Abstract
Ionizing radiation is an important environmental factor affecting the dynamics of biospheric processes in the past and present, as well as limiting the spread of life outside the Earth. The effect of radiation on microorganisms has been studied for decades, but studies of the response of natural microbial ecosystems are still scarce. We have studied the effect of 100 kGy gamma irradiation under low pressure (1 Torr) and low temperature (–50°C) on microbial community of the ancient Antarctic permafrost sedimentary rock. After irradiation, the total number of prokaryotic cells determined by epifluorescence microscopy, as well as the number of metabolically active bacterial and archaeal cells detected by fluorescence in situ hybridization remained at the control level, while the number of cultured heterotrophic bacteria decreased by an order of magnitude. Using the multisubstrate testing method, it has been found that the microbial complex retained a high potential metabolic activity and functional diversity after exposure to a combination of extreme physical factors. The resistance demonstrated by the microbial community significantly exceeded the generally accepted estimates of the prokaryotes’ radioresistance and indicated an underestimation of the microorganisms' radioresistance in natural habitats and the important role of mineral heterophase environment and irradiation conditions (pressure, temperature). The study confirmed the potential for long-term cryopreservation of viable terrestrial-like microorganisms in the Martian regolith, as well as the possibility of transferring anabiotic life forms as a part of small bodies in the space environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Ionizing radiation is an important factor affecting the dynamics of biospheric processes in the past and present [6, 14]. As civilization develops, the need for assessment the radiation effect on the environment increases due to nuclear power generation, accumulation of radioactive waste, and increasing radioactive load on the biosphere. Possibilities for humans to leave our planet and prospects to reach other planets of the Solar System also require improving the knowledge on radioactive environment and survival there of the life forms known on the Earth.
The effect of radiation on microorganisms has been studied for decades, but many problems remain unsolved. First of all, the limits are not clear of the existence of microorganisms in natural environment affected by ionizing irradiation. It was reported that most radioresistant species of prokaryotes can withstand in pure culture the impact of doses about 20–25 kGy [25]. However, the radioresistance of microorganisms can change significantly under the conditions of natural heterophase organomineral ecotopes (soils, rocks). High biodiversity of natural microbial communities, diversity of ecological niches (microzones) characterized by different physicochemical parameters, duplication of environmental functions by microorganisms, intra- and interpopulation interactions, presence of the pool of metabolites, and protective properties of heterophase environment serve to perpetuate homeostasis of ecosystems and their functioning and survival under extreme loads [11, 35]. Microorganisms of natural habitats have developed interrelated mechanisms of response to different stresses [18] and increased adaptive capacity [20], and this can correct their resistance to irradiation effects.
Radioresistance of microorganisms in natural environment is relatively poorly known. According to the data of different authors, sterilizing doses for soils and grounds vary from ~6 to 65 kGy [22, 25, 30, 31]. In most works on the study of radioresistance of microbial communities in situ, the detection of living cells was carried out only with culture technique. At the same time, it is known that microorganisms can form nonculturable and dormant forms under stress conditions [12, 30]. These physiological rearrangements are very important for the strategy of survival of microorganisms and microbial communities in soils and rocks and especially in extreme habitats [34]. Transition of microorganisms to dormant or nonculturable state can be misunderstood as their death, and the size of sterilizing dose of radiation can be underestimated. It is obvious that such studies require simultaneous use of both cultural and in situ methods. Sterilizing doses of radiation for natural microbial communities as well as the factors affecting the resistance of microorganisms in situ are not determined for now.
Ionizing irradiation is considered as the main factor limiting the existence and distribution of life outside our planet [3, 27]. The results of the study of microorganisms’ radioresistance are used in planning the space missions to assess the possibility of finding living organisms and biomolecules in different space objects and in constructing models of life origin and evolution. Radiation effects can be modified significantly, when a cell is exposed to radiation in extraterrestrial environment. Particularly, temperature, pressure, atmosphere composition, presence of water, and other factors affect the number of radiation-induced defects in the cells [3, 10]. In this relation it is important to study synergistic action of the totality of extraterrestrial conditions on complex heterogenic bio-abiotic systems (soils, rocks, sediments), which are terrestrial analogs of hypothetic ecosystems of another planets.
