Abstract
A method for the preparation of samples from a biological material for the detection of upconversion nanoparticles (UNPs) contained in it is proposed on the basis of confocal microscopy techniques. The method was tested by monitoring the natural excretion of an injected colloidal solution of YVO4:Yb, Er nanoparticles with sizes from 10 to 700 nm (0.2 mL, containing 15 mg UNPs and with a dosage of 600 mg/kg) into the body of Helix lucorum. The experiments showed that the animal excreted the nanoparticles from the body in a natural way within 3 days, while the excretion rate decreased over time. The sensitivity threshold in the experiments was one nanoparticle per 1 µL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Successes in the use of noninvasive fluorescent nanoprobes have become a reliable foundation for the development of a wide range of biological fields. There is no doubt that such methods will become basic tools in the study of physiological principles of intracellular activity in the near future. Various compounds, such as organic dyes, quantum dots, fluorescent proteins, and luminescent transition metal complexes, are used as bioprobes [1–6]. As a rule, UV radiation is used for their excitation, which is strongly scattered and absorbed by biological tissues. It causes the self-fluorescence, photodestruction, and heating of cells, which greatly limits the possibilities of application. The low photostability of organic fluorophores, an undesirably wide emission spectrum of fluorescent proteins, flickering and toxicity of quantum dots, and a number of other undesirable factors should also be noted [7–11].
To avoid the problems in the application of downconversion fluorescent nanosensors, we use a fundamentally different approach based on the upconversion phenomenon. In this approach, upconversion nanoparticles (UNPs) doped with rare-earth ions act as nanosensors that exhibit bright visible luminescence when excited by radiation in the near-infrared range (transparency window of biological tissues). The photostability of UNPs, the absence of parasitic autofluorescence [12–14], the large depth of excitation radiation penetration into tissues [15], the virtual absence of local heating, and the extremely low photodestruction of biomolecules [16] are indisputable advantages of this approach. The narrow luminescence lines of rare-earth ions and the very low toxicity of UNPs in some cases should also be mentioned [15, 17–19]. These features explain the growing interest in UNPs as promising bionanoprobes in solving numerous biological problems (bioimaging, bionanosensing, drug transport, theranostics, phototherapy, optogenetics, etc.) [20, 21].
Dopants based on Yb3+ and Er3+ ions are the most popular ones. This is due to the fact that Yb3+ ions have a large absorption cross section at a wavelength of 980 nm and are capable of efficiently transferring energy to the resonance levels of Er3+ ions. Since metastable ion levels are involved in the energy transfer, this ensures a high probability of two quantum processes [22, 23]. In the role of a matrix, we use YVO4 nanocrystals with a phonon energy of 880 cm–1, which is substantially higher than a phonon energy of 350 cm–1 in the frequently used β-NaYF4 matrix [24, 25]. The advantage of YVO4:Yb, Er nanoparticles over fluoride counterparts is their low sensitivity to the action of surface quenchers in biological media. In addition, oxide nanoparticles are considered to be of low toxicity [26], which together makes them attractive for use in biological media. Our recent bioimaging experiments on snails Helix lucorum showed the promise of their use as fluorescent nanoprobes [27, 28]. It should be noted that land snails are a traditional object in studies of the nervous system [29–31]. They are actively used when studying the mechanisms of learning and memory because of a relatively simple nervous system with a rich variety of behavioral responses, which are determined by the complex interaction of unconditioned reflexes modulated by the habituation, sensitization, and associative learning processes. In [28], we performed a comparative analysis of the behavioral activity of animals injected with UNPs and control animals without UNPs, which did not reveal any deviations. It was found that UNPs (injected into the bodies of land snails at a dose of up to 200 mg/kg of animal weight) were almost completely excreted by animals in a natural way within a day.
It is crucial to recognize that information about the rate of natural excretion of YVO4:Yb, Er nanoparticles is extremely important for the further development of strategies for their use as fluorescent nanoprobes. To this end, we have developed a method for the control of UNPs in animal feces. The sample preparation method proposed by us also makes it possible to detect UNPs in biological materials prepared from individual organs. This method was tested on land snail Helix lucorum. As a result, we found that the snail was able to completely remove UNPs from the body in 3 days despite the high administered dose (600 mg/kg). Samples with snail secretions on the fourth day and beyond did not contain UNPs. At the same time, we did not notice any deviations in the behavioral reactions of snails after the injection. After 7 days, samples of individual organs were prepared from the snail. The experiments showed the absence of UNPs in these samples.
