Abstract
In the world practice, the organic carbon content (Corg) in the soils containing carbonates is measured in different ways. We have analyzed the methods for solving this problem including the state-of-the-art approaches, such as thermogravimetry, differential scanning calorimetry, and spectroscopy. As is shown, the presence of CaCO3 does not prevent the Corg measurement with dichromatometric method (Tyurin and Walkley–Black variants). The disadvantages of this method comprise the laborious analysis, constant presence of operator, incomplete oxidation of organic compounds, and environmental pollution. The measurement of soil weight loss-on-ignition (LOI) is economical and rapid but overestimates Corg content because of inadequacy of the conversion factor of 1.724, the presence of adsorbed and chemically bound water, as well as mineral components decomposing at T = 105–550°C. The most relevant solution for assaying the Corg content in carbonate soils is to use an analyzer and a calcimeter although the accuracy of Corg measurements in the presence of carbonates is significantly reduced because the errors of two methods are quadratically summed. A high cost of the device, maintenance, verification, and repair limit its widespread use in soil laboratories. The content of soil carbonates can be measured using both gravimetric (LOI) and volumetric (calcimeter) methods. The latter method is preferable for the soils with the prevalence of CaCO3 in carbonates. The preliminary removal of carbonates from soil samples is labor-intensive and can cause a partial loss of Corg via acid extraction. A high cost of the instruments and the absence of the libraries of soil spectra hinder the development of Vis-NIR and MIR spectroscopy as an alternative to wet chemistry methods. Further comparative studies will give a deeper insight into the spatial patterns in the distribution of soil organic carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In soil, carbon is present in the organic matter and individual organic compounds (Corg), as well as in different species of carbonates, mainly those of calcium and magnesium (Cinorg). On a global scale, carbonate soils cover over 30% of the Earth’s surface [27, 48, 79] and approximately one-third of the total carbon is represented by its inorganic species [13].
Carbon continuously circulates in the biosphere as a result of chemical, physical, and biological processes. Two forms of carbon are tightly interrelated [88, 96]. On the one hand, carbonates slow down the cycling of organic carbon via Corg stabilization by the physical protection (occlusion) against degradation [64]. On the other hand, the organic acids formed via functioning of biota [5] dissolve carbonates. Carbon as the carbon dioxide formed in the reaction is eliminated from the system [7, 8, 58, 60, 80, 109]. A tight correlation between the contents of Corg and Cinorg (r = –0.983, p < 0.000, n = 9 [6]), characteristic of the soils of the Polar Urals (Bolshoi Paipudynsky Ridge) confirms the course of these processes (Fig. 1).
Correlation between the organic and inorganic carbon contents, ω(Corg) and ω(Cinorg), in the mountain–tundra carbonate soils of the Polar Urals (according to [6]). Hereinafter, ω(Cinorg) is measured volumetrically.
The literature sources give controversial information about the correlation between different carbon species, reporting both positive and negative (less tight) correlations as well as its absence. This is determined by soil properties and genesis, origins of organic and inorganic compounds, climate, land use and management practices [51, 56, 107]. The example of the Bolshoi Paipudynsky Ridge is unique because the soils there have developed on the eluvial–colluvial derivates of calcareous rocks with a uniform composition and the content (ω) of CaCO3 reaching 100% (correspondingly, ω(Cinorg) = 12%).
The organic and inorganic carbon cycle responds to climate change and contributes to its regulation. Carbonates are regarded as a reliable paleoecological indicator and are used in regional paleoclimatic studies [108, 112]. The system of soil Cinorg is a key sink of atmospheric CO2 [24, 44, 59, 110, 111]. On the other hand, voluminous data set demonstrates a lateral removal of dissolved carbon of carbonates. Note that the scale of Cinorg aqueous export as H2CO3, \({\text{HCO}}_{3}^{ - }\), and \({\text{CO}}_{3}^{{2 - }}\) can severalfold exceed the level of its stabilization [43, 66, 77].
Thus, the assessment of both soil carbon species within the total pool of this element becomes ever more relevant in the epoch of global changes [27, 98]. The analysis of the papers in this area demonstrates a disproportionally larger number of the studies on the methods for measuring Corg in carbonate-free soils. However, the comparison of the approaches to measure the Corg content in the presence of carbonates is rather ignored [34, 87]. Meantime, such these studies are necessary to integrate the global data aiming to monitor the current state of soil carbon and forecast its changes caused by natural and anthropogenic factors [72, 86]. The absence of the comparable data is one of the reasons underlying a poor accuracy of the global maps for the stock of soil organic carbon, including the carbonate-containing soils [40].
The efforts of the Global Soil Laboratory Network (GLOSOLAN) with the Global Soil Partnership of the Food and Agricultural Organization (FAO) are aimed at resolving the problems in standardization and unification of the methods for quantification soil properties. Over 5 years, the worldwide work of scientists brought about more than 20 protocols for harmonizing soil analytical data (https://www.fao.org/global-soil-partnership/glosolan/), and the work is continuing.
The goal of this work was to critically analyze the techniques for measuring the Corg content in carbonate soils including their advantages and disadvantages.
FACTORS THAT INFLUENCE THE MEASUREMENT ACCURACY OF CARBON CONTENT IN THE SOIL
In the world practice, the organic and inorganic carbon content in soils is measured with different methods. When choosing a particular method, the specific chemical features of assayed soil, the necessary measurement accuracy, the time necessary for analysis, its cost, ecological friendliness (safety for engineers and environment), and the possibility to measure other characteristics in the same sample (for example, N, S, O, and H) are taken into account.
All techniques for determination of Corg content are based on its oxidation to carbon oxide (IV). Some methods utilize the combustion of organic compounds (dry technique) based of measuring the amount of CO2 and the Corg oxidation methods in solutions (wet techniques). The latter imply the assessment of the amount of oxidizing agent necessary to transform Corg into CO2 or the amount of the reduced species of the used oxidizer formed in redox reaction [3].
Different equipment levels of chemical laboratories limit the comparison of the Corg contents measured with alternative methods. The change and development of analytical methods also contribute to the problems in a long-term monitoring of individual areas [49, 50]. However, the use of different methods is not the only reason for the discrepancies of results [67].
Free rendering of analytical protocols resulting from the absence of comprehensive descriptions for all procedures causes serious difficulties in the interpretation of the time series of soil carbon dynamics. Other factors also contribute to the incompatibility of soil data, in particular,
(1) Changes in field sampling schemes [69] and natural heterogeneity of soil formation conditions [23, 74];
(2) Different sample preparation procedures, including the volume of undecomposed plant residues removed [35, 45, 106], choice of particle size (sieve mesh size), and insufficient sample uniformity [36, 40];
(3) Selection of the weight of the sample that determines the accuracy of measurement result (Horwitz’s trumpet, that is, an increase in the measurement error with a decrease in the concentration of the assayed substance in the analyzed sample) [104];
(4) Change in operators in laboratory; and
(5) Duration and conditions of soil storage. This factor requires more attention in future studies [16]. The change in sample composition during storage can result from microbiological activity. According to Blake at al. [19], the repeated analysis of the soils stored for approximately 30 years in closed glass containers did not show any changes in the total carbon content. However, another research team [32, 103] reported a decrease in carbon content caused by continuous CO2 emission.
METHODS FOR MEASURING CARBON CONTENT IN SOILS
Carbon high-temperature catalytic oxidation (HTCO) in analyzers. This method for measuring total carbon (Ctot) content in soil in a TOC analyzer (combustion of organic compounds and decomposition of carbonates at a temperature over 1000°C) is regarded as a gold standard [26, 31, 36].
This method has a number of advantages over other techniques for assaying soil carbon, in particular,
(1) It guarantees a complete oxidation of soil organic carbon and decomposition of inorganic carbon;
(2) It is selective since the intensity of analytical signal (area of CO2 peak in a chromatographic pattern) is independent of the presence of other soil components;
(3) It is suitable for a wide range of carbon content (from 0.1 to 100%);
(4) Reference samples are available for calibration of analyzers (both individual organic compounds, state (SRS) and departmental (DRS) reference samples of soils, plant materials, and other solid objects are used);
(5) It is rapid and, thus, allows a batch of soil samples (up to 100 samples) placed in a sampler to be assayed in an automated mode; and
(6) It has a high measurement accuracy, which depends on the carbon content in soil. The relative errors (δ) of ω(Ctot) amount to ±δ = 23, 15, 10, and 3.5% for the ω(Ctot) ranges of 0.1–2, 2–5, 5–30, and >30%, respectively.Footnote 1
Correspondingly, the method for assessing the total soil carbon content in an analyzer is frequently used as the reference technique when comparing the experimental data on the carbon content measured with different methods [86].