It was demonstrated recently that microbial communities of ancient (about 2 million years) frozen Arctic rocks in situ can withstand the effect of gamma irradiation in the doses up to 100 kGy under low pressure and low temperature [7]. Such high radioresistance exceeds significantly the existing beliefs concerning the resistance of microorganisms. Revealing mechanisms responsible for the stability of natural ecosystems under the influence of gamma irradiation requires: (i), comparative study of radioresistance of microbial communities in so called “extreme habitats” and biotopes not subjected to permanent high stress load with evaluation of the effect on stress resistance of their physicochemical characteristics and genesis; (ii), comparative analysis of the data obtained earlier for the other extreme natural objects in order to find out how high radioresistance of microbial communities in situ is a phenomenon, or it is a specific characteristics of extreme ecotopes.
We studied the integral effect of physical factors imitating primary conditions of Martian surface [gamma irradiation (100 kGy), low pressure (1 Torr), and low temperature (–50°С)] on the viability of natural microbial community in the permafrost sedimentary Antarctic rock. Permafrost and arid polar deserts of Antarctica are considered to be most close in the totality of physicochemical conditions to the known characteristics of Martian regolith. This is the cause to carry out microbiological study of astrobiological character in the most extreme regions of Antarctica, and particularly in the region of Dry Valleys [13, 16, 32]. The dose of irradiation used in the experiments corresponded to the long-lasting effect of cosmic radiation on Martian regolith, and accumulation of radiation-induced defects by cells supposedly preserved in an anabiotic state in Martian ground. Such approach, when determining the limiting dose, allows assessing the geological time of preservation the viability by microorganisms and microbial communities of terrestrial type in the regolith after loss by Mars of most part of its atmosphere and formation of recent conditions on this planet.
Consumption (optical density in the wells of MST-pad) of nominal groups of substrates by the community before and after ray treatment: P, pentoses; H, hexoses; O, oligosaccharides; A, alcohols; AA, amino acids, OA, salts of organic acids; Pm, polymers; AAN, amins, amides, and nucleosides. Samples: 1, control; 2, irradiated sample no. 1; 3, irradiated sample no. 2; 4, irradiated sample no. 3.
MATERIALS AND METHODS
The sample of Antarctic frozen sedimentary rock (the sample А-6/99-6) was used in the study. The sample was taken from the depth 1.3–1.5 m in the borehole 6/99, drilled on the plain in the Beacon Valley (77°50′ S, 160°36′ E, 1270 m a.s.l.) [16]. The region of Dry Valleys in Antarctica, where McMurdo Station is situated, is studied intensely and described in details [5]. The age of permafrost studied in this work is estimated as 50–300 thousand years [16]. The sedimentary rock is coarse sand with pebbles cemented by ice into a massive cryogenic mass not less than 20 m in depth (it was not drilled deeper). Maximal temperature below zero recorded in the studied frozen rocks was –18.5°C. Concentrations of \({\text{NO}}_{{\text{3}}}^{ - },\) Cl–, \({\text{CO}}_{{\text{3}}}^{{{\text{2}} - }},\) \({\text{NH}}_{{\text{4}}}^{ + },\) Na+, Mn2+, Mg2+, K+ and that of Fe2+ + Fe3+ ions in the sample were 0.78, 62.3, 172.39, 2.56, 915.15, 331.35, 10.47, 106.98, and 34.22 mg/kg respectively. Concentration of Сorg was 0.01%, рН of water extract 8.21 [16]. The methods of sampling and transportation to the laboratory were described in details earlier [16]. After carrying to the laboratory and up to carrying out the experiment, the samples were stored under –18°С.
The sample of frozen rock was incubated before ray treatment without adding of any substances under the temperature +28°С during 10 days in order to reactivate the microbial community; then the sample was dried to air-dry state during 24 hours under the same temperature. Then the sample was divided to 4 portions, one of which was not subjected to irradiation and served as control, 3 portions were subjected to irradiation. For the purpose of irradiation, the samples were placed into earlier described climate chamber [28], which allows supporting stable low pressure 1 Torr and temperature –50°С during the whole time of irradiation. Ray treatment was carried out in gamma device К-120000 with sources of 60Со under irradiation intensity of 5 kGy/h. The samples were stored after ray treatment up to analyses under –18°С.
