7.40 (m, 3H, indole-H), 7.46 (d, 1H, J = 8.14 Hz, indole-H), 7.52 (t, 1H, Ar–H), 7.59 (s, 1H, pyrim-H), 7.66–7.71 (m, 2H, Ar–H), 7.76 (d, 1H, J = 8.94 Hz, Ar–H), 7.87 (s, 1H, indole-H), 9.95 (bs, 1H, indole-H); MS (ESI, m/z): 321 [M + H]+; Anal. calcd. for C18H13N4Cl: C, 67.40; H, 4.08; N, 17.47. Found: C, 67.39; H, 4.06; N, 17.46.
4-(3-Fluorophenyl)-6-(1H-indol-3-yl) pyrimidin-2-amine (Ve): 211 mg in 50% yield; mp: 252–254°C; (FT-IR) (KBr, cm–1): 3568, 1648, 1267, 1178, 1056, 983; 1H NMR (300 MHz, DMSO-d6): δ 6.75 (s, 2H, ‒NH2), 7.38 (d, 1H, J = 8.57 Hz, indole-H), 7.45 (d, 1H, J = 8.57 Hz, indole-H), 7.56 (t, 2H, indole-H), 7.62 (s, 1H, pyrim-H), 7.76 (s, 1H, Ar–H), 7.82–7.86 (m, 2H, Ar–H), 7.88 (d, 1H, J = 8.96 Hz, Ar–H), 7.93 (s, 1H, indole-H), 10.05 (bs, 1H, –NH); MS (ESI, m/z): 305 [M + H]+; Anal. calcd. for C18H13N4F: C, 71.04; H, 4.31; N, 18.41. Found: C, 71.00; H, 4.29; N, 18.39.
4-(3-(Benzyloxy)phenyl)-6-(1H-indol-3-yl)pyrimidin-2-amine (Vf): 229 mg in 42% yield; mp: 261–263°C; (FT-IR) (KBr, cm–1): 3578, 1662, 1740, 1241, 1156, 1012, 945; 1H NMR (300 MHz, DMSO-d6): δ 5.24 (s, 2H, –CH2), 6.74 (s, 2H, –NH2), 7.23 (s, 1H, Ar–H), 7.29 (t, 1H, Ar–H), 7.36 (t, 1H, Ar–H), 7.45 (d, 1H, J = 8.12 Hz, Ar–H), 7.47–7.52 (m, 5H, Ar–H), 7.58 (d, 1H, J = 8.24 Hz, indole-H), 7.62 (d, 1H, J = 8.45 Hz, indole-H), 7.65–7.69 (m, 2H, indole-H), 7.89 (s, 1H, pyrim-H), 8.10 (s, 1H, indole-H), 10.20 (bs, 1H, –NH); MS (ESI, m/z): 393 [M + H]+; Anal. calcd. for C25H20N4O: C, 76.51; H, 5.14; N, 14.28. Found: C, 76.50; H, 5.14; N, 14.27.
4-(1-Methyl-1H-indol-3-yl)-6-phenylpyrimidin-2-amine (Vg): 240 mg in 64% yield; mp: 218–220°C; (FT-IR) (KBr, cm–1): 3510, 1622, 1216, 1123, 1042, 934; 1H NMR (300 MHz, DMSO-d6): δ3.78 (s, 3H, ‒CH3), 6.59 (s, 2H, –NH2), 7.05 (d, 1H, J = 8.34 Hz, indole-H), 7.10 (d, 1H, J = 8.52 Hz, indole-H), 7.19 (q, 2H, indole-H), 7.30 (d, 1H, J = 8.52 Hz, Ar–H), 7.38 (d, 1H, J = 8.58 Hz, Ar–H), 7.56–7.62 (m, 3H, Ar–H),7.69 (s, 1H, indole-H), 7.81(s, 1H, pyrim-H); MS (ESI, m/z): 301 [M + H]+; Anal. calcd. for C19H16N4: C, 75.98; H, 5.37; N, 18.65. Found: C, 75.96; H, 5.34; N, 18.63.