A disadvantage of this method is that the conditions for separate measurements of Corg and Cinorg are not worked out in detail for most of the analyzer types. That is why, an additional estimation of carbon in carbonate ions is necessary when assaying carbonate soils. In this case, the accuracy of ω(Corg) measurements decreases because the errors of two methods are quadratically summed:
where δ is the relative measurement error for Corg content and ∆, absolute measurement fraction error for Corg content.
Note that the relative error of the characteristic calculated according to the difference between two values is this case depends in a statistically significant manner on the ratio of minuend and subtrahend. Find below the two methods for solving this problem: preliminary removal of carbonates from soil samples and the measurement of the carbon in inorganic compounds by volumetric method (VMM) using a calcimeter.
Implementation of the target problem using carbon HTCO in an analyzer in the presence of carbonates implies three measuring devices: analyzer, calcimeter (or incinerator), and analytical balance. Together with the cost of expendables, maintenance, testing, and repair, carbon HTCO technique in analyzers may well be rather expensive and financially unavailable for many laboratories [61].
Applied soil science actively uses the analogs of analyzers. In particular, Dokuchaev Soil Science Institute tested AN-7529 rapid analyzer (Gomel) for assaying total carbon as early as in the beginning of the 1980s [2]. As compared with CNS analyzers, the soil assay with this device was considerably (at least tenfold) cheaper; however, it could not simultaneously determine the contents of other elements. In addition, the upper limit of ω(Corg) determination was only 10%. In this approach, the problem of additional Cinorg determination was solved by measuring the CO2 formed when treating the other soil weighed sample with HClO4 (coulometric titration).
Removal of carbonates from the soil sample. To assay soil in an analyzer, excess hydrochloric acid is added in a dropwise manner to a weighed sample (1.5–20 mg depending on the Corg content) until the gas (CO2) ceases emitting. The assay is conducted in silver containers. Two weighed samples are used when it is necessary to assess ω(Cinorg) by measuring Ctot in the initial sample and Corg after the removal of carbonate anions.
A similar procedure of carbonate removal from soil sample is also used in other methods [11, 34, 63, 83]. Different acids (HCl, H2SO3, and H3PO4) are used to decompose carbonates [22]. However, the preliminary removal of carbonates is laborious and can result in a partial loss of Corg because of the acid extraction when removing the excess of acid by decantation [10, 84]. Centrifugation is recommended to minimize the losses in organomineral colloid particles [22]. Another acidification method is to fumigate the soil sample placed into a capsule with acid fumes [34, 52].
The technique of in situ acidification consists in the treatment of soil samples with acid solution followed by their drying to avoid the loss in acid-soluble components. The in situ acidification methods allow the losses in acid-soluble Corg to be prevented because they do not require the repeated weighing of the samples treated with the acid [95].
Acidimetry, utilizing the back-titration of residual acid after the reaction with assayed sample, is another possible approach [71].
Gravimetric measurements (weight loss-on-ignition, LOI) with CO2 emission was also proposed as a more rapid, inexpensive, and accurate variant for determining the carbonate carbon [47, 78]. All acid-soluble compounds are removed after soil treatment with the acid solution when eliminating its excess and the carbonate anions are replaced by anions of the used acid when using its minimum amount; correspondingly, the nature of the compounds via LOI is vague.
Taking into account that all approaches are based on the interaction of soil carbonates with soil, the limitations include different duration of the reaction for different carbonate types, which depends on their reactivity. In addition, these methods are often insufficiently accurate at a low content of carbonates [71].
Volumetric methods for measuring soil inorganic carbon content with the help of calcimeter. In this approach, the carbonates present in soil are decomposed with hydrochloric acid solution. The pressure in the reaction container connected with burette increases with CO2 emission and the water level in the burette rises. The change in water level is the measure of CO2 amount. Calcimeter is calibrated using carbonate weighed samples. The calcimeter by Eijkelkamp (The Netherlands)Footnote 2 has shown a good performance; this device allows for simultaneous measurement of carbonates in five samples [6]. Calcimeter is rather simple in use. The results of measurements are related to CaCO3 content; correspondingly, calcimeter is preferable when analyzing soils with the prevalence of CaCO3 among carbonates. However, the problems associated with the soils containing MgCO3 and Na2CO3 are solvable by expressing the measurement results as Cinorg content. Note that the compounds that interact with HCl solution with emission of gas products (SO2, H2S, and so on) can interfere with the measurements.
The relative error when measuring the content of calcium carbonate and, correspondingly, Cinorg, for ω(CaCO3) = 0.5–5% and ω(Cinorg) = 0.06–0.6% amounts to δ = 20%; for ω(CaCO3) = 5–15% and ω(Cinorg) = 0.6–1.8% amounts to δ = 15%; and for ω(CaCO3) > 15% and ω(Cinorg) > 1.8%, amounts to δ = 10%.
As is shown above (Eq. (1)), the estimation of ω(Corg) according to the difference between Ctot and Cinorg contents gives an increased relative error of Corg content. As an example, Table 1 lists the theoretical (calculated according to the errors of the corresponding methods) values of the relative error in ω(Corg) measurements in carbonate-rich soils [87]. The error depends on the share of Cinorg in the total carbon content \(\left( {\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{inorg}}}}}} \right)}}{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{tot}}}}}} \right)}}} \right)\). At \(\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{inorg}}}}}} \right)}}{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{tot}}}}}} \right)}}\) ≤ 40%, the error of Corg measurement does not exceed 20%. In turn, the error of 70–290% is characteristic of the soils with the prevalence of carbonates (samples 18–23, Table 1), where \(\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{inorg}}}}}} \right)}}{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{tot}}}}}} \right)}}{{\;}}\) = 80–90%).
Dichromatometric method (modifications of Tyurin and Walkley–Black techniques). The disadvantages here comprise laboriousness, constant presence of operator, and environmental pollution [98].
The limitations of this method are the presence of Сl–, Mn2+, and Fe2+ in soils, as well as of the components resistant to oxidation, such as charcoal, and uncertainty of the conventional zero degree of organic carbon oxidation in the reaction with potassium dichromate [38, 39].
However, the evident difficulties and limitations do not prevent an active applied use of dichromatometric method implemented in two variants the most widespread in world soil science—the Tyurin and Walkley–Black protocols. According to the reference document,1 the organic carbon or organic matter (OM) is measured by the oxidation with dichromate anions in the range of ω(Corg) = 0.17–8.7% (ω(OM) = 0.3–15%).
According to this reference protocol, the Corg nonoxidizable by potassium dichromate at a measurement accuracy of 20% is taken into account with the help of correction factors amounting to 1.15 and 1.31 (Tyurin and Walkley–Black methods, respectively). In our view, the discrepancy of correction factors for two variants of the wet method is associated with a higher dispersion of the soil solid phase and the heating duration of the system. These factors lead to more efficient oxidation of organic carbon in the Tyurin variant as compared with Walkley–Black one [4, 86].
Although several authors recommend using a universal correction factor for the Walkley–Black method [49, 50, 61], a considerable number of their opponents deny the universality of this factor. Numerous published sources propose other values of correction factors from 1.2 to 1.85 [68, 75, 83, 86]. Many experts [25, 33, 49, 55, 61] believe that the value of correction factor depends on the soil type, Corg range, and particle size composition. A low level of carbon reduction can be associated with the local factors, such as a considerable amount of charred organic matter after fires [91]. In addition, some researchers believe that the value of correction factors in some samples depends on the climatic conditions of a particular year. Reithmaier et al. [77] believe that the search for correction factors for a particular plot and year is the best strategy for increasing the data quality. However, this solution requires additional financial and human resources.
There are different expert opinions on the effect of carbonates on the results of ω(Corg) dichromatometric assessment. Apesteguia et al. [10] regard the presence of carbonates as the limitation of this method. According to Vorob’eva [1], the carbonates of alkaline-earth metals react with the chromic mixture (neutralize the acid) and as a rule do not interfere with the measurement of organic carbon. The current state standardFootnote 3 has no information about the specificity of analyzing carbonate-containing soils. The Walkley–Black protocol implies the limitation on assays for soils containing considerable amounts of carbonized materials [37]; however, the threshold limit CaCO3 content is not indicated.