EXPERIMENTAL
The UNPs were synthesized as described earlier in [10] in accordance with the following procedures published in [32–34]. An aqueous solution that contained Y(NO3)3, Er(NO3)3, and Yb(NO3)3 (the concentrations were c = 0.1, 0.002, and 0.02 mol/L, respectively) was slowly added to an aqueous solution of Na3VO4 (c = 0.1 mol/L) under constant stirring at room temperature. A white precipitate was formed as a result, which indicates the presence of YVO4:Yb, Er nanoparticles. Next, the solution was dried to a powder state. Colloidal silicon dioxide was prepared by heating tetraethoxysilane, ethanol, and distilled water at pH 1.25 and T = 60°C for 1 h. After that, the nanoparticles were coated with colloidal silicon dioxide and dispersed polymer (PE6800) (molar ratio V : Si : PE6800 = 1 : 5 : 0.05). After drying, a mesoporous silicon network structure containing nanoparticles was obtained. The sample was calcined at 500°C for 1 h, and then the nanoparticles were annealed for 10 min at a temperature of 1000°C. Afterward, the silicon matrix was removed by 3-hour treatment in hydrofluoric acid with a molar ratio of HF : Si = 9 : 1. According to electron-microscopy data (see Fig. 1, left image), the size of the obtained UNPs is in the range of 10–700 nm.
The method was tested on a land snail with a weight of 25 g (see Fig. 1, right image). Before starting the experiments, the animal was active for at least 2 weeks, had abundant food, and was kept in a humid atmosphere at ambient temperatures (18–22°C). Aqueous colloidal solutions of UNPs were injected into the internal cavity of the animal through the region of the sinus node of the cochlea that do not contain pain receptors. To prepare a colloidal solution of UNPs, distilled water and a 10-min ultrasonic dispersion procedure were used right before injection. The colloidal solution contained 15 mg UNPs in 0.2 mL H2O (dosage 600 mg per 1 kg of animal weight).
After the administration, the animal was kept in a terrarium for 7 days with daily selection of secretions, from which samples were prepared in the following fashion. The daily secretions of the snail were carefully collected from the walls of the terrarium and the body of the snail. Thereafter, the collected biomaterial was annealed at a temperature of 500°C for 5 min, which yielded gray ash. The latter was then dissolved in a 0.06 N solution of hydrochloric acid and washed twice with water to settle in a microcentrifuge. At this stage, the sample was a colloidal solution of unburned and insoluble precipitate in water with a volume of 2 mL. To perform optical experiments, a drop of 1 μL of the solution was placed on a glass slide by means of a dispenser, which was spread over an area of approximately 12.5 mm2 and dried.
The luminescence of UNPs was recorded using a confocal microscope under excitation by a diode laser with a wavelength of 980 nm. The spatial resolution of the microscope was 1 μm. The use of single-photon detectors for detecting radiation made it possible to reliably detect luminescence from single UNPs. The glass slide with the test sample was placed in front of the objective, after which the object was scanned using a galvoscanner that moved the beam over the sample. An image was obtained as a result, which was a gradient of the intensity of the scanned surface. To confirm that the glowing dots are the desired UNPs, the spectrum of each glowing particle was recorded. It should be noted that the area of one scan in a confocal microscope is 0.068 mm2.
RESULTS
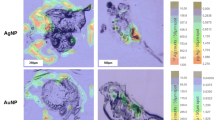
A typical scan of a sample prepared from snail secretions for the first day after injection is shown in Fig. 2 (left image). This scan shows regions with intense luminescence, which are shown in blue in the image. Their luminescence spectrum shown in Fig. 2 (left image) is a characteristic emission spectrum of Er3+ ions in UNPs, which indicates the presence of UNPs in these regions. The rest of the scans, taken at random locations over the sample, contain three to seven bright luminescence regions. In general, this result indicates the presence of UNPs in the natural excretions of the animal during the first day.
Figure 3 (left image) shows a scan of a sample from snail secretions of the second day. It shows three regions of intense luminescence, the spectrum of which (see Fig. 3, right image) indicates the presence of UNPs. Other scans of this sample contain up to five regions containing UNPs. This case is characterized by a substantial decrease in the size of the luminescent regions compared to the previous sample.
In the sample prepared from the secretions of the snail from the third day, it was possible to detect only a single luminescent region that is shown in Fig. 4 (left image). The luminescence spectrum (see Fig. 4, right image) confirms the presence of UNPs in it. The remaining scans do not contain UNP signals. The relatively large sizes of the region should be noted, which are comparable with the sizes of the largest regions in the first sample (see Fig. 2).
Single confocal microscope scan (on the left) taken from a sample of collected secretions on the third day after UNP injection, which contains a single luminescent spot. A luminescence spectrum (on the right) that confirms the presence of UNPs in the single region highlighted in blue in the confocal microscope scan.