The number of cultured heterotrophic aerobic bacteria was determined by inoculation of every sample from the series of dilutions into solid nutrient media: glucose-peptone-yeast (GPY) (peptone 2 g/L, glucose 1 g/L, yeast extract 1 g/L, casein hydrolysate 1 g/L, СаСО3 1 g/L, agar-agar 20 g/L) and 1/2 R2A [R2A agar (Difco, USA) 9.1 g/L, agar-agar 15 g/L] [1]. The cultures were grown under the temperature +28°С. The total number of prokaryotes in the samples was determined with epifluorescence microscopy (EFM) method with acridine orange [1]. The number of bacteria and archaea was estimated with the help of fluorescence in situ hybridization (FISH) with rRNA-specific fluorescently labeled oligonucleotide probes ARCH915 and EUB338 + EUB338I (Sintol, Russia), which are specific for representatives of Archaea and Bacteria domains, respectively. The analysis was carried out according to the procedure described earlier [21]. Potential metabolic activity of microbial communities was studied with the method of multisubstrate testing according to earlier described procedure [2] with modifications [7]. Statistical treatment of data was carried out using program packages STATISTICA 8.0, Microsoft Office Excel 2007, and original software Eco-Log [2].
RESULTS AND DISCUSSION
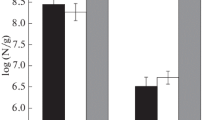
The number of CFU of heterotrophic bacteria decreased after irradiation by an order of magnitude (Table 1). However, the number of cultured cells of bacteria remained at high level: tens of millions CFU/g. Total number of cells of prokaryotes, determined with EFM method with acridine orange, became 1.5–1.8 times smaller after ray treatment. Total number of prokaryotes and the number of CFU of bacteria in all studied samples were slightly greater those in the other samples of permafrost rocks from Dry Valleys and Beacon Valley [16]. This was apparently connected with the increase of population density of microorganisms during sample incubation. Both bacteria and archaea were found in microbial communities in situ with FISH method. The number of metabolically active cells of bacteria and archaea changed insignificantly (the change did not exceed the measurement error) after irradiation, varying of parameters of cell number in subjected to irradiation sample was also insignificant, and this shows high repeatability of results of measurements.
Studied microbial community of ancient Antarctic permafrost has demonstrated high radioresistance in situ in comparison with the known data. Particularly, it was demonstrated earlier that the number of CFU of bacteria in surface samples of frozen rock from the same region (McMurdo, Dry Valleys) decreased by more than two orders, when the samples were subjected to irradiation in the dose 6 kGy under normal conditions [25]. There is no information about survival of prokaryotes irradiated by doses above 80 kGy [22, 25, 31], except for the above-mentioned results of our study [7].
Depending on the medium of growing, the number of CFU in control sample accounted for 2.3–7.3% of total number of prokaryotes (EFM) and 32–100% of the number of metabolically active cells of bacteria (FISH). These data in irradiated samples accounted for 0.1–1.6 and 3.6–14.5% respectively. It can be expected that the decrease of the fraction of cultured cells was connected with the change of physiological status of microorganisms in situ and inhibition of replication processes. The transition is possible of a part of populations into the nonculturable state, and this response of prokaryotes to stress effect is known [12]. Such result (the decrease of the number of CFU and small change of total number of prokaryotes) could be explained also by the transition of microorganisms into dormant state, but dormant cells as a rule are not determined with FISH method without adding special procedures [8]. The ratio between the number of metabolically active bacteria and total number of prokaryotes practically did not change after ray treatment. The effect of transition of soil bacteria into the nonculturable state after irradiation was observed earlier by Pitonzo et al. [30]. These authors demonstrated that the transition of bacteria from the nonculturable state and restoration of capability for growth are possible under favorable conditions.
Analysis of the main parameters of functional diversity and metabolic activity of microbial communities after ray treatment of samples, using the method of multisubstrate testing, demonstrated the increase of rank distribution coefficients of substrate consumption ranges (d), and this attested to some community destabilization. The diversity of consumed substrates (N) decreased, and specific metabolic work (W) increased (Table 2). The values of Shannon (H) and evenness (E) indices were close to control values. Average utilization of pentoses, alcohols, oligosaccharides, and salts of organic acids was close to utilization of these groups of substrates in control, whereas consumption of hexoses, polymers, and amino acids increased 1.5, 2.1, and 2.7 times, respectively. Hence, the increase of potential metabolic activity with small decrease of functional diversity was observed after irradiation under given modelled conditions. Microbial community of Antarctic frozen rock more actively utilized amino acids and polymers after ray treatment in comparison with the control community (Fig. 1). However, the metabolic “fingerprint” of community after irradiation in dose 100 kGy under given conditions did not change significantly.