4-(2-Fluoro-4-(trifluoromethyl)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine(Vh): 248 mg in 51% yield; mp: 266–268°C; (FT-IR) (KBr, cm–1): 3587, 1665, 1245, 1145, 1067, 979; 1H NMR (300 MHz, DMSO-d6): δ3.75 (s, 3H, –CH3), 6.72 (s, 2H, –NH2), 7.32 (d, 1H, J = 8.96 Hz, indole-H), 7.43 (d, 1H, J = 8.96 Hz, indole-H), 7.47 (t, 1H, indole-H), 7.52 (t, 1H, indole-H), 7.70 (s, 1H, Ar–H), 7.74 (d, 1H, J = 9.20 Hz, Ar–H), 7.82 (d, 1H, J = 9.20 Hz, Ar–H), 7.92 (s, 1H, pyrim-H), 7.96 (s, 1H, indole-H); MS (ESI, m/z): 387 [M + H]+; Anal. calcd. for C20H14N4F4: C, 62.18; H, 3.65; N, 14.50. Found: C, 62.16; H, 3.63; N, 14.48.
4-(4-(Trifluoromethyl) phenyl)-6-(1-methyl-1H-indol-3-yl) pyrimidin-2-amine (Vi): 210 mg in 45% yield; mp: 268–270°C; (FT-IR) (KBr, cm–1): 3564, 1636, 1256, 1123, 1046, 957; 1H NMR (300 MHz, DMSO-d6): δ 3.83 (s, 3H, –CH3), 6.34 (s, 2H, –NH2), 7.23–7.45 (m, 4H, indole-H), 7.68 (d, 2H, J = 8.56 Hz), 7.73 (d, 2H, J = 8.78 Hz), 7.75 (s, 1H, pyrim-H),7.78 (s, 1H, indole-H); MS (ESI, m/z): 369 [M + H]+; Anal. calcd. for C20H15N4F3: C, 65.21; H, 4.10; N, 15.21. Found: C, 65.19; H, 4.10; N, 15.20.
4-(3-(Benzyloxy)phenyl)-6-(1-methyl-1H-indol-3-yl)pyrimidin-2-amine (Vj): 217 mg in 42% yield; mp: 248–250°C; (FT-IR) (KBr, cm–1): 3535, 1645, 1767, 1216, 1120, 1057, 968; 1H NMR (300 MHz, DMSO-d6): δ 3.81 (s, 3H, –CH3), 5.18 (s, 2H, –CH2), 5.68 (s, 2H, –NH2), 7.24 (d, 1H, J = 8.56 Hz, indole-H), 7.31 (d, 1H, J = 8.69 Hz, indole-H), 7.36 (t, 2H, indole-H), 7.56 (s, 1H, Ar–H), 7.60 (s, 1H, pyrim-H), 7.63–7.70 (m, 5H, Ar–H), 7.73 (d, 1H, J = 8.34 Hz Ar–H), 7.77 (d, 1H, J = 8.54 Hz, Ar–H), 7.81 (t, 1H, Ar–H), 7.89 (s, 1H, indole-H); MS (ESI, m/z): 407 [M + H]+; Anal. calcd. for C26H22N4O: C, 76.83; H, 5.46; N, 13.78. Found: C, 76.83; H, 5.45; N, 13.78.
4-(4-Chlorophenyl)-6-(1-methyl-1H-indol-3-yl)-pyrimidin-2-amine (Vk): 236 mg in 56% yield; mp: 254–256°C; (FT-IR) (KBr, cm–1): 3567, 1656, 1235, 1145, 1087, 969; 1H NMR (300 MHz, DMSO-d6): δ 3.82 (s, 3H, –CH3), 5.33 (s, 2H, –NH2), 7.34 (d, 1H, J = 8.76 Hz, indole-H), 7.44 (d, 1H, J = 8.82 Hz, indole-H), 7.47(t, 1H, indole-H), 7.54 (t, 1H, indole-H), 7.68 (d, 2H, J = 8.23 Hz, Ar–H), 7.74 (d, 2H, J = 8.33 Hz, Ar–H),7.86 (s, 1H, pyrim-H), 7.92 (s, 1H, indole-H); MS (ESI, m/z): 335 [M + H]+; Anal. calcd. for C19H15N4Cl: C, 68.16; H, 4.52; N, 16.73. Found: C, 68.15; H, 4.50; N, 16.73.
