Abstract—
Based on the analysis of dose–response relationships under exposure to a wide range of copper sulfate concentrations, the metal tolerance of seed progeny was assessed using the root elongation test in two morphological forms of Taraxacum officinale Wigg. s.l. growing in background and technogenically transformed areas (industrial waste dumps). Since previous studies in the same areas showed that these forms differed in their abundance and ratio in the coenopopulations and in the level of copper accumulation, it was assumed that they would also differ in the metal tolerance of seed progeny. It was found that the average values of effective copper sulfate concentrations inhibiting root growth in seedlings by 10, 50, and 90%, did not differ between the study areas and between the morphological forms of dandelion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The ability of plant populations to maintain their abundance in an environment polluted with heavy metals (HMs) is widely known [1–4, etc.]. Although the operation period of industries as sources of environmental pollution is negligible on the evolutionary time scale, the results of studies on this problem indicate that plants adapt to anthropogenic transformation of the environment [5, 6]. Such an adaptation is based on reduction in the permeability of the root barrier upon the input of toxicants into the plant organism [7, 8] and increase in fertility, reproduction capacity, and other properties. Many studies [9–11] are devoted to the identification of differences between background and metal-tolerant populations and testing of the hypothesis that pollution can lead to morphological or physiological differentiation due to reproductive isolation, founder effect, or selection. However, there are only a few studies comparing the pathways of adaption between intraspecific forms [10, 14], which could have provided evidence for possible microevolutionary processes in cenopopulations under extreme environmental conditions.
For more than 20 years we have been studying Taraxacum officinale Wigg. s.l. cenopopulations in technologically transformed territories. In this course, the question has arisen as to what is the basis of their long-term functioning and stability of population parameters such as abundance, density, and age composition. Possibly, this may be due to a certain correction in physiological parameters related to tolerance for excess of HM and to selection for metal resistance.
The purpose of this study was to reveal differences in the response to copper sulfate concentrations suppressing root growth by 10, 50 and 90% (ЕСх) between morphological forms and cenopopulations of dandelion based on the analysis of dose–response relationships. Two hypotheses are discussed: (1) metal tolerance (i.e., ECx) increases along the gradient of technogenic transformation, and (2) metal tolerance differs between T. officinale morphological forms. The second hypothesis is based on the fact that the abundance ratio of the dandelion forms in the study cenopopulations differs between technologically transformed and background habitats.
MATERIALS AND METHODS
Object of study. Common dandelion (Taraxacum officinale Wigg. s.l.), family Asteraceae Dumort (Compositae Giseke) was used as a model species. With respect to the type of sexual reproduction, dandelion is an optional apomict, a triploid [15]. As a ruderal species, it has high seed productivity and viability of progeny. The study cenopopulations contain two morphological forms of dandelion: T. off. f. dahlstedtii Lindb. fil. and T. off. f. pectinatiforme Lindb. fil.
Characteristics of test plots. The seed material was collected in the zone exposed to airborne pollutant emissions from ferrous metallurgy enterprises in Nizhny Tagil (the Middle Urals). The sampled cenopopulations grow in areas that represent a gradient of technogenic transformation and differ in a number of physicochemical parameters of the soil, including the contents of mobile HM forms [16]. The chemical composition of the soil was analyzed by standard methods in the certified laboratory of the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences (certificate no. ROSS RU. 0001.515630). Based on the concentrations of heavy metals in soils and the calculated pollution index (total concentration factor Z expressed in the units relative to background values), technogenic load zones with test plots located within their limits were distinguished: background zone (plot F: Z = 1.0 rel. units), buffer zone (plot B: Z = 6.2 rel. units), and impact zone (plot I: Z = 22.8 rel. units). The zones are named in accordance with the UNEP nomenclature [17].
According to landscape and soil conditions, plot F is classified as agrozem. It is located in agrolandscapes with sod-podzolic soils (a fallow over 20 years old) characterized by medium fertility and base saturation, low to medium contents of mobile phosphorus and potassium, and medium to low content of hydrolysable nitrogen. Plots B and I (technozems) are located in technogenic landscapes, on industrial waste dumps over 45 years old. These are young soils formed akin to the burozem and lithozem types, with higher fertility. Base saturation is high; calcium prevails in the exchangeable complex; the contents of exchangeable phosphorus and potassium are high to very high; nitrogen content is low if sod is weak and high if sod is well developed. A detailed description of the landscapes, the agrochemical properties of the soils and subsoils in the study areas, and the content of heavy metals in the soil have been published previously [16].