Earlier published researches [23, 29] reported that treatment of soil by gamma irradiation in the dose about 10 kGy under normal conditions resulted in more significant inhibition of microbial communities in situ than in our experiment: potential metabolic activity decreased 6 times and more, the diversity of utilized substrates decreased 4 times and more up to complete inhibition of activity. The possibility was demonstrated of restoration of metabolic activity and functional diversity as the result of moistening and subsequent incubation of irradiated samples [23].
Hence, combined action of high dose of gamma irradiation, low temperature, and low pressure did not result in the death of prokaryotic complexes formed under extreme conditions of Antarctic permafrost. However, such effect can cause the change of reproductive and metabolic activity of functioning population. The main mechanism of quick response to such effect is apparently the transition into the nonculturable and/or dormant state.
The results of this study agree with earlier obtained data about the resistance of prokaryotes from ancient frozen rock of the Arctic region to similar effect [7] despite the essentially different genesis of rocks and significant difference in temperature conditions and time of rock being in frozen state. Identical response was demonstrated by microbial communities to complex influence of the main physical factors of Martian regolith: significant decrease of the number of CFU and preservation of total number of prokaryotic cells and the number of metabolically active cells at the level close to initial one, preservation of metabolic “fingerprint’’ of microbial complex with some decrease of diversity of utilized substrates and increase of potential metabolic activity with active consumption of amino acids. At the same time, the community of Antarctic permafrost according to the parameters of total population density determined with EFM method appeared slightly less resistant than earlier studied microbial complex of frozen Arctic rock and demonstrated the decrease of total abundance of cells after irradiation treatment. Total number of prokaryotes in Arctic permafrost remained at control level [7]. It is possible that the difference had an effect in the age of studied frozen rocks, i.e. in time of their being frozen. Microorganisms of polar regions of the Earth can accumulate the radiation injuries caused by very high doses of ionizing irradiation during long cryopreservation over the geological time [15]. The total accumulated dose apparently did not exceed 4–6 kGy for earlier studied Arctic permafrost and was apparently and order of magnitude lower for younger Antarctic rocks. It was demonstrated experimentally that bacteria can obtain increased radioresistance after ray treatment [17]. The temperature of sedimentary layer, which is 10–15°С lower in Antarctica, can be another factor to discuss some difference in resistance. If the temperature in permafrost of the East Siberia accounts for –7–12°С and allows cells preserving their physiological activity and repairing the injuries, so it accounts in permafrost of Antarctica in the place of sampling for –18–27°С [15, 16], i.e. it inhibits metabolic activity of cells to minimal level and consequently inhibits the reparation processes. However, our data in general suggest that high radioresistance in situ is typical for microorganisms survived in extremely cold ecotopes.
The resistance demonstrated by microbial communities of permafrost in situ exceeds significantly commonly adopted estimates of radioresistance of microorganisms. As it was noted, the radioresistance was determined by the conditions, under which the ray treatment was carried out: low temperature, low pressure, and consequently low concentration of oxygen and water content. It is known that temperature fall and the decrease of concentration of the sources of free radicals (oxygen and water) decreases significantly the number of radiation injuries of a cell [3, 10]. However, the obtained data allow believing that radioresistance of microorganisms in natural environment is underestimated. This is confirmed in part by the data about survival of Cryomyces antarcticus fungus after treatment with gamma irradiation in the dose 117 kGy under normal conditions [26]. It should be noted that eukaryotes are generally believed to be more radiosensitive in comparison with prokaryotes [22].
Currently, the ideas on extremophily and resistance of microorganisms change essentially. More and more resistant microorganisms are found, taxonomic diversity of resistant forms increases. Particularly, the information is supplemented about temperature limits of growth of microorganisms [33], about resistance to high and low pressure [28, 33], strong oxidizers [4], and new radioresistant species of microorganisms were found [24, 31]. The range of cell viability appeared to be much wider than it was believed earlier.