We also prepared samples from the secretions in the period from the fourth to the seventh day. The performed spectroscopic studies did not make it possible to detect the signal of UNP luminescence in them. This indicates either an absence or an extremely low content of UNPs falling beyond the sensitivity of our method.
We used this method of preparing samples from a biomaterial to search for UNPs in the biologicals prepared from fragments of the snail body (liver, lungs, heart, nervous system, hemolymph, body, leg) on the seventh day after injection. The performed studies did not allow us to detect UNP signals in these samples. This means either the absence of UNPs in the body of the animal or their low concentration beyond the sensitivity limit of the method.
DISCUSSION
The method we proposed for preparing samples from biomass makes it possible to reliably detect the UNPs contained in them. This method was successfully tested on a snail that was injected with an aqueous solution of UNP (a 0.2 mL colloidal aqueous solution containing 15 mg of UNP at a dosage 600 mg/kg). The data obtained from monitoring the natural excretion of UNPs from the body of the snail indicate that the animal was able to get rid of foreign oxide nanoparticles within 3 days. We were unable to detect a luminescent signal in the samples prepared from snail secretions for the fourth day and further up to the seventh day. Also, UNPs were not found in samples prepared from individual body fragments.
At this point, it is important to make some estimates of the sensitivity of the applied method. The confocal spectroscopy technique reliably detects the luminescence of a single UNP. This means that the samples under study, with which no luminescent signal was detected, do not contain UNPs. However, the sensitivity of the method is severely limited by the sample preparation method, which involves the procedure of applying and drying 1 µL of the solution onto a glass slide. Accordingly, the accuracy of the method cannot exceed one nanoparticle per 1 µL. Apparently, merely this limit of accuracy was reached in the sample on the third day of the experiment when a single luminescent region was found. It should be noted that the absence of a luminescent signal in the sample indicates a concentration of less than one UNP per 1 µL.
The next limitation of the method is associated with the correct estimation of the absolute amount of UNPs in the sample. This is determined by the difficulty in determining the number of UNPs from their combined luminescent signal. To appreciate this possibility, the method with the involvement of additional experiments—for example, with the use of electron microscopy—needs to be developed. However, some observations can be made at this stage from the analysis of confocal microscope scans. Although we cannot obtain absolute data on the number of UNPs in the samples, we can confidently speak of a trend toward a decrease in the number of UNPs excreted from the body over time in the interval of the first 3 days. Since all samples were prepared according to a single method, comparison of the relative concentrations of luminescent regions containing UNPs by comparing scans of a confocal microscope is correct and reflects the general trend. It is also of interest to inspect the shapes of the luminescent regions in the scans. Recall that the sizes of injected UNPs are 10–700 nm. We believe that the bright shapeless luminescent regions in the scan shown in Fig. 2 may be agglomerates of the smallest UNPs that were excreted by animals on the first day. Separate small luminescent spots in Fig. 3 are likely to be larger UNPs that are excreted more slowly from the body. Finally, the only luminescent region in Fig. 4 may be one large UNP, the excretion of which took place in the most difficult way. It should be noted that these arguments are of a qualitative nature and require additional experimental verification.
CONCLUSIONS
Using the technique of confocal microscopy—which makes it possible to reliably detect the upconversion luminescence of a single UNP—we have developed a method for determining the rate of natural excretion of oxide UNPs from the body of a snail. At the same time, the accuracy of the method is limited mainly by the method used for sample preparation and in our experiments did not exceed one UNP per 1 μL of a solution volume. To test the method, land snails were chosen. The experiments show that the animal naturally excreted UNP from the body in sizes from 10 to 700 nm within 3 days after the injection of a colloidal solution of UNPs at a dosage concentration of 600 mg/kg, while the number of UNPs in the samples decreased over time. It is also shown that UNPs are absent in the body of the snail on the seventh day after administration, or their concentration is beyond the sensitivity of the method. It should be noted that the proposed method can be used with any other animals. Experiments with a single snail are not sufficient to fully assess the toxicity of UNPs. Nevertheless, the results obtained in this study point to the emerging possibilities of using UNPs as minimally invasive luminescent nanoprobes to solve a wide range of biological problems.
REFERENCES
J. S. Y. Lau, P. K. Lee, and K. H. K. Tsang, Inorg. Chem. 48, 708 (2009).
N. Malkani and J. A. Schmid, PLoS One 6, e18586 (2011).
L. Medintz, H. T. Uyeda, E. R. Goldman, and H. Mattoussi, Nat. Mater. 4, 435 (2005).
C. Wang, Q. Ma, W. C. Dou, and S. Kanwal, Talanta 77, 1358 (2009).
M. X. Yu, Q. Zhao, L. X. Shi, et al., Chem. Commun. 2008, 2115 (2008).
Q. Zhao, M. X. Yu, and L. X. Shi, Organometallics 29, 1085 (2010).
S. A. Hilderbrand, F. W. Shao, C. Salthouse, U. Mahmood, and R. Weissleder, Chem. Commun. 2009, 4188 (2009).
D. R. Larson, W. R. Zipfel, R. M. Williams, et al., Science 300, 1434 (2003).
F. van de Rijke, H. Zijlmans, S. Li, et al., Nat. Biotechnol. 19, 273 (2001).
V. G. Nikiforov, A. V. Leontyev, A. G. Shmelev, D. K. Zharkov, V. S. Lobkov, and V. V. Samartsev, Laser Phys. Lett. 16 (6), 065901 (2019).