Syntaxonomic characteristics of communities:F, unranked community Elytrigia repens [Stellarietea mediae/Molinio-Arrhenatheretea]; B, unranked community Trifolium pratense-Festuca pratensis [Arrhenatheretalia]; I, unranked community Tussilago farfara–Calamagrostis arundinacea [Dauco-Melilotion/Agropyrion repentis]. Herbaceous communities are serial, developing on fallows and waste dumps. Species richness of communities: F, 25 species; B, 47 species; I, 42 species. Total projective coverage of all species: F, 103.1%, B, 148.4%, I, 82.9%. According to the floristic composition, the community of plot B is at the meadow stage of succession, and that of plot I, at the grass stage. Differences in the species richness and coverage between the communities are apparently due not only to their successional age but also to the unfavorable edaphic conditions of plot I. Thus, the study cenopopulations grow in areas with different edaphic and phytocenotic conditions.
Evaluation of metal tolerance. Seeds were collected in June 2015 from 20 plants of each morphological form growing within the studied cenopopulations (120 plants in total). The progeny of a single plant was regarded as a family. Germination of seeds in roll culture was performed after the latent period [18], beginning from the 8th month after sampling. Experiments were carried out in 50-mL vessels with copper sulphate solutions at 22–24°C and illumination with fluorescent lamps for 12 h.
Due to the large amount of work, the study was divided into two experiments (March and May 2016). The first experiment included five families of each form and cenopopulation (30 in total), with one vessel containing 40 seeds used in each variant of treatment (family × concentration of copper sulfate). In the second experiment, five families of each form and cenopopulation (30 in total) and three vessels with 15 seeds per variant were used. Nine families were included in both experiments (to reveal possible systematic error).
As a control solution, 5 × 10-4 М Са(NO3)2 + 1 × 10–3 M KCl was used, in which all progeny were cultivated for the first 7 days. After measuring the maximum root length, the plants were divided into the control and experimental groups. Experimental solutions were prepared by supplementing the control solution with the following amounts of СuSO4: 0.08; 0.10; 0.13; 0.18; 0.26; 0.31; 0.34; 0.45; 0.51 mg/L. After culturing for 10 days, the maximal root length of each seedling was measured again, and root increment over this period was taken as the resultant parameter. A total of 12 539 seedlings were studied.
Statistical analysis was performed in R v. 3.2.3. Since any mathematical model is an approximation of a real biological process, the same data set can be equally well approximated by different models, each describing a certain part of the toxicant concentration range. As a result, a multimodel approach has become standard in modern toxicology. Its essence is that several competing models are used and their compliance with the original data is rigorously tested, e.g., by the Akaike information criterion (AIC). The average values of toxicometric parameters obtained with different models are calculated, and the goodness of fit is evaluated for each model. The use of only one model for the description of different data arrays may lead to errors, since the form of the dose–response relationship is usually individual.
In our case, the dependence of root growth on copper sulfate concentration for each family was approximated by the following functions: Weibull model with different asymmetric forms, log-normal model, Cedergreen–Ritz–Streibig model, Brain–Cousens model, and exponential decay model [19] using the package drc v. 2.2-1 [20]. The lower limit of root growth was taken as zero. The correspondence of each model to the initial data was evaluated using AIC values transformed into AIC weights indicative of the probability that a given model is the best fit among the models studied [21]. According to the simulation results, the list of the best-fit models for 42 families included particular cases of the Cedergreen-Ritz-Streibig model, the exponential attenuation model, and the Weibull model with different forms of asymmetry. For seven families, a successful approximation of relationship was possible only by using a linear model.
Based on each of the models, the EC10, EC50 and EC90 were calculated for an individual family (Fig. 1) and their average values were determined taking into account the AIC weights of the models used. Since each ECx sample consisted of nine to ten families, which was insufficient for parametric analysis, ECx for different dandelion forms and cenopopulations were compared using the Mann-Whitney test with false discovery rate (FDR) control in multiple testing.
An example of root growth–copper sulfate concentration relationship and its approximation by (1) Cedergreen–Ritz–Streibig, (2) linear, (3) Weibull I, (4 ) Weibull II, and (5) exponential decay models for one of the T. officinale families. Projections on the X axis are indicated for EC10 (dotted line), EC50 (dashed line), and EC90 (solid line) obtained with different models.