The results of our study allow assessing more adequately the possibility of finding viable microorganisms in Martian ground. It is supposed that Mars had the climate favorable for biosphere development at initial stages of planet development [13]. The hypothetic biosphere could be cryopreserved similarly to microbial communities of ancient permafrost later, when climate changed [16]. Time of biosphere preservation in viable state is inevitably limited by accumulation of radiation damages in cells, because the rate of repairing the injuries by cell is very low under low temperatures [3, 27, 33]. Taking into account the radiation intensity in Martial regolith [9, 19], the dose 100 kGy is accumulated during 1.3 million years in the surface layer of regolith and during 20 million years at the depth of 5 m, obtained data allow assuming the possibility of preservation of viable microorganisms and relic ecosystems in Martian regolith at least during these time periods after the loss by Mars of significant part of its atmosphere and approaching to recent conditions. It should be emphasized again that microbial community of permafrost preserved its metabolic activity in situ after irradiation, and this was confirmed by FISH data. Consequently, microorganisms can perform the reparation of injuries after the impact of such high doses of radiation. This fact suggests that the time of preservation the hypothetic ecosystems in Martial regolith can be increased significantly. Moreover, it follows from the results of our study that studied under the conditions of low pressure and low temperature dose of irradiation was lower than the sterilizing dose.
REFERENCES
D. G. Zvyagintsev, Methods of Soil Microbiology and Biochemistry (Moscow State Univ., Moscow, 1991) [in Russian].
M. V. Gorlenko and P. A. Kozhevin, Multisubstrate Testing of Natural Microbial Communities (MAKS Press, Moscow, 2005) [in Russian].
C. Baumstark-Khan and R. Facius, “Life under conditions of ionizing radiation,” in Astrobiology: The Quest for the Conditions of Life (Springer-Verlag, Berlin, 2002), pp. 261–284). https://doi.org/10.1007/978-3-642-59381-9_18
K. Beblo-Vranesevic, H. Huber, and P. Rettberg, “High tolerance of Hydrogenothermus marinus to sodium perchlorate,” Front. Microbiol. 8, 1369 (2017). https://doi.org/10.3389/fmicb.2017.01369
J. G. Bockheim, I. B. Campbell, and M. McLeod, “Permafrost distribution and active-layer depths in the McMurdo Dry valleys, Antarctica,” Permafrost Periglacial Process. 18 (3), 217–227 (2007). https://doi.org/10.1002/ppp.588
A. R. Brown, C. Boothman, S. M. Pimblott, and J. R. Lloyd, “The impact of gamma radiation on sediment microbial processes,” Appl. Environ. Microbiol. 81 (12), 4014–4025 (2015). https://doi.org/10.1128/AEM.00590-15
V. S. Cheptsov, E. A. Vorobyova, N. A. Manucharova, M. V. Gorlenko, A. K. Pavlov, M. A. Vdovina, V. N. Lomasov, and S. A. Bulat, “100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions,” Extremophiles 21 (6), 1057–1067 (2017). https://doi.org/10.1007/s00792-017-0966-7
H. Daims, K. Stoecker, and M. Wagner, “Fluorescence in situ hybridization for the detection of prokaryotes,” in Molecular Microbial Ecology (Taylor and Francis, London, 2005), pp. 192–212.
L. R. Dartnell, L. Desorgher, J. M. Ward, and A. J. Coates, “Martian sub-surface ionizing radiation: biosignatures and geology,” Biogeosci. Discuss. 4 (1), 455–492 (2007).
L. R. Dartnell, S. J. Hunter, K. V. Lovell, A. J. Coates, and J. M. Ward, “Low-temperature ionizing radiation resistance of Deinococcus radiodurans and Antarctic Dry Valley bacteria,” Astrobiology 10 (7), 717–732 (2010). https://doi.org/10.1089/ast.2009.0439
G. I. El-Registan, A. L. Mulyukin, Y. A. Nikolaev, I. Y. Stepanenko, A. N. Kozlova, E. I. Martirosova, E. F. Shanenko, M. G. Strakhovskaya, and A. A. Revina, “The role of microbial low-molecular-weight autoregulatory factors (alkylhydroxybenzenes) in resistance of microorganisms to radiation and heat shock,” Adv. Space Res. 36 (9), 1718–1728 (2005). https://doi.org/10.1016/j.asr.2005.02.070
G. I. El-Registan, A. L. Mulyukin, Yu. A. Nikolaev, N. E. Suzina, V. F. Gal’chenko, and V. I. Duda, “Adaptogenic functions of extracellular autoregulators of microorganisms,” Microbiology (Moscow) 75, 380–389 (2006). https://doi.org/10.1134/S0026261706040035
A. G. Fairén, A. F. Davila, D. Lim, N. Bramall, R. Bonaccorsi, J. Zavaleta, E. R. Uceda, C. Stoker, J. Wierzchos, J. M. Dohm, R. Amils, D. Andersen, and C. P. McKay, “Astrobiology through the ages of Mars: the study of terrestrial analogues to understand the habitability of Mars,” Astrobiology 10 (8), 821–843 (2010). https://doi.org/10.1089/ast.2009.0440
G. Giardino, I. Pillitteri, F. Favata, and G. Micela, “The X-ray luminosity of solar-mass stars in the intermediate age open cluster NGC 752,” Astron. Astrophys. 490 (1), 113–123 (2008). https://doi.org/10.1051/0004-6361:200810042
D. Gilichinsky, “Permafrost as a microbial habitat,” in Encyclopedia of Environmental Microbiology (Wiley, New York, 2002), pp. 932–956.