A. V. Leontyev, A. G. Shmelev, D. K. Zharkov, V. G. Nikiforov, V. S. Lobkov, V. V. Samartsev, and L. A. Nurtdinova, Laser Phys. Lett. 16 (1), 015901 (2019).
N. M. Idris, Z. Q. Li, L. Ye, et al., Biomaterials 30, 5104 (2009).
N. J. J. Johnson, N. M. Sangeetha, J. C. Boye, and F. C. J. M. van Veggel, Nanoscale 2, 771 (2010).
X. J. Wu, Q. B. Zhang, X. Wang, H. Yang, and Y. M. Zhu, Eur. J. Inorg. Chem. 2011, 2158 (2011).
D. K. Chatterjee, A. J. Rufaihah, and Y. Zhang, Biomaterials 29, 937 (2008).
S. Jiang and Y. Zhang, Langmuir 26, 6689 (2010).
Y. I. Park, J. H. Kim, K. T. Lee, et al., Adv. Mater. 21, 4467 (2009).
A. R. Jalil and Y. Zhang, Biomaterials 29, 4122 (2008).
L. Q. Xiong, T. S. Yang, Y. Yang, C. J. Xu, and F. Y. Li, Biomaterials 31, 7078 (2010).
F. Jia, G. Li, B. Yang, et al., Nanotechnol. Rev. 8, 1 (2019).
M. V. Da Costa, S. Doughan, Y. Han, et al., Anal. Chim. Acta 832, 1 (2014).
D. R. Gamelin and H. U. del Gu, Top. Curr. Chem. 214, 1 (2001).
F. Auzel, Chem. Rev. 104, 139 (2004).
M. F. Joubert, Opt. Mater. 11, 181 (1999).
P. Goldner and F. Pellé, J. Lumin. 55, 197 (1993).
P. E. Petrochenko, Q. Zhang, H. Wang, T. Sun, B. Wildt, M. W. Betz, P. L. Goering, and R. J. Narayan, Int. J. Appl. Ceram. Technol. 9, 881 (2012).
D. K. Zharkov, A. G. Shmelev, A. V. Leontyev, et al., Bull. Russ. Acad. Sci. Phys. 84 (12), 1486 (2020). https://doi.org/10.3103/S1062873820120400
A. G. Shmelev, V. G. Nikiforov, D. K. Zharkov, et al., Bull. Russ. Acad. Sci. Phys. 84 (12), 1439 (2020). https://doi.org/10.3103/S1062873820120357
P. M. Balaban, Neurosci. Biobehav. Rev. 26 (5), 597 (2002).
T. Kiss, Z. Pirger, and G. Kemenes, Neurobiol. Learn. Mem. 92, 114 (2009).
V. V. Andrianov, T. Kh. Bogodvid, I. B.Deryabina, et al., Front. Behav. Neurosci. 9, 1 (2015).
G. Mialon, S. Turkcan, A. Alexandrou, et al., J. Phys. Chem. C 113, 18699 (2009).
G. Mialon, S. Turkcan, G. Dantelle, et al., J. Phys. Chem. C 114, 22449 (2010).
M. H. Alkahtani, F. S. Alghannam, C. Sanchez, et al., Nanotechnology 27, 485501 (2016).
Funding
The synthesis of oxide UNPs was supported by a grant from the Ministry of Education and Science of the Russian Federation under agreement no. 075-15-2021-623 with Federal Research Center Kazan Scientific Center, Russian Academy of Sciences. Spectroscopic measurements were supported by the Russian Foundation for Basic Research within the frameworks of grants nos. 19-02-00569 A and 20-52-04018 Bel_mol_ai no. 20-02-00545 A. Animal experiments were carried out with financial support from the PRIORITET-2030 Strategic Academic Leadership Program for Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Shmelev, A.G., Nikiforov, V.G., Zharkov, D.K. et al. Monitoring of the Natural Excretion of YVO4:Yb, Er Upconversion Nanoparticles from a Land Snail. Tech. Phys. 67, 283–288 (2022). https://doi.org/10.1134/S1063784222050097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784222050097