RESULTS AND DISCUSSION
The stable presence of Taraxacum officinale Wigg. s.l. in the test plots is indicative of sufficient seed productivity in all cenopopulations, allowing the species to maintain its abundance at a necessary level. The average long-term density of plants in cenopopulations (ind./0.25 m2) varied as follows: F, from 14.22 ± 1.87 to 44.67 ± 5.77; B, from 13.67±1.94 to 85.89±17.1; I, rom 11.44 ± 2.88 to 56.78 ± 4.52. Regardless of the level of pollution over a long period of time (11 observation years), cenopopulations were characterized as young normal [22]. Cyclic changes in the age composition were usually not accompanied by a transition of cenopopulations from one class to another and were oscillatory. It is only in some years that fluctuations in the age composition of cenopopulations resulted in their transition from the young to the maturing class. The stable predominance of young ontogenetic groups and the maintenance of ontogenetic spectra close to the baseline, regardless of the level of technogenic soil transformation, indicates the high stability of the ontogenetic structure of cenopopulations to different edaphic conditions and weather factors.
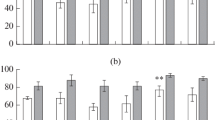
In the structure of all cenopopulations, the T. off. f. dahlstedtii form was more numerous. In the gradient of change in the quality of the environment, its proportion decreased from 84.3 ± 1.2% in F to 70.7 ± 3.0% in B and to 62.5 ± 3.7% in I. Morphological forms differed in the levels of accumulation of HMs, including copper (Table 1). Although the concentrations of copper ions in the underground and aboveground organs of T. officinale were not maximal for this species, they were comparable to the concentrations in plants from habitats polluted by emissions from motor vehicles [23, 24, etc.] or by the steel and coke industries [25]. The study of the intraspecific differentiation of plants with respect to their accumulative features showed that at low concentrations of copper in the soil (0–100 µg/g) its contents in the roots did not differ significantly between the two dandelion forms. At higher copper concentrations in the soil (above 100 µg/g), its contents in both roots and leaves were significantly higher in f. pectinatiforme (p < 0.05).
Differences in HM accumulation by plants along the gradient of technogenic load reflects the response of the population system as a whole to changes in the quality of the environment. Since the studied areas differ in the level of HM in the soils, the question remains open as to whether there is selection of seeds for metal tolerance.
The viability and metal tolerance of seed progeny in Taraxacum officinale Wigg. s.l. were already studied previously (1997–1998) in the same zone near Nizhny Tagil, in similar plots with different levels of soil pollution with HMs [26]. Using the root elongation test in roll culture treated with the same dose of CuSO4 in aqueous solution (100 mg/L) selected in a preliminary experiment, statistically significant differences in the root length of seedlings were revealed between the two morphological forms of this species growing in the gradient of soil metal pollution. However, a more correct assessment of the metal tolerance of plants is possible by using the root elongation test to evaluate the dose-effect relationships under exposure to a wide range of metal concentrations [6, 27, 28]. This approach makes it possible to estimate heterogeneity of seed progeny with respect to the proportion of metal-resistant seeds it contains.
The example of nine families included in both variants of the experiment showed the absence of a systematic error in the estimates of effective concentrations, which could have resulted from possible differences between the conditions of different experiments (nonparametric sign test: Z = 0.76; p = 0.44). The number of surviving seedlings remained unchanged as copper sulfate concentration increases up to the maximum. The mean values of the same effective concentrations for both T. officinale forms did not differ between the plots with different levels of transformation (p > 0.91; Fig. 2). This fact indicates that the seed progeny of plants from even the most polluted site does not possess an increased tolerance to excess copper concentrations. This fact is in agreement with the equal levels of copper accumulation in the seeds of both T. officinale forms from the background and impact areas (Table 1).
No significant differences in metal tolerance were revealed between different T. officinale forms within each plot (p > 0.94), despite different copper contents in their underground and aboveground organs under impact conditions (Table 1, Fig. 2). It is possible that the absence of differences in the response of the seedling root system between the two forms of dandelion is due to the fact that the limiting dose of copper (ED90) in this study is almost an order of magnitude lower than the dose used previously [26]. It is not excluded that such differences may manifest themselves in root elongation tests with significantly higher concentrations of metal that exceed their real concentrations in the soil. It should be taken into account that the high variability of responses from the dandelion root systems has also been noted by other authors, according to which the reproductive characteristics of this species may change by a factor of several tens depending on weather and time parameters [29].
It is also possible that, at the soil pollution levels observed in the study areas, an important role in the formation of tolerance to excess HMs is played by mycorrhizal associations with stress-resistant mycobionts. This hypothesis was proposed previously [30] on the basis of microscopic analysis of roots in T. officinale from polluted habitats.