D. A. Gilichinsky, G. S. Wilson, E. I. Friedmann, C. P. McKay, R. S. Sletten, E. M. Rivkina, T. A. Vishnivetskaya, L. G. Erokhina, N. E. Ivanushkina, G. A. Kochkina, V. A. Shcherbakova, V. S. Soina, E. V. Spirina, E. A. Vorobyova, D. G. Fyodorov-Davydov, et al., “Microbial populations in Antarctic permafrost: biodiversity, state, age and implication for astrobiology,” Astrobiology 7 (2), 275–311 (2007). https://doi.org/10.1089/ast.2006.0012
D. R. Harris, S. V. Pollock, E. A. Wood, R. J. Goiffon, A. J. Klingele, E. L. Cabot, W. Schackwitz, J. Martin, J. Eggington, T. J. Durfee, C. M. Middle, J. E. Norton, M. C. Popelars, H. Li, S. A. Klugman, et al., “Directed evolution of ionizing radiation resistance in Escherichia coli,” J. Bacteriol. 191 (16), 5240–5252 (2009). https://doi.org/10.1128/JB.00502-09
J. P. Harrison, N. Gheeraert, D. Tsigelnitskiy, and C. S. Cockell, “The limits for life under multiple extremes,” Trends Microbiol. 21 (4), 204–212 (2013). https://doi.org/10.1016/j.tim.2013.01.006
D. M. Hassler, C. Zeitlin, R. F. Wimmer-Schweingruber, B. Ehresmann, S. Rafkin, J. L. Eigenbrode, D. E. Brinza, G. Weigle, S. Böttcher, E. Böhm, S. Burmeister, J. Guo, J. Köhler, C. Martin, G. Reitz, et al., “Mars’ surface radiation environment measured with the Mars Science Laboratory’s Curiosity rover,” Science 343, 1244797 (2014). https://doi.org/10.1126/science.1244797
N. A. Kryazhevskikh, E. V. Demkina, N. G. Loiko, R. V. Baslerov, T. V. Kolganova, V. S. Soina, N. A. Manucharova, V. F. Gal’chenko, and G. I. El’-Registan, “Comparison of the adaptive potential of the Arthrobacter oxydans and Acinetobacter lwoffii isolates from permafrost sedimentary rock and the analogous collection strains,” Microbiology (Moscow) 82, 29–42 (2013). https://doi.org/10.1134/S0026261713010050
N. A. Manucharova, A. N. Vlasenko, E. V. Men’ko, and D. G. Zvyagintsev, “Specificity of the chitinolytic microbial complex of soils incubated at different temperatures,” Microbiology (Moscow) 80, 205–215 (2011). https://doi.org/10.1134/S002626171102010X
N. P. McNamara, H. I. J. Black, N. A. Beresford, and N. R. Parekh, “Effects of acute gamma irradiation on chemical, physical and biological properties of soils,” Appl. Soil Ecol. 24 (2), 117–132 (2003). https://doi.org/10.1016/S0929-1393(03)00073-8
N. P. McNamara, R. I. Griffiths, A. Tabouret, N. A. Beresford, M. J. Bailey, and A. S. Whiteley, “The sensitivity of a forest soil microbial community to acute gamma-irradiation,” Appl. Soil Ecol. 37 (1–2), 1–9 (2007). https://doi.org/10.1016/j.apsoil.2007.03.011
A. Mirzaie, J. F. Mehrabadi, N. Amirmozafari, and T. Nejadsattari, “Isolation and characterization of a new gamma and UV radiation resistant bacterium from soil samples of an Iranian radioactive site and analysis of its pigment,” Microbiology (Moscow) 84 (3), 449–452 (2015). https://doi.org/10.1134/S0026261715030133
M. Musilova, G. Wright, J. M. Ward, and L. R. Dartnell, “Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance,” Astrobiology 15 (12), 1076–1090 (2015). https://doi.org/10.1089/ast.2014.1278
C. Pacelli, L. Selbmann, L. Zucconi, M. Raguse, R. Moeller, I. Shuryak, and S. Onofri, “Survival, DNA integrity, and ultrastructural damage in Antarctic cryptoendolithic eukaryotic microorganisms exposed to ionizing radiation,” Astrobiology 17 (2), 126–135 (2017). https://doi.org/10.1111/1758-2229.12632