A high variability of the measured organic carbon content is reported for the case when the inorganic carbon content is considerably higher than the organic carbon because carbonates are rather recalcitrant [68]. However, the experimental data demonstrate that carbonates do not influence the dichromatometric ω(Corg) assessment [87]. This suggests that the measurement limits and the error characteristic of the Tyurin method modification and Walkley–Black method1 are applicable to the carbonate soils as well.
The first evidence is the comparison of ω(Corg) in carbonate-free reference soil samples (state reference sample GSO 10413-2014 and departmental reference samples OSO 11201, OSO 21401, and OSO 29106, Russia) and their mixtures with CaCO3. A threefold excess of carbon amount in carbonates over the organic carbon has no effect on the measured Corg content using the Tyurin and Walkley–Black methods [87]. Presumably, this fact is associated with the specific fabric of calcareous rocks.
The other evidence is the comparison of ω(Corg) in the soils initially containing carbonates and the samples, in which carbonates are removed with a sufficient amount of sulfuric acid. The Tyurin and Walkley–Black methods were used for measuring ω(Corg) and the difference between the measured ω(Ctot) by HTCO in analyzer and ω(Cinorg) by VMM in calcimeter was taken as the reference ω(Corg) value (Fig. 2). As is evident from Fig. 2, the ω(Corg) values measured with the Tyurin and Walkley–Black methods in the initial soils and the samples with removed carbonates do not deviate from the reference values by more than 35%. The difference of the values for the initial sample and the sample after removal of carbonates by both the Tyurin and Walkley–Black methods does not exceed 7%.
Correlation of the organic carbon content ω(Corg)2 measured by (1) Walkley–Black and (2) Tyurin methods with the reference value ω(Corg)1 = ω(Ctot) – ω(Cinorg)1 in (a) initial soil samples and (b) the carbonate-free samples. Dashed lines show the boundaries of relative error δ = ±35%. Hereinafter, ω(Ctot) is measured with high-temperature catalytic oxidation.
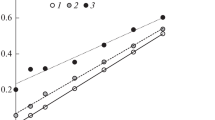
In the current worldwide practice in soil science, the ever more attention is paid to the assurance of soil data quality, both external (interlaboratory comparisons or loopback tests) and internal (https://www. fao.org/global-soil-partnership/glosolan/). As is known, the latter is implemented using either an alternative (reference) method or reference samples. The range of the certified Corg values for reference samples is ω(Corg) = 0.2–9%. Any state and departmental reference samples for higher Corg values are absent. Correspondingly, another approach (HTCO together with VMM [87]) was used to assess the measurement quality of dichromatometric Corg assessment in the carbonate soils with a wide range of Corg content by elevating the upper limit of the Corg content from ω(Corg) = 8.7% (ω(OM) = 15%)1 to ω(Corg) = 46% (ω(OM) = 79%). The overall data array for ω(Corg) was divided into two subranges, namely, 0.17–10% (99 samples; of them, 18 ones containing carbonates) and 10–46% (54 samples; of them, four containing carbonates) (Fig. 3). The content of soil inorganic carbon falls into the range of 0.3–10%.
Correlation between the organic carbon content ω(Corg)2 measured with the Walkley–Black method and the reference value ω(Corg)1 = ω(Ctot) – ω(Cinorg)1: (1) carbonate-free soils with ω(Corg)1 of 0.17–10% and (2) 10–46% and carbonate soils with ω(Corg)1 of (3) 2–10% and (4) 10–46%. Dashed lines show the boundaries of relative error δ = ±25%.
In the first subrange, the relative deviation of the Corg values measured with the Walkley–Black method from the difference between Ctot and Cinorg contents amounts to over 25% for the samples with ω(Corg) ≤ 2% (Fig. 4a). Thus, the error of the reference method exceeds the specified error for dichromatometric measurement, amounting to 20%,1 which is unacceptable. In this case, the quality of the ω(Corg) measurement in carbonate soils with the Walkley–Black method1 using two devices (analyzer and calcimeter) is assessable only for ω(Corg) > 2%. This experimental conclusion confirms and refines the calculation of theoretical relative errors (taking into account the errors of all methods): the measurement quality can be controlled for the samples with the lower ω(Corg) content of 2% and \(\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{inorg}}}}}} \right)}}{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{tot}}}}}} \right)}}\) not exceeding 30% (braced in Fig. 5). Consequently, the quality of dichromatometric data for the carbonate soils with ω(Corg) = 0.17–2% should be controlled only using the reference samples certified according to this method. However, the state reference samples of this type have been never met during long-term work of the analytical laboratory.
Calculated relative error (δ) of the measured ω(Corg) contents of (1) 1%, (2) 2%, and (3) 5% in carbonate-containing soils using two methods depending on \(\frac{{{{\omega }}{{{\left( {{{{\text{C}}}_{{{\text{inorg}}}}}} \right)}}_{1}}}}{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{tot}}}}}} \right)}}\).
As for the carbonate-free soils and the carbonate soils with ω(Corg) > 2%, including the soils of the second subrange with ω(Corg) > 10%, the deviation in most cases (93 of 99) does not exceed 20% (Fig. 4b). Moreover, the examined soils with ω(Corg) > 10% displayed a high correlation of the measured characteristics (r > 0.9, p < 0.000, and n = 54) with the reference values (the difference between HTCO and VMM values). Consequently, an additional metrological study of soil organic horizons is promising to certify the Tyurin and Walkley–Black methods for assessing the Corg content in soil at ω(Corg) > 8.7% (ω(OM) > 15%).
Note that the majority of the Corg values measured with Walkley–Black method at its content exceeding 2% are overestimated as compared with the reference value (Fig. 5b). The factors that take into account an incomplete oxidation of organic carbon by dichromate ions under the Walkley–Black conditions amount on the average to 1.24 rather than 1.3. Dichromate ions oxidize even higher part of organic compounds in the range of ω(Corg) = 10–46% (Fig. 3, deviation from the y = x line). In this case, the correction line decreases from 1.3 to 1.15. This additionally confirms the variability in the value of this correction factor, which depends on the nature of soil organic compounds and, consequently, on the soil type, conditions of OM formation, range of ω(Corg) values in sample, and so on.
Note in addition that the absolute deviation for the results of ω(Corg)2 obtained with the Walkley–Black method (Fig. 4) in carbonate-containing soils is determined by the errors of three methods: θ(∆) = ω(Ctot) – ω(Cinorg) – ω(Corg)2. That is why, the relative deviation θ(δ) is that high at a low Corg content (Fig. 4a).
Gravimetric method (soil weight LOI) is regarded as an economical, ecologically friendly, rapid, simple, and relatively inexpensive technique for assessing the content of both carbon species [15, 100, 105, 108]. This method requires conventional equipment available in the majority of laboratories: analytical balance, drying cabinet, incinerator, and porcelain crucibles. This method is based on soil ignition at a high temperature for a certain time interval and is mainly used to measure the OM content in soils at its content over 15% (mats, litters, peat, and so on) [1], as well as to recalculate the data of elemental analysis per incinerated soil.
The adherence to temperature regime is the most important requirement especially when measuring ω(Corg) in carbonate-containing soils. However, the relevant literature and reference protocols lack any uniform recommendations for heating conditions. As it is believed, the optimal temperature is determined, on the one hand, by the degree of OM removal (T = 500–600°C) and, on the other hand, by minimization of the loss in weight caused by the decomposition of carbonates (T = 750–850°C). Some guidelines recommend the temperature of 450°C [42] or 500°C [63]. However, a complete decomposition of soil organic compounds is reached at T= 550°C [54]. The presence of copper, iron, manganese, and magnesium carbonates as well as complex carbonates decomposing at T = 380–600°C interferes with the accuracy in measuring ω(Corg) [53, 73, 101].