CONCLUSIONS
Analysis of the results based on a multimodel approach showed that high concentrations of copper (up to and above 900 μg/g) in soils have no direct influence on the development of increased metal tolerance (ECx) in the seed progeny of Taraxacum officinale. Apparently, the ratios of the two morphological forms of Taraxacum officinale f. dahlstedtii and T. off. f. pectinatiforme in cenopopulations growing in the gradient of technogenic transformation of the environment are accounted for not only by differences in the metal tolerance of their seed progeny. Changes in the intraspecific structure of populations are probably explained by changes in edaphic and cenotic conditions, including those caused by elevated levels of HMs in the soils. This indicates the need for further research on the intraspecific variability of T. officinale, in particular with respect to indices of cenotic competitiveness and tolerance to abiotic environmental factors (moisture, soil fertility, etc.).
REFERENCES
Pozolotina, V.N., Antonova, E.V., Bezel’, V.S., et al., Pathways of adaptation of common dandelion cenopopulations to long-term chemical and radiation effects, Russ. J. Ecol., 2006, vol. 37, no. 6, pp. 402–407. https://doi.org/10.1134/S1067413606060063
Trubina, M.R., The survival strategy of Crepistectorum L. under conditions of chronic atmospheric pollution, Russ. J. Ecol., 2011, vol. 42, no. 2, pp. 103–109. https://doi.org/10.1134/S1067413611020135
Kaznina, N.M., Titov, A.F., Batova, Y.V., and Laidinen, G.F., The resistance of plants Setariaveridis (L.) Beauv. to the influence of cadmium, Biol. Bull., 2014, vol. 41, no. 5, pp. 428–433. https://doi.org/10.7868/S000233291405004.x
Pozolotina, V.N., Antonova, E.V., and Shimalina, N.S., Adaptation of greater plantain, Plantago major L., to long-term radiation and chemical exposure, Russ. J. Ecol., 2016, vol. 47, no. 1, pp. 1–10. https://doi.org/10.1134/S1067413616010124
Ernst, W.H.O., Evolution of metal tolerance in higher plants, For. Snow Landsc. Res., 2006, vol. 80, no. 3, pp. 251–274.
Dulya, O.V., Mikryukov, V.S., and Vorobeichik, E.L., Strategies of adaptation to heavy metal pollution in Deschampsia caespitosa and Lychnis flos-cuculi: Analysis based on dose–response relationship, Russ. J. Ecol., 2013, vol. 44, no. 4, pp. 271–281. https://doi.org/10.1134/S1067413613040036
Bezel, V.S., Zhuikova, T.V., and Pozolotina, V.N., The structure of dandelion cenopopulations and specific features of heavy metal accumulation, Russ. J. Ecol., 1998, vol. 29, no. 5, pp. 331–337.
Jin, X.F., Yang, X.O., Islam, E., et al., Effects of cadmium on ultrastructure and antioxidative defense system in hyperaccumulator and non-hyperaccumulator ecotypes of Sedum alfredii Hance, J. Hazard. Mater., 2008, vol. 156, nos. 1–3, pp. 387–397. https://doi.org/10.1016/j.jhazmat.2007.12.064
Baker, A.J.M. and Dalby, D.H., Morphological variation between some isolated populations of Silene maritima within the British-Isles with particular reference to inland populations on metalliferous soils, New Phytol., 1980, vol. 84, no. 1, pp. 123–138.
Pozolotina, V.N. and Severyukhina, O.A., The reproductive capacity of plants in a gradient of chemical environmental pollution, Russ. J. Ecol., 2002, vol. 33, no. 6, pp. 407–412. https://doi.org/10.1051/radiopro/2002193
Jimenez-Ambriz, G., Petit, C., Bourrie, I., et al., Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: Role of gene flow, selection and phenotypic plasticity, New Phytol., 2007, vol. 173, no. 1, pp. 199–215.
Abratowska, A., Wasowicz, P., Bednarek, P.T., et al., Morphological and genetic distinctiveness of metallicolous and non-metallicolous populations of Armeria maritimas L. (Plumbaginaceae) in Poland, Plant Biol., 2012, vol. 14, no. 4, pp. 586–595. https://doi.org/10.1111/j.1438-8677.2011.00536.x
Dulya, O.V. and Mikryukov, V.S., Changes in the shape and size of leaves in Lychnis flos-cuculi L. along a polymetallic pollution gradient, Vestn. Tomsk. Gos. Univ., 2013, vol. 18, no. 3, pp. 778–782.