A. K. Pavlov, A. V. Blinov, and A. N. Konstantinov, “Sterilization of Martian surface by cosmic radiation,” Planet. Space Sci. 50 (7–8), 669–673 (2002). https://doi.org/10.1016/S0032-0633(01)00113-1
A. K. Pavlov, V. N. Shelegedin, M. A. Vdovina, and A. A. Pavlov, “Growth of microorganisms in Martian-like shallow subsurface conditions: laboratory modeling,” Int. J. Astrobiol. 9 (1), 51–58 (2010). https://doi.org/10.1017/S1473550409990371
B. J. Pitonzo, P. S. Amy, and M. Rudin, “Effect of gamma radiation on native endolithic microorganisms from a radioactive waste deposit site,” Radiat. Res. 152 (1), 64–70 (1999). https://doi.org/10.2307/3580050
B. J. Pitonzo, P. S. Amy, and M. Rudin, “Resuscitation of microorganisms after gamma irradiation,” Radiat. Res. 152 (1), 71–75 (1999). https://doi.org/10.2307/3580051
F. A. Rainey, K. Ray, M. Ferreira, B. Z. Gatz, M. F. Nobre, D. Bagaley, B. A. Rash, M.-J. Park, A. M. Earl, N. C. Shank, A. M. Small, M. C. Henk, J. R. Battista, P. Kämpfer, and M. S. da Costa, “Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample,” Appl. Environ. Microbiol. 71 (9), 5225–5235 (2005). https://doi.org/10.1128/AEM.71.9.5225-5235.2005
E. Rivkina, A. Abramov, E. Spirina, L. Petrovskaya, A. Shatilovich, L. Shmakova, V. Scherbakova, and T. Vishnivetskaya, “Earth’s perennially frozen environments as a model of cryogenic planet ecosystems,” Permafrost Periglacial Process. 29 (4), 246–256 (2018). https://doi.org/10.1002/ppp.1987
J. D. Rummel, D. W. Beaty, M. A. Jones, C. Bakermans, N. G. Barlow, P. J. Boston, V. F. Chevrier, B. C. Clark, J.-Pi. P. de Vera, R. V. Gough, J. E. Hallsworth, J. W. Head, V. J. Hipkin, T. L. Kieft, A. S. McEwen, et al., “A new analysis of Mars “special regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2),” Astrobiology 14 (11), 887–968 (2014). https://doi.org/10.1089/ast.2014.1227
V. S. Soina, A. L. Mulyukin, E. V. Demkina, E. A. Vorobyova, and G. I. El-Registan, “The structure of resting bacterial populations in soil and subsoil permafrost,” Astrobiology 4 (3), 345–358 (2004). https://doi.org/10.1089/ast.2004.4.345
V. S. Soina and E. A. Vorobyova, “Adaptation of bacteria to the terrestrial permafrost environment,” in Cellular Origin, Life in Extreme Habitats and Astrobiology (Springer-Verlag, Dordrecht, 2004), pp. 427–444. https://doi.org/10.1007/1-4020-2522-X_26
ACKNOWLEDGMENTS
We express our respect in memoriam of David A. Gilichinsky, who kindly presented us the samples of Antarctic frozen sedimentary rocks.
Funding
Financial support of the study was provided from Russian Science Foundation, project no. 17-12-01184, and in part from Russian Foundation for Basic Research, project no. 20-02-00470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Chicheva
Rights and permissions
About this article
Cite this article
Cheptsov, V.S., Vorobyova, E.A., Manucharova, N.A. et al. Prokaryotic Community of the Ancient Antarctic Permafrost after Irradiation with Gamma Rays under Simulated Martian Conditions. Eurasian Soil Sc. 54, 417–423 (2021). https://doi.org/10.1134/S1064229321030030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229321030030