This method is used to directly measure the soil weight LOI (as is believed, all organic compounds incinerate at this temperature); correspondingly, the share of Corg in the removed compounds is necessary to calculate ω(Corg) (Fig. 5). According to the review by Pribyl [75], the commonly recognized Corg share in the so-called OM of \(\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{org}}}}}} \right)}}{{{{\omega }}\left( {{\text{OM}}} \right)}}\) = 0.58 (1/1.724) is not universal [63]. Its values according to different published sources can be pooled into the range of 0.4–0.71 [56, 75, 81, 87]. The variability of the measured \(\frac{{{{\omega }}\left( {{{{\text{C}}}_{{{\text{org}}}}}} \right)}}{{{{\omega }}\left( {{\text{OM}}} \right)}}\) ratio is associated with different natures of organic compounds in soils.
Shamrikova et al. [87] analyzed 153 soil samples using gravimetric technique. The contents of organic compounds measured by this method in the ω(Corg) range of 0.2–5% are overestimated by 85 to 18%, respectively. Consequently, the lower limit of ω(Corg) measurement is 5% (further, the samples with ω(Corg) ≤ 5% were omitted). A comparison of the ω(Corg) measured by different methods and the OM amount determined according to soil weight lost-in-ignition (72 soil samples were carbonate-free and 16 contained carbonates; Fig. 6-I) allowed for the assessment of ω(Corg) in the organic compounds removed from the soil heated to T = 550°C, which amounted to 0.43–0.48 (reference value ω(Corg) = ω(Ctot) – ω(Cinorg); reference method, Walkley–Black technique).
Correlations of (Ia and Ib) the organic and (II) inorganic carbon contents measured by different methods: calculation according to high-temperature catalytic oxidation in combination with volumetric technique, ω(Corg)1 = ω(Ctot) – ω(Cinorg)1; Walkley–Black method, ω(Corg)3; gravimetric method, ω(Corg)2 and ω(Cinorg)2; and volumetric method, ω(Cinorg)1.
In addition to the decomposition of organic compounds, other processes take place during soil heating. This factor to a greater degree depends on soil mineralogical composition and the content of clay and colloid fractions, for example, removal of chemically bound water. Crystalline hydrates lose water when heated (T = 105–550°C) [100]. The carbonates with higher volatility decompose in the same temperature range [20]; in particular, azurite, malachite, siderite, and rhodochrosite (T = 380–500°C); sodium hydrocarbonate (T = 60–200°C) [20, 53, 101]; and magnesite and dolomite (T = 600–800°C). Magnesium carbonate commences decomposing at T = 500°C. Gypsum and sulfide minerals can emit gas products at high temperatures [21, 82, 100]. Thus, the presence of copper, iron, manganese, magnesium, and sodium carbonates in soils, as well as complex carbonates can distort the results of ω(Corg) measurements.
Gravimetric method makes it possible to assess ω(Cinorg) too by sequentially heating a soil weighed sample at T = 550°C and then at T ≥ 800°C assuming that only gas decomposition product of carbonates, CO2, is removed at T ≥ 800°C. However, on the one hand, halides decompose at T ≥ 800°C as well and, on the other hand, sodium carbonate completely decompose only at T = 1000°C [57, 75, 92]. Correspondingly, T = 925°C, which was proposed to assess the content of carbonates [18], looks debatable.
A comparison of volumetric and gravimetric methods for assessing the inorganic carbon content in 23 soil samples for the ω(Cinorg) range of 0.3 to 2% demonstrated that the latter method overestimated its value by 160 to 17%, respectively. In the ω(Cinorg) range of 2 to 4%, the overestimation considerably decreased (by 28 and 8%, respectively). As for the higher contents, the discrepancy between the values obtained by these two methods did not exceed 4% (Fig. 6-II).
At an insignificant difference between the costs of a calcimeter and an incinerator, a smaller number of factors (except for random) affects the data obtained using calcimeter and decreases the accuracy of ω(Cinorg) quantification as compared with gravimetric method [87].
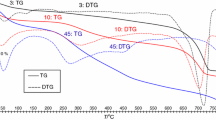
Instrumental methods for thermal decomposition of carbon-containing compounds. Recently, soil research practice adopts alternative methods for quantification of different carbon species making it possible to resolve the problems with incomplete oxidation and removal of carbonates and exclude the use of harmful reagents. These are examples of the methods utilizing the thermolability of organic and inorganic compounds [10, 48, 102]. The combination of various methods for thermal analysis (thermogravimetry, differential scanning calorimetry, and gas emission analysis) in a single approach makes them a useful tool for soil research [85].
Vuong et al. [99] tested the capabilities of thermal imaging in quantifying both carbon species in soils and artificial samples and inferred that this method could be more reliable as compared with the traditional techniques. Thermal analysis was implemented for soil studies [10] using a Netzsch STA 409 PC Luxx synchronous thermal analyzer. The Corg content was assessed according to CO2 emission in the range of T = 120–550°C and Cinorg, T = 550–850°C. The authors believe that the additional advantages of this method are rapid assay, relatively low cost, and simplicity. The point of its inexpensiveness is rather dubious because not any chemical laboratory can afford a derivatograph.
Pillot et al. [73] demonstrated the feasibility of carbonate quantification in soils using Rock-Eval pyrolysis. The Rock-Eval 6 pyrolyzer measures the content of CO2 emitted by decomposed carbonates of different metals based on the temperature gradient and identifies the nature of metal carbonate according to decomposition temperature.
Thus, the thermal analysis of soils retain the limitations mentioned when characterizing gravimetric method.
Spectroscopy. New methods for quantification of both soil carbon species–reflectance spectroscopy in the visible–infrared (Vis-NIR, 400–2500 nm) and mid-infrared (MIRS, 4000–400 cm–1) with measuring diffuse reflectance coefficient [14, 30, 46, 93] are actively developed in the last 30 years.
A number of advantages of soil spectroscopic analysis are undeniable as compared with the traditional wet chemistry methods [12, 48]. The data recording in both ranges is very rapid (one scanning takes several seconds); sample preparation is mainly reduced to soil drying and grinding; any destruction of analyzed material is unnecessary; and soil is not spent during scanning. In addition, the spectrum of a single scanning allows several soil parameters (pH, total nitrogen, particle size composition, cation exchange capacity, and so on) to be simultaneously assessed. Correspondingly, spectroscopy makes it possible to avoid the potential variation in the soil characteristics associated with sampling [90].
Development of the technology of infrared spectroscopy set the stage for the accumulation of soil spectral libraries covering the territories of different spatial scales [17, 30, 62, 70]. Some calibration databases accumulate the information about soils on a national (France [46] and China [89]), continental (Europe [94]), and global [97] levels.
Spectrometers are rather expensive. In particular, an ASD Labspec spectral radiometer for Vis-NIR soil assay reaches $65 000. However, the cost efficiency is very high because tens of thousands of soil samples are analyzed, which gives a considerable payoff as compared with the initial investment [9, 29]. In total, these advantages provide a higher performance and lower cost of soil quantitative analysis as compared with the routine laboratory techniques [41].
This forms the background for an optimistic assertion [48] on the expected displacement of traditional physicochemical methods in soil analysis by spectroscopic approaches the availability of comprehensive spectral databases provided. The experts worldwide unite their efforts to reach this goal. The Global Soil Laboratory Network is a major contributor to the development of all kinds of spectroscopy for soil analysis.
CONCLUSIONS
In the epoch of global changes in environment, the monitoring of organic carbon is most relevant, including its content in soils. The demand for an adequate assessment of soil organic matter content as a key characteristic of soil fertility is ever increasing in terms of the problems in food safety as well. The integration of the data obtained by different methods frequently requires the use of correction factors. Correspondingly, the comparison of the methods for quantification of soil characteristics becomes most important for the integrated databases, inventory of soil resources, mapping, and prediction changes in the soil cover.
Our comparative analysis of the advantages and disadvantages of the corresponding methods can enhance the informed choice of the approaches to quantification of organic carbon in carbonate-rich soils. The need to further compare the data for soils in different geographic zones remains evidently relevant. In terms of the search for correction coefficients, it is important to use reference methods. The external (assurance of the qualification of soil laboratories via interlaboratory comparisons and loopback tests) and internal controls, as well as prevention of changes in the methodical standards will improve the measurement quality. Elaboration of standardized and comprehensive protocols comprising the sampling strategy (design, depth, and time), storage conditions, and analytical techniques, as well as harmonization of the collected data remains in demand.