Vorobyev, G.V., Alyabyev, A.Y., Ogorodnikova, T.I., Khamidullin, A.F., et al., Adaptive properties of the dandelion (Taraxacum officinale Wigg. s.l.) under conditions of air pollution by motor vehicle exhausts, Russ. J. Ecol., 2014, vol. 45, no. 2, pp. 90–94. https://doi.org/10.7868/S0367059714020103
Zhuikova, T.V., Severyukhina, O.A., Bezel’, V.S., and Prushinskaya, N.M., Responses of Taraxacum officinale s.l. male gametophyte to chemical pollution of the environment, Sib. Ekol. Zh., 2007, no. 3, pp. 511–516.
Zhuikova T.V., Meling E.V., Kaigorodova S.Y. et al., Specific features of soils and herbaceous plant communities in industrially polluted areas of the Middle Urals, Russ. J. Ecol., 2015, vol. 46, no. 3, pp. 213–221. https://doi.org/10.7868/S0367059715030130
Global Environmental Monitoring System (GEMS): Action Plan for Phase I. SCOPE Rep. 3, Toronto, Canada, 1973.
Shkol’nik, M.Ya. and Alekseeva-Popova, N.V., Rasteniya v ekstremal’nykh usloviyakh mineral’nogo pitaniya: ekologo-fiziologicheskie issledovaniya (Plants under Extreme conditions of Mineral Nutrition: Ecophysiological Studies), Leningrad: Nauka, 1983.
Ritz, C., Toward a unified approach to dose–response modeling in ecotoxicology, Environ. Toxicol. Chem., 2010, vol. 29, no. 1, pp. 220–229. https://doi.org/10.1002/etc.7
Ritz, C. and Streibig, J.C., Bioassay analysis using R, J. Stat. Softw., 2005, vol. 12, no. 5, pp. 1–22.
Burnham, K.P. and Anderson, D.R., Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, New York: Springer, 2002.
Zhuikova, T.V., Responses of cenopopulations and herbaceous plant communities to chemical pollution of the environment, Extended Abstract of Doctoral (Biol.) Dissertation, Yekaterinburg, 2009.
Petrova, S., Yurkova, L., and Velcheva, I., Taraxacum officinale as a biomonitor of metals and toxic elements (Plovdiv, Bulgaria), Bulg. J. Agric. Sci., 2013, vol. 19, no. 2, pp. 241–247.
Giacomino, A., Malandrino, M., Colombo, M.L., et al., Metal content in dandelion (Taraxacum officinale) leaves: Influence of vehicular traffic and safety upon consumption as food, J. Chem., 2016, vol. 2016, p. 9. https://doi.org/10.1155/2016/9842987
Królak, E., Accumulation of Zn, Cu, Pb and Cd by dandelion (Taraxacum officinale Web.) in environments with various degrees of metallic contamination, Pol. J. Environ. Stud., 2003, vol. 12, no. 6, pp. 713–721.
Zhuikova, T.V., Pozolotina, V.N., and Bezel’, V.S., Different strategies of plant adaptation to toxic environmental pollution with heavy metals: An example of Taraxacum officinale s.l., Russ. J. Ecol., 1999, vol. 30, no. 3, pp. 189–196.
Shitikov, V.K., Terekhova, V.A., Uzbekov, B.A., et al., Dose–response models for ecological risk assessment under technogenic soil pollution, Printsipy Ekologii, 2015, no. 3, pp. 73–88.
Kopittke, P.M., Blamey, F.P., Asher, C.J., and Menzies, N.W., Trace metal phytotoxicity in solution culture: A review, J. Exp. Bot., 2010, vol. 61, no. 4, pp. 945–954.
Pozolotina, V.N. and Antonova, E.V., Temporal variability of the Taraxacum officinale seed progeny from the East-Ural Radioactive Trace: Is there an interaction between low level radiation and weather conditions?, Int. J. Radiat. Biol., 2017, vol. 93, no. 3, pp. 330–339.
Maleci, L., Buffa, G., Wahsha, M., and Bini, C., Morphological changes induced by heavy metals in dandelion (Taraxacum officinale Web.) growing on mine soils, J. Soils Sediments, 2014, vol. 14, no. 4, pp. 731–743. https://doi.org/10.1007/s11368-013-0823-y
Funding
The work was performed under state assignment for the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences and supported by the Integrated Research Program of the Ural Branch of the Russian Academy of Sciences (project no. 18-4-4-9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by V. Mittova
Rights and permissions
About this article
Cite this article
Bezel’, V.S., Zhuikova, T.V., Dulya, O.V. et al. Intraspecific Variability of Metal Tolerance in Taraxacum officinale Wigg. s.l. Seed Progeny: Analysis Based on Dose–Response Relationship. Russ J Ecol 50, 331–336 (2019). https://doi.org/10.1134/S1067413619040052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413619040052