MTT assay: In vitro anti-cancer activity of the synthesized compounds (Va–k) was tested using MTT colorimetric assay as per ATCC protocol. Cell lines that were used for testing in vitro cytotoxicity included HeLa, MCF-7 and HEK 293 T was received with job number 1767 from NCCS, Pune. Cell lines were maintained at 37°C in a humidified 5% CO2 incubator using suitable media prescribed in NCCS Protocol. Decontaminated flasks were incubated for subculture. Cells were passed by 12 numbers. After getting 70% confluence; from culture flasks take 100 µL cell suspension and make a cell count using haemocytometer and found 5 000–6 000 per well in a 96-well plate. Cell suspension was mixed thoroughly by pippetting several times to get a uniform single cell suspension. Different dilutions of drugs solutions 3, 10, 30, 100 µg/mL were made in media with final 0.5% DMSO. 100 µL of cell suspension was transferred aseptically to each well of a 96 well plate and to it 100 µL of drug solution in (quadruplicate) in media was added. The plate was then incubated at 37°C for 72 hours in CO2 incubator. After 48 hours of incubation, 20 µL of MTT was added to each well. The plate was again incubated for 2 hours, 80 µL of analysis buffer was added to each well the plate was wrapped in aluminum foil to prevent the oxidation of the dye and the plate was placed on a shaker for 30 minutes. The absorbance were recorded on the ELISA reader (Biotech EL×800) at 570 nm wavelength. We will calculate % inhibition by following formula.
% inhibition = Control ODs-Test ODs+Control ODs× 100 and finally IC50 Values to asses anti-cancer activity. Doxorubicin was used as the standard drug in the assay.
Antimicrobial activity: Antimicrobial activities of compounds (Va–k) were carried out by agar well diffusion [37] method against test organisms. Using the sterile cork borer, wells 6 mm were made in to each Petri-plate. DMSO used as a negative control. The test compounds and standard drugs are dissolved in DMSO of specific concentrations 600 and 900 µg/mL test compounds are filled in the wells and incubated at 37°C and the diameter of the inhibition zones were measured after 24 hours in case of bacteria and after 48 hours in case of fungi. After appropriate incubation, the diameter of zone of inhibition of each well was measured. Ciprofloxacin was used as reference drug for antibacterial activity agent. Itrazole drug was used as reference for anti fungal agent. DMSO used as a negative control.Duplicate were maintained and the average values were calculated for eventually antibacterial and antifungal activity.
CONCLUSIONS
The presented work described an efficient synthesis of new indole-pyrimidine (Va–k) derivatives by applying a multi-component one-pot method. The target molecules investigated for their in vitro cytotoxicity and anti-microbial properties by MTT assay and broth dilution method [37] respectively. The most susceptible cell lines comprised of HeLa, HEK 293T and MCF-7 with majority of compounds showing higher inhibition against these cell lines. The compounds (Vj) and (Vk) showed good to moderate activity against MCF-7, whereas compound (Vj) exhibit moderate activity against HaLa cell line. Whereas the compound (Vg) showed potent activity on HEK 293T and the remaining compounds (Vj), (Vk) and (Va) exhibited moderate activity against HEK 293T. The other hand, in case of anti-bacterial studies, compound (Vj) shows more potent activity compared to standard, where as the remaining analogues (Vg), (Vc), (Vh), (Vi), (Va), (Ve) and (Vk) exhibited well to moderate activity compared to standard. Anti-fungal screening results suggest that the compounds (Vk), (Vd), (Vc) and (Vb) showed potent activity. They may be considered as a promising leads for future design of potent and selective antimicrobial agents.

Scheme 1 . Reagents and conditions: (i) CH3I, Cs2CO3, CH3CN, reflux, 3 h. (ii) EtOH, aq. NaOH, reflux, 3 h.