Notes
Vanchikova, E.V., Kondratenok, B.M., and Tumanova, E.A. Measurement protocol no. 88-17641-004-2016 (FR.1.31.2016.23502). Soils; grounds; bottom sediments; peat and its products; solid materials of plant, animal, natural, and industrial origins; and chemical compounds. Protocol for measurement of nitrogen, carbon, and organic matter in EA 1110 (CHNS-O) elemental analyzer. Institute of Biology, Komi Scientific Center, Ural Branch, Russian Academy of Sciences: Syktyvkar, 2016. 29 p.
ISO 10693:1995. Soil quality—Determination of carbonate content—Volumetric method, 1995, p. 9.
State standard GOST 26213-91 Soils. Methods for Determination of Organic Matter, Moscow: Izd. Standartov, 1992.
REFERENCES
L. A. Vorob’eva, Chemical Analysis of Soils (Mosk. Univ., Moscow, 1998) [in Russian].
B. M. Kogut, E. Yu. Milanovskii, and Sh. A. Khamatnurov, “On methods for determining the content of organic carbon in soils (critical review),” Byull. Pochv. Inst. im. V. V. Dokuchaeva, No. 114, 5–28 (2023). https://doi.org/10.19047/0136-1694-2023-114-5-28
V. V. Ponomareva and T. A. Plotnikova, Humus and Soil Formation (Nauka, Leningrad, 1980) [in Russian].
E. V. Shamrikova, E. V. Vanchikova, B. M. Kondratenok, E. M. Lapteva, and S. N. Kostrova, “Problems and limitations of the dichromatometric method for measuring soil organic matter content: a review,” Eurasian Soil Sci. 55 (7), 861–867 (2022). https://doi.org/10.1134/s1064229322070092
E. V. Shamrikova, V. V. Punegov, I. V. Gruzdev, E. V. Vanchikova, and A. A. Vetoshkina, “Individual organic compounds in water extracts from podzolic soils of the Komi Republic,” Eurasian Soil Sci. 45 (10), 939–946, (2012). https://doi.org/10.1134/S1064229312100080
E. V. Shamrikova, E. V. Zhangurov, E. E. Kulyugina, M. A. Korolev, O. S. Kubik, and E. A. Tumanova, “Soils and the soil cover of mountainous tundra landscapes on calcareous rocks in the Polar Urals: diversity, taxonomy, and nitrogen and carbon patterns,” Eurasian Soil Sci. 53 (9), 1206–1221 (2020). https://doi.org/10.1134/S106422932009015X
E. V. Shamrikova, O. S. Kubik, S. V. Deneva, and V. V. Punegov, “Composition of the water-soluble soil fraction on the Barents Sea coast: organic carbon and nitrogen, low-molecular weight components,” Eurasian Soil Sci. 52 (11), 1347–1362 (2019). https://doi.org/10.1134/S1064229319110103
E. V. Shamrikova, O. S. Kubik, V. V. Punegov, and I. V. Gruzdev, “Effect of the biota diversity on the composition of low-molecular-weight water-soluble organic compounds in southern tundra soils,” Eurasian Soil Sci. 47 (3), 173–181 (2014). https://doi.org/10.1134/S1064229314030077
Z. U. Ahmed, P. B. Woodbury, J. Sanderman, B. Hawke, V. Jauss, D. Solomon, and J. Lehmann, “Assessing soil carbon vulnerability in the Western USA by geospatial modeling of pyrogenic and particulate carbon stocks,” J. Geophys. Res. Biogeosci. 122, 354–369 (2017). https://doi.org/10.1002/2016JG003488
M. Apesteguia, A. F. Plante, and I. Virto, “Methods assessment for organic and inorganic carbon quantification in calcareous soils of the Mediterranean region,” Geoderma Reg. 12, 39–48 (2018). https://doi.org/10.1016/j.geodrs.2017.12.001
S. Bao, Soil Agricultural Chemistry Analysis (China Agriculture Press, Beijing, 2000), pp. 178–200 [in Chinese].
B. G. Barthès, E. Kouakoua, P. Moulin, K. Hmaidi, T. Gallali, M. Clairotte, M. Bernoux, E. Bourdon, J. Toucet, and T. Chevallier, “Studying the physical protection of soil carbon with quantitative infrared spectroscopy,” J. Near Infrared Spectrosc. 24, 199–214 (2016). https://doi.org/10.1255/jnirs.1232
N. H. Batjes, “Total carbon and nitrogen in the soils of the world,” Eur. J. Soil Sci. 47, 151–163 (1996).
V. Bellon-Maurel and A. B. McBratney, “Near-infrared (NIR) and mid-infrared (MIR) spectroscopic techniques for assessing the amount of carbon stock in soils—critical review and research perspectives,” Soil Biol. Biochem. 43, 1398–1410 (2011). https://doi.org/10.1016/j.soilbio.2011.02.019
E. Ben-Dor and A. Banin, “Determination of organic matter content in arid-zone soils using a simple “loss-on-ignition” method," Commun. Soil Sci. Plant Anal. 20 (15–16), 1675–1695 (1989). https://doi.org/10.1080/00103628909368175
E. L. Bergh, F. J. Calderon, A. K. Clemensen, L. Durso, J. O. Eberly, J. J. Halvorson, V. L. Jin, A. J. Margenot, C. E. Stewart, S. V. Pelt, and M. A. Liebig, “Time in a bottle: Use of soil archives for understanding long-term soil change,” Soil Sci. Soc. Am. J. 1–8, 520–527 (2022). https://doi.org/10.1002/saj2.20372
J. K. M. Biney, M. Saberioon, L. Borůvka, J. Houška, R. Vašát, P. C. Agyeman, et al., “Exploring the suitability of UAS based multispectral images for estimating soil organic carbon: comparison with proximal soil sensing and spaceborne imagery,” Remote Sens. 13, 1–19 (2021). https://doi.org/10.3390/rs13020308
I. Bisutti, I. Hilke, J. Schumacher, and M. Raessler, “A novel single-run dual temperature combustion (SRDTC) method for the determination of organic, in-organic and total carbon in soil samples,” Talanta 71 (2), 521–528 (2007).
L. Blake, K. W. T. Goulding, C. J. B. Mott, and P. R. Poulton, “Temporal changes in chemical properties of air-dried stored soils and their interpretation for long-term experiments,” Eur. J. Soil Sci. 51, 345–353 (2000). https://doi.org/10.1046/j.1365-2389.2000.00307.x
J. F. Boyle, “A comparison of two methods for estimating the organic matter content of sediments,” J. Paleolimnol. 31, 125–127 (2004). https://doi.org/10.1023/B:JOPL.0000013354.67645.DF
A. Brauer, J. Mingram, U. Franc, C. Gunter, G. Schettler, S. Wulf, B. Zolitschka, and J. F. W. Negendank, “Abrupt environmental oscillations during the Early Weichselian recorded at Lago Grande di Monticchio, southern Italy,” Quat. Int. 73–74, 79–90 (2000). https://doi.org/10.1016/S1040-6182(00)00066-5
C. R. Brodie, M. J. Leng, J. S. L. Casford, C. P. Kendrick, J. M. Lloyd, Z. Yongqiang, and M. I. Bird, “Evidence for bias in C and N concentrations and δ13C composition of terrestrial and aquatic organic materials due to pre-analysis acid preparation methods,” Chem. Geol. 282 (3–4), 67–83 (2011). https://doi.org/10.1016/j.chemgeo.2011.01.007
D. J. Brus, “Statistical approaches for spatial sample survey: persistent misconceptions and new developments,” Eur. J. Soil Sci. 72, 686–703 (2021). https://doi.org/10.1111/ejss.12988
M. A. Bughio, P. Wang, F. Meng, C. Qing, Y. Kuzyakov, X. Wang, and S. A. Junejo, “Neoformation of pedogenic carbonates by irrigation and fertilization and their contribution to carbon sequestration in soil,” Geoderma 262, 12–19 (2016). https://doi.org/10.1016/j.geoderma.2015.08.003
K. Y. Chan, M. K. Conyers, G. D. Li, K. R. C. Helyar, G. Poile, and A. Oates, “Soil carbon dynamics under different cropping and pasture management in temperate Australia: results of three long—term experiments,” Soil Res. 49, 320–328 (2011). https://doi.org/10.1071/SR10185
A. Chatterjee, R. Lal, R. K. Shrestha, and D. A. N. Ussiri, “Soil carbon pools of reclaimed minesoils under grass and forest landuses,” Land Degrad. Dev. 20, 300–307 (2009). https://doi.org/10.1002/ldr.916
L. Chen, X. Jing, D. F. B. Flun, Y. Shi, P. Kuhn, T. Scholten, and J. S. He, “Changes of carbon stocks in alpine grassland soils from 2002 to 2011 on the Tibetan Plateau and their climatic causes,” Geoderma 288, 166–174 (2017). https://doi.org/10.1016/j.geoderma.2016.11.016