REFERENCES
Unger-Saldaña, K., World. J. Clin. Oncol., 2014, vol. 5, pp. 465–477. https://doi.org/10.5306/wjco.v5.i3.465
Mora-Bermúdez, F. and Huttner, W.B., Mol. Biol. Cell., 2015, vol. 26, pp. 4302–4306. https://doi.org/10.1091/mbc.E15-03-0152
Kaur, R., Kaur, G., Gill, R.K., Soni, R., and Bariwal, J., Eur. J. Med. Chem., 2014, vol. 87, pp. 89–124. https://doi.org/10.1016/j.ejmech.2014.09.051
Driowya, M., Leclercq, M.J., Verones, V., Barczyk, A.M., Lecoeur, N., Renault, N., Flouquet, A., Ghinet P., and Berthelot, N., Eur. J. Med. Chem., 2016, vol. 115, pp. 393–405. https://doi.org/10.1016/j.ejmech.2016.03.056
Z. Gan-Or, G.A., Rouleau, E.E., and Benarroch, Neurology, 2016, vol. 87, pp. 2173–2184. https://doi.org/10.1212/wnl.0000000000003444
Kumar, A.S., Reddy, M.A., Jain, N., Kishor, C., Murthy, T.R., Ramesh, B.D., Supriya, B., Addlagatta, A., Kalivendi, S.V., and Sreedhar, B., Eur. J. Med. Chem., 2013, vol. 60, pp. 305–324. https://doi.org/10.1016/j.ejmech.2012.12.008
Strzyz, P., Nat. Rev. Mol. Cell Biol., 2016, vol. 17, pp. 333–341. https://doi.org/10.1038/nrm.2016.63
Dumontet, C. and Jordan, M.A., Nat. Rev. Drug Discov., 2010, vol. 9, pp. 790–803. https://doi.org/10.1038/nrd3253
Shelke, S.H., Mhaske, P.C., Kasam, S.K., and Bobade, V.D., J. Heterocycl. Chem., 2014, vol. 51, pp. 1893–1897. https://doi.org/10.1002/jhet.1910
Singh, P., Eur. J. Med. Chem., 2014, vol. 74, pp. 440–450. https://doi.org/10.1016/j.ejmech.2013.12.047
Sharma, S.K., Kumar, P., Narasimhan, B., Ramasamy, K., Mani, V., Mishra, K.R., Majeed, A., Eur. J. Med. Chem., 2012, vol. 48, pp. 16–25. https://doi.org/10.1016/j.ejmech.2011.11.028
Mehndiratta, S., Hsieh, Y.L., Liu, Y.M., and Wang, A.W., Eur. J. Med. Chem., 2014, vol. 85, pp. 468–479. https://doi.org/10.1016/j.ejmech.2014.08.020
Liew, L.P.P., Fleming, J., Longeon, A., Mouray, E., and Florent, I., Tetrahedron, 2014, vol. 70, pp. 4910–4920. https://doi.org/10.1016/j.tet.2014.05.068
Yamuna, E., Kumar, R.A., and Zeller, M., Eur. J. Med. Chem., 2012, vol. 47, pp. 228–238. https://doi.org/10.1016/j.ejmech.2011.10.046
Comín-Anduix, B., Agell, N., Bachs, O., and Ovadi, J., Mol. Pharmacol., 2001, vol. 60, pp. 1235–1242. https://doi.org/10.1124/mol.60.6.1235
Nassar, E., J. Am. Sci., 2010, vol. 6, pp. 463–471. https://doi.org/10.1134/S0003683810050017
Nagarapu, L., Vanaparthi, S., Bantu, R., and Kumar, C.G., Eur. J. Med. Chem., 2013, vol. 69, pp. 817–822. https://doi.org/10.1016/j.ejmech.2013.08.024
Pingxian Liu, Yang Yang, Yunxiang Tang, Tao Yang, Zitai Sang, and Zhiyong Liu, Eur. J. Med. Chem., 2019, vol. 163, pp. 169–182. https://doi.org/10.1016/j.ejmech.2018.11.054
Ballell, A.F., Robert, G.A.C., and Chung Young, R., Bioorg. Med. Chem. Lett., 2007, vol. 17, pp. 1736–1740. https://doi.org/10.1016/j.bmcl.2006.12.066
Gorlitzer, K., Herbig, S., and Walter, R.D., Org. Chem. Int., 1997, vol. 52, pp. 670–672. https://doi.org/10.1155/2013/582079
Malik, V., Singh, P., and Kumar, S., Tetrahedron, 2006, vol. 62, pp. 5944–5951. https://doi.org/10.1016/j.tet.2006.04.018
Al-Issa, A.S., Saudi Pharm. J., 2013, vol. 21, pp. 305–316. https://doi.org/10.1016/j.jsps.2012.09.002
Wagner, E., Al-Kadasi, K., Zimecki, M., and Sawka-Dobrowolska, W., Eur. J. Med. Chem., 2008, vol. 43, pp. 2498–2504. https://doi.org/10.1016/j.ejmech.2008.01.035
Miyazaki, Y., Matsunaga, S., Tang J., Maeda, Y., Nakano, M., and Philippe, R.J., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 2203–2207. https://doi.org/10.1016/j.bmcl.2005.03.034
Christopherson, R.I. and Lyons, S.D., Med. Res. Rev., 1990, vol. 10, pp. 505–548. https://doi.org/10.1002/med.2610100406
Raffa, D., Daidone, G., Maggio, B., and Cascioferro S., II Farmaco Sci., 2004, vol. 59, pp.451–455. https://doi.org/10.1016/j.farmac.2003.10.006
Yang, W., Ruan, Z., Wang, Y., Van-Kirk, K., Ma, Z., and Arey, B.J., J. Med. Chem., 2009, vol. 52, pp. 1204–1208. https://doi.org/10.1021/jm801178c
Hanselmann, R., Job, G.E., Johnson, G., Lou, R.L., and Martynow, J.G., Org. Process Res. Dev., 2010, vol. 14, p. 152. https://doi.org/10.1021/op900252a
Nikhila, G., Dayakumar, D., and Manjunatha Kumsi., J. Saudi Chem. Soc., 2017, vol. 7, pp. 761–775. https://doi.org/10.1016/j.jscs.2015.09.003
Biradar, J.S. and Somappa, S.B., Pharm. Lett., 2012, vol. 4, pp. 344–348. http://scholarsresearchlibrary.com/archive.html
Tanveer Mahamad Alli, S. and Habtamu D., J. Chem., 2020, vol. 2020, pp. 1–9. https://doi.org/10.1155/2020/4358453
Mohamed, M.S., Youns, M.M., and Ahmed, N.M., Med. Chem. Res., 2014, vol. 23, pp. 3374–3388. https://doi.org/10.1007/s00044-014-0916-1
Akue-Gedu, R., Debiton, E., Ferandin, Y., and Meijer, L.M., Bioorg. Med. Chem., 2009, vol. 17, pp. 4420–4424. https://doi.org/10.1016/j.bmc.2009.05.017
Radwan, M.A. and El-Sherbiny, M., Bioorg. Med. Chem., 2007, vol. 15, pp. 1206–1211. https://doi.org/10.1016/j.bmc.2006.11.023
Selvam, T.P., James, C.R., Dniandev, P.V., and Valzita, S.K., Res. Pharm., 2012, vol. 2, pp. 01–09. https://doi.org/10.24959/ophcj.14.804
Mosmann, T., J., Immunol. Methods, 1983, vol. 65, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
National Committee for Clinical Laboratory, Nat. Commun. Clin. Lab. Stands., 1982, vol. 33, p. 242.
Romagnoli, R., Prencipe, F., Lopez-Cara, L.C., and Oliva, P., J. Enzyme Inhib. Med. Chem., 2018, vol. 33, pp. 727–742. https://doi.org/10.1080/14756366.2018.1450749
ACKNOWLEDGMENTS
The authors are thankful to the director of Indian Institute of Chemical Technology in Hyderabad for providing spectral data, thankful to chairman (Chaitanya Deemed to be University) for providing financial support, Warangal. The authors are thankful to the head, Department of Bio-technology, Kakatiya University Warangal for providing data of biological activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Juluru Bhaskar, Srinivas, B., Gouthami, D. et al. One-Pot Multi-Component Synthesis and Biological Evaluation of Novel Indole-Pyrimidine Derivatives as Potent Anti-Cancer and Anti-Microbial Agents. Russ J Bioorg Chem 47, 954–962 (2021). https://doi.org/10.1134/S106816202104004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202104004X