Y. Chen and P. Barak, “Iron nutrition of plants in calcareous soils,” Adv. Agron. 35, 217–240 (1982).
M. Clairotte, C. Grinand, E. Kouakoua, A. Thébault, N. P. A. Saby, M. Bernoux, and B. G. Barthès, “National calibration of soil organic carbon concentration using diffuse infrared reflectance spectroscopy,” Geoderma 276, 41–52 (2016). https://doi.org/10.1016/j.geoderma.2016.04.021
J. P. Comstock, S. R. Sherpa, R. Ferguson, S. Bailey, J. P. Beem-Miller, F. Lin, J. Lehmann, and D. W. Wolfe, “Carbonate determination in soils by mid-IR spectroscopy with regional and continental scale models,” PLoS One 14 (2), e0210235 (2019). https://doi.org/10.1371/journal.pone.0210235
M. R. Davis, B. J. R. Alves, D. L. Karlen, K. L. Kline, M. Galdos, and D. Abulebdeh, “Review of soil organic carbon measurement protocols: A US and Brazil comparison and recommendation,” Sustainability 10, 4–8 (2018). https://doi.org/10.3390/su10010053
M. De Nobili, M. Contin, and P. C. Brookes, “Microbial biomass dynamics in recently air-dried and rewetted soils compared to others stored air-dry for up to 103 years,” Soil Biol. Biochem. 38, 2871–2881 (2006). https://doi.org/10.1016/j.soilbio.2006.04.044
B. De Vos, S. Lettens, B. Muys, and J. A. Deckers, “Walkley-Black analysis of forest soil organic carbon: recovery, limitations and uncertainty,” Soil Use Manage. 23, 221–229 (2007). https://doi.org/10.1111/j.1475-2743.2007.00084.x
G. S. Dhillon, B. Y. Amichev, R. Freitas, and K. Rees, “Accurate and precise measurement of organic carbon content in carbonate-rich soils,” Commun. Soil Sci. Plant Anal. 46 (21), 2707–2720 (2015). https://doi.org/10.1080/00103624.2015.1089271
M. Díaz-Zorita, J. H. Grove, and E. Perfect, “Sieving duration and sieve loading impacts on dry soil fragment size distributions,” Soil Tillage Res. 94, 15–20 (2007). https://doi.org/10.1016/j.still.2006.06.006
FAO, A Protocol for Measurement, Monitoring, Reporting and Verification of Soil Organic Carbon in Agricultural Landscapes-GSOC-MRV Protocol (Rome, 2020a). https://doi.org/10.4060/cb0509en
FAO, Global Soil Laboratory Network. Standard Operating Procedure for Soil Calcium Carbonate Equivalent. Volumetric Calcimeter Method (FAO, Rome, 2020). https://www.fao.org/3/ca8620en/ca8620en.pdf.
FAO, Global Soil Laboratory Network. Standard Operating Procedure for Soil Organic Carbon Tyurin Spectrophotometric Method (FAO, Rome, 2021). https://www. fao.org/3/cb4757en/cb4757en.pdf.
FAO, Global Soil Laboratory Network. Standard Operating Procedure for Soil Organic Carbon Walkley-Black Method: Titration and Colorimetric Method (FAO, Rome, 2020c). https://www.fao.org/3/ca7471en/ ca7471en.pdf.
FAO, Soil Organic Carbon Mapping Cookbook, 2nd Ed. (FAO, Rome, 2018). https://www.fao.org/3/I8895EN/ i8895en.pdf.
FAO, Soil Spectroscopy Training Material: A Primer on Soil Analysis using Visible and Near-Infrared (vis-NIR) and Mid-Infrared (MIR) Spectroscopy (FAO, Rome, 2022). https://www.fao.org/3/cb9005en/cb9005en.pdf.
F. Fordyce, N. Brereton, J. Hughes, G. Reay, L. Thomas, A. Walker, W. Luo, and J. Lewis, The Selenium Content of Scottish Soil and Food Products (Food Standards Agency, Scotland, 2009).
S. D. Friesen, C. Dunn, and C. Freeman, “Decomposition as a regulator of carbon accretion in mangroves: a review,” Ecol. Eng. 114, 173–178 (2018). https://doi.org/10.1016/j.ecoleng.2017.06.069
Y. Gao, J. Tian, Y. Pang, and J. Liu, “Soil inorganic carbon sequestration following afforestation is probably induced by pedogenic carbonate formation in Northwest China,” Front. Plant Sci. 8, 1282 (2017). https://doi.org/10.3389/fpls.2017.01282
Z. Ge, T. An, R. Bol, S. Li, P. Zhu, C. Peng, Y. Xu, N. Cheng, T. Li, Y. Wu, N. Xie, and J. Wang, “Distributions of straw-derived carbon in Mollisol’s aggregates under different fertilization practices,” Sci. Rep. 11, 1–9 (2021). https://doi.org/10.1038/s41598-021-97546-3
F. Gogé, R. Joffre, C. Jolivet, I. Ross, and L. Ranjard, “Optimization criteria in sample selection step of local regression for quantitative analysis of large soil NIRS database,” Chemom. Intell. Lab. Syst. 110, 168–176 (2012).
T. B. Goh, R. J. S. Arnaud, and A. R. Mermut, “Carbonates,” in Soil Sampling and Methods of Analysis (Lewis Publishers, CRC Press, Boca Raton, 2013), pp. 177–185.
C. Gomez, T. Chevallier, P. Moulin, I. Bouferra, K. Hmaidi, D. Arrouays, and B. G. Barthès, “Prediction of soil organic and inorganic carbon concentrations in Tunisian samples by mid-infrared reflectance spectroscopy using a French national library,” Geoderma 375, 114469 (2020). https://doi.org/10.1016/j.geoderma.2020.114469
K. Grahmann, J. A. Terra, R. Ellerbrock, V. Rubio, R. Barro, A. Caamaño, and A. Quincke, “Data accuracy and method validation of chemical soil properties in long-term experiments: standard operating procedures for a non-certified soil laboratory in Latin America,” Geoderma Reg. 28, e00487 (2022). https://doi.org/10.1016/j.geodrs.2022.e00487
K. Grahmann, M. Zwink, D. Barkusky, G. Verch, and M. Sommer, “The dilemma of analytical method changes for soil organic carbon in long-term experiments,” Eur. J. Soil Sci. 74, e13362 (2023). https://doi.org/10.1111/ejss.13362
Y. Guo, X. Wang, X. Li, J. Wang, M. Xu, and D. Li, “Dynamics of soil organic and inorganic carbon in the cropland of upper Yellow River Delta, China,” Sci. Rep. 6, 36105 (2016).
D. Harris, W. R. Horwáth, and C. Van Kessel, “Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis,” Soil Sci. Soc. Am. J. 65 (6), 1853–1856 (2001). https://doi.org/10.2136/sssaj2001.1853
M. Hartman, K. Svoboda, M. Poherely, and M. Syc, “Thermal decomposition of sodium hydrogen carbonate and textural features of its calcines,” Ind. Eng. Chem. Res. 52, 10619–10626 (2013). https://doi.org/10.1021/IE400896C
M. J. J. Hoogsteen, E. A. Lantinga, E. J. Bakker, and P. A. Tittonell, “An evaluation of the loss-on-ignition method for determining the soil organic matter content of calcareous soils,” Commun. Soil Sci. Plant Anal. 49 (13), 1541–1552 (2018). https://doi.org/10.1080/00103624.2018.1474475
J. L. Jensen, B. T. Christensen, P. Schjonning, C. W. Watts, and L. J. Munkholm, “Converting loss-on-ignition to organic carbon content in arable topsoil: pitfalls and proposed procedure,” Eur. J. Soil Sci. 69, 604–612 (2018). https://doi.org/10.1111/ejss.12558
A. Kamara, E. R. Rhodes, and P. A. Sawyerr, “Dry combustion carbon, Walkley-Black carbon, and loss on ignition for aggregate size fractions on a toposequence,” Commun. Soil Sci. Plant Anal. 38 (15–16), 2005–2012 (2007). https://doi.org/10.1080/00103620701548639
M. Kristl, M. Muršec, V. Šuštar, and J. Kristl, “Application of thermogravimetric analysis for the evaluation of organic and inorganic carbon contents in agricultural soils,” J. Therm. Anal. Calorim. 123, 2139–2147 (2016). https://doi.org/10.1007/s10973-015-4844-1
R. J. Lal and M. Kimble, “Pedogenic carbonates and the global C cycle,” in Global Climate Change and Pedogenic Carbonates (Lewis Publishers, Boca Raton, 2000), pp. 1–14.
R. Lal, “Sequestering carbon in soils of agro-ecosystems,” Food Policy 36, S33–S39 (2011). https://doi.org/10.1016/j.foodpol.2010.12.001
J. Lehmann and M. Kleber, “The contentious nature of soil organic matter,” Nature 528, 60–68 (2015). https://doi.org/10.1038/nature16069
S. Lettens, B. Vos, P. Quataert, et al., “Variable carbon recovery of Walkley-Black analysis and implica-tions for national soil organic carbon accounting,” Eur. J. Soil Sci. 58, 1244–1253 (2007). https://doi.org/10.1111/j.1365-2389.2007.00916.x
M. Leue, C. Hoffmann, W. Hierold, and M. Sommer, “In-situ multi-sensor characterization of soil cores along an erosiondeposition gradient,” Catena 82, 104140 (2019). https://doi.org/10.1016/j.catena.2019.104140
N. Li, D. Sack, J. Sun, S. Liu, B. Liu, J. Wang, G. Gao, D. Li, Z. Song, and D. Jie, “Quantifying the carbon content of aeolian sediments: which method should we use?,” Catena 185, 104276 (2020). https://doi.org/10.1016/j.catena.2019.104276
L. Lopez-Sangil and P. Rovira, “Sequential chemical extractions of the mineral-associated soil organic matter: an integrated approach for the fractionation of organo-mineral complexes,” Soil Biol. Biochem. 62, 57–67 (2013). https://doi.org/10.1016/j.soilbio.2013.03.004
T. Lu, X. Wang, M. Xu, Z. Yu, Y. Luo, and P. Smith, “Dynamics of pedogenic carbonate in the cropland of the North China Plain: influences of intensive cropping and salinization,” Agric. Ecosyst. Environ. 292, 106820 (2020).
D. T. Maher, I. R. Santos, L. Golsby-Smith, J. Gleeson, and B. D. Eyre, “Groundwater derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: the missing mangrove carbon sink?,” Limnol. Oceanogr. 58 (2), 475–488 (2013). https://doi.org/10.4319/lo.2013.58.2.0475
A. Menditto, M. Patriarca, and B. Magnusson, “Understanding the meaning of accuracy, trueness and precision,” Accredit. Qual. Assur. 12, 45–47 (2007). https://doi.org/10.1007/s00769-006-0191-z
A. F. Navarro, A. Roig, J. Cegarra, and M. P. Bernal, “Relationship between total organic carbon and oxidizable carbon in calcareous soils,” Commun. Soil Sci. Plant Anal. 24 (17–18), 2203–2212 (1993). https://doi.org/10.1080/00103629309368949
K. R. Olson, M. M. Al-Kaisi, R. Lal, and B. Lowery, “Experimental consideration, treatments, and methods in determining soil organic carbon sequestration rates,” Soil Sci. Soc. Am. J. 78, 348–360 (2014). https://doi.org/10.2136/sssaj2013.09.0412
S. M. O'Rourke, D. A. Angers, N. M. Holden, and A. B. McBratney, “Soil organic carbon across scales,” Global Change Biol. 21, 3561–3574 (2015). https://doi.org/10.1111/gcb.12959
M. Pansu and J. Gautheyrou, “Carbonates,” in Handbook of Soil Analysis (Springer-Verlag, Berlin, 2006), pp. 593–604.
G. Peralta, L. Di Paolo, I. Luotto, C. Omuto, M. Mainka, K. Viatkin, and Y. Yigini, Global Soil Organic Carbon Sequestration Potential Map (GSOCseq v1.1). Technical Manual (FAO, Rome, 2022). https://doi.org/10.4060/cb2642en
D. Pillot, E. Deville, and A. Prinzhofer, “Identification and quantification of carbonate species using rock-eval pyrolysis,” Oil & Gas Science and Technology – Rev. IFP Energies nouvelles 69 (2), 341–349 (2014). https://doi.org/10.2516/ogst/2012036
C. Poeplau, M. A. Bolinder, and T. Kätterer, “Towards an unbiased method for quantifying treatment effects on soil carbon in long-term experiments considering initial within-field variation,” Geoderma 267, 41–47 (2016). https://doi.org/10.1016/j.geoderma.2015.12.026
D. W. Pribyl, “A critical review of the conventional SOC to SOM conversion factor,” Geoderma 156, 75–83 (2010). https://doi.org/10.1016/j.geoderma.2010.02.003
G. E. Rayment, R. O. Miller, and E. Sulaeman, “Proficiency testing and other interactive measures to enhance analytical quality in soil and plant laboratories,” Commun. Soil Sci. Plant Anal. 31, 1513–1530 (2000). https://doi.org/10.1080/00103620009370523
G. M. S. Reithmaier, D. T. Ho, S. G. Johnston, and D. T. Maher, “Mangroves as a source of greenhouse gases to the atmosphere and alkalinity and dissolved carbon to the coastal ocean: a case study from the Everglades National Park, Florida,” J. Geophys. Res. Biogeosci. 125 (12), e2020JG005812 (2020). https://doi.org/10.1029/2020JG005812
J. B. Rodriguez, J. R. Self, and F. J. Arriaga, “A simple, gravimetric method to quantify inorganic carbon in calcareous soils,” Soil Sci. Soc. Am. J. 80 (4), 1107–1113 (2016).
J. Romanyà and P. Rovira, “An appraisal of soil organic C content in Mediterranean agricultural soils,” Soil Use Manage. 27, 321–332 (2011). https://doi.org/10.1111/j.1475-2743.2011.00346.x
J. Romanyà and P. Rovira, “Organic and inorganic P reserves in rain-fed and irrigated calcareous soils under longterm organic and conventional agriculture,” Geoderma 151, 378–386 (2009). https://doi.org/10.1016/J.GEODERMA.2009.05.009
W. R. Roper, W. P. Robarge, D. L. Osmond, and J. L. Heitman, “Comparing four methods of measuring soil organic matter in North Carolina soils,” Soil Sci. Soc. Am. J. 83 (2), 466–474 (2019). https://doi.org/10.2136/SSSAJ2018.03.0105
M. R. Rosen, C. Chague-Goff, P. Esre, and L. Goshell, “Utilisation of the sedimentological and hydrochemical dynamics of the Stump Bay Wetland along Lake Taupo, New Zealand, for the recognition of paleo-shoreline indicators,” Sediment. Geol. 148, 357–371 (2002). https://doi.org/10.1016/S0037-0738(01)00226-3
C. Santi, G. Certini, and L. P. D' Acqui, “Direct determination of organic carbon by dry combustion in soils with carbonates,” Commun. Soil Sci. Plant Anal. 37 (1–2), 155–164 (2006). https://doi.org/10.1080/00103620500403531
H. R. Schulten, “Direct pyrolysis-mass spectrometry of soils: a novel tool in agriculture, ecology, forestry, and soil science,” in Mass Spectrometry of Soils (Marcel Dekker, New York, 1996), pp. 373–436.
D. Sebag, E. P. Verrecchia, L. Cecillon, T. Adatte, R. Albrecht, M. Aubert, F. Bureau, G. Cailleau, Y. Copard, T. Decaens, J. R. Disnar, M. Hetenyi, T. Nyilas, and L. Trombino, “Dynamics of soil organic matter based on new Rock-Eval indices,” Geoderma 284, 185–203 (2016).
E. V. Shamrikova, B. M. Kondratenok, E. A. Tumanova, E. V. Vanchikova, E. M. Lapteva, T. V. Zonova, E. I. Lu-Lyan-Min, A. P. Davydova, Z. Libohova, and N. Suvannang, “Transferability between soil organic matter measurement methods for database harmonization,” Geoderma 412, 115547 (2022a). https://doi.org/10.1016/j.geoderma.2021.115547
E. V. Shamrikova, E. V. Vanchikova, E. I. Lu-Lyan-Min, O. S. Kubik, and E. V. Zhangurov, “Which method to choose for measurement of oranic and inorganic carbon content in carbonate-rich soils? Advantages and disadvantages of dry and wet chemistry,” Catena 228, 107151 (2023). https://doi.org/10.1016/j.catena.2023.107151
E. Shamrikova, E. Yakovleva, D. Gabov, E. Zhangurov, M. Korolev, and E. Zazovskaya, “Polyarenes distribution in the soil-plant system of reindeer pastures in the Polar Urals,” Agronomy 12 (2), 372–389 (2022b). https://doi.org/10.3390/agronomy12020372
Z. Shi, W. Ji, R. A. Viscarra-Rossel, S. Chen, and Y. Zhou, “Prediction of soil organic matter using a spatially constrained local partial least squares regression and the Chinese vis–NIR spectral library,” Eur. J. Soil Sci. 66, 679–687 (2015). https://doi.org/10.1111/ejss.12272
K. Singh, B. W. Murphy, and B. P. Marchant, “Towards cost-effective estimation of soil carbon stocks at the field scale,” Soil Res. 50 (8), 672–684 (2013). https://doi.org/10.1071/SR12119
J. O. Skjemstad and J. A. Taylor, “Does the Walkley-Black method determine soil charcoal?,” Commun. Soil Sci. Plant Anal. 30, 2299–2310 (1999). https://doi.org/10.1080/00103629909370373
S. Sleutel, S. De Neve, B. Singier, and G. Hofman, “Quantification of organic carbon in soils: a comparison of methodologies and assessment of the carbon content of organic matter,” Commun. Soil Sci. Plant Anal. 38, 2647–2657 (2007). https://doi.org/10.1080/00103620701662877
J. M. Soriano-Disla, L. J. Janik, R. A. Viscarra Rossel, L. M. MacDonald, and M. J. McLaughlin, “The performance of visible, near-, and mid-infrared reflectance spectroscopy for prediction of soil physical, chemical, and biological properties,” Appl. Spectrosc. Rev. 49 (2), 139–186 (2014). https://doi.org/10.1080/05704928.2013.811081
A. Stevens, M. Nocita, G. Toth, L. Montanarella, and B. van Wesemael, “Prediction of soil organic carbon at the European scale by visible and near infrared reflectance spectroscopy,” PLoS One 8 (6), e66409 (2013). https://doi.org/10.1371/journal.pone.0066409
D. J. Verardo, P. N. Froelich, and A. McIntyre, “Determination of organic carbon and nitrogen in marine sediments using the Carlo Erba NA-1500 analyzer,” Deep Sea Res., Part I 37 (1), 157–165 (1990).
I. Virto, R. Antón, and A. Plante, “Role of carbonates in the physical stabilization of soil organic matter in agricultural Mediterranean soils,” in Geology, Chapter 9 (2018), pp. 121–136. https://doi.org/10.1016/B978-0-12-812128-3.00009-4
R. A. Viscarra Rossel, T. Behrens, E. Ben-Dor, D. J. Brown, J. A. M. Demattê, K. D. Shepherd, Z. Shi, et al., “A global spectral library to characterize the world’s soil,” Earth Sci. Rev. 155, 198–230 (2016). https://doi.org/10.1016/j.earscirev.2016.01.012
C. Vitti, A. M. Stellacci, R. Leogrande, M. Mastrangelo, E. Cazzato, and D. Ventrella, “Assessment of organic carbon in soils: a comparison between the Springer-Klee wet digestion and the dry combustion methods in Mediterranean soils (Southern Italy),” Catena 137, 113–119 (2016). https://doi.org/10.1016/j.catena.2015.09.001
T. X. Vuong, F. Heitkamp, H. F. Jungkunst, A. Reimer, and G. Gerhard, “Simultaneous measurement of soil organic and inorganic carbon: evaluation of a thermal gradient analysis,” J. Soils Sediments 13 (7), 1133–1140 (2013). https://doi.org/10.1007/s11368-013-0715-1
Q. Wang, Y. Li, and Y. Wang, “Optimizing the weight loss-on-ignition methodology to quantify organic and carbonate carbon of sediments from diverse sources,” Environ. Monit. Assess. 174, 241–257 (2011). https://doi.org/10.1007/s10661-010-1454-z
Q. Wang, K. Seki, T. Miyazaki, and Y. Ishihama, “The causes of soil alkalinization in the Songnen Plain of Northeast China,” Paddy Water Environ. 7 (3), 259–270 (2009). https://doi.org/10.1007/s10333-009-0166-x
X. Wang, J. Wang, and J. Zhang, “Comparisons of three methods for organic and inorganic carbon in calcareous soils of northwestern China,” PLoS One 7 (8), e44334 (2012). https://doi.org/10.1371/journal.pone.0044334
T. Włodarczyk, P. Szarlip, W. Kozieł, M. Nosalewicz, M. Brzeziñska, M. Pazur, and E. Urbanek, “Effect of long storage and soil type on the actual denitrification and denitrification capacity to N2O formation,” Int. Agrophys. 28, 371–381 (2014). https://doi.org/10.2478/intag-2014-0027
J. Workman and H. Mark, “Limitations in analytical accuracy, part I: Horwitz’s trumpet,” Spectroscopy 21 (9), 19–24 (2006).
A. L. Wright, Y. Wang, and K. R. Reddy, “Loss-on-ignition method to assess soil organic carbon in calcareous everglades wetlands,” Commun. Soil Sci. Plant Anal. 39 (19–20), 3074–3083 (2008). https://doi.org/10.1080/00103620802432931
S. Xu, M. L. Silveira, L. W. Ngatia, A. E. Normand, L. E. Sollenberger, and K. Ramesh Reddy, “Carbon and nitrogen pools in aggregate size fractions as affected by sieving method and land use intensification,” Geoderma 305, 70–79 (2017). https://doi.org/10.1016/j.geoderma.2017.05.044
Y. Yang, J. Fang, C. Ji, W. Ma, S. Su, and Z. Tang, “Soil inorganic carbon stock in the Tibetan alpine grasslands,” Global Biogeochem. Cycles 24 (4), GB4022 (2010). https://doi.org/10.1029/2010GB003804
Y. Yang, X. Ma, L. Wang, X. Fu, and J. Zhang, “Evaluation of three methods used in carbonate content determination for lacustrine sediments,” J. Lake Sci. 28 (4), 917–924 (2016). [in Chinese].https://doi.org/10.18307/2016.0426
K. Zamanian, K. Pustovoytov, and Y. Kuzyakov, “Pedogenic carbonates: forms and formation processes,” Earth Sci. Rev. 157, 1–17 (2016). https://doi.org/10.1016/j.earscirev.2016.03.003
K. Zamanian, M. Zarebanadkouki, and Y. Kuzyakov, “Nitrogen fertilization raises CO2 efflux from inorganic carbon: a global assessment,” Global Change Biol. 24 (7), 2810–2817 (2018). https://doi.org/10.1111/gcb.14148
K. Zamanian, J. Zhou, and Y. Kuzyakov, “Soil carbonates: the unaccounted, irrecoverable carbon source,” Geoderma 384, 114817 (2021). https://doi.org/10.1016/j.geoderma.2020.114817
J. Zhao, X. Luo, Y. Ma, T. Shao, and Y. Yue, “Soil characteristics and new formation model of loess on the Chinese Loess Plateau,” Geosci. J. 21, 607–616 (2017). https://doi.org/10.1007/s12303-016-0069-y
Funding
The work was supported by the state budget (project no. 122040600023-8 “Cryogenesis as a Factor of Soil Formation and Evolution in the Arctic and Boreal Ecosystems of European Northeast under Conditions of Modern Anthropogenic Impacts and Global and Regional Climate Trends”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by G. Chirikova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shamrikova, E.V., Vanchikova, E.V., Kyzyurova, E.V. et al. Methods for Measuring Organic Carbon Content in Carbonate-Containing Soils: A Review. Eurasian Soil Sc. 57, 380–394 (2024). https://doi.org/10.1134/S1064229323603104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229323603104