Abstract
The entrance of Pt marker nanoparticles (NPs) into the hydrosphere leads to association with unicellular algae Chlorella (the bioaccumulation factor reaches 10 000). There is a significant elimination time (t1/2 = 7 days) even with single contamination of the hydrosphere. Pt NPs, entering the hydrosphere, accumulate in the organism Daphnia magna Straus in large numbers (bioaccumulation factor of 1000–2000), starting from the first day, which can be dangerous for consumers of a higher trophic level. The accumulation of NPs occurs both in the digestive tract and on the surface of the body (t1/2 = 3 h). The accumulation of NPs during transmission through the food chain with chlorella contaminated with nanoparticles exceeds accumulation from the environment by 4 times, which is associated with the preliminary accumulation of NPs by food (chlorella) and absorption by Daphnia of NPs in a concentrated form. NPs accumulate in fish from the environment (bioaccumulation factor of up to 2500) and along the food chain (bioaccumulation factor of up to 350). Purification from NPs during accumulation along the food chain occurs much more slowly than in the series with the accumulation of NPs from the environment. When using fish products contaminated with NPs, such organs and tissues as skin, muscles and skeleton are of greatest risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, due to the numerous ways of releasing nanomaterials into the environment, their impact on ecosystems and human health is growing [1–3]. It has been established that in addition to the toxic effect on bacteria [4–6], lower and higher plants [7–9], some nanoparticles (NPs) based on metals and the metal ions released from them can be adsorbed in aquatic organisms and transferred through the food chains of the aquatic ecosystem. Adsorption, which significantly depends on the surface properties of NPs, is the first and most important step in the interaction between NPs and aquatic-animal species. After adsorption, NPs can accumulate on the cell surface or pass into the intracellular medium by diffusion or endocytosis [10–12].
NP adsorption induces cell-wall damage and causes severe acute toxicity in freshwater and marine algae: Raphidocelis subcapitata, Phaeodactylum tricornutum, Chlorella sp., Dunaliella tertiolecta [13, 14]. The attachment of NPs (NP TiO2, Al2O3, multi-walled carbon nanotubes) to the outer surface of Daphnia magna, Ceriodaphnia dubia, Artemia salina, and Danio rerio is observed during the first 48 h from the onset of exposure and accumulation in the intestine, lipid vesicles, phagocytes, followed by a decrease in their content occurs after 48 h of exposure [15–17]. The concentration in Daphnia magna increased with increasing concentration of TiO2 NPs in the environment [18]. The rate of removal of Ag NPs in Daphnia is much lower than that of Ag ions, which indicates the slower elimination of Ag NPs [19]. When Ceriodaphnia dubia were exposed for 6 h to dispersed media with different concentrations (1–50 mg/L) of Fe2O3 NPs, the maximum accumulation of NPs was observed at a concentration of 20 mg/L [20]. When nekton organisms, such as fish Danio rerio, are exposed to dispersed systems of TiO2 NPs with concentrations of 0.1 and 1.0 mg/L, they can bioaccumulate NPs with a bioaccumulation coefficient (BAC) of 25.38 and 181.38, respectively [21]. However, after 25 days of carp (Cyprinus carpio) exposure to dispersed systems of TiO2 NPs with concentrations of 3 and 10 mg/L, the concentration dependence of the BAC was not so pronounced, and it was equal to 675 and 595, respectively [22].
Upon accumulation in certain species of phytoplankton and zooplankton, NPs are transferred along the food chain, which leads to significant biomagnification: the transfer of quantum dots from algae has been verified (Pseudokirchneriella subcapitata) to zooplankton (Ceriodaphnia dubia) after their treatment by algae [23]; CdSe quantum dots accumulating in bacteria (Pseudomonas aeruginosa) are transferred to Tetrahymena thermophila [24]; a high content of Au NPs in species of the primary trophic level of the food chain leads to a high content of Au in the primary consumer Daphnia magna [21]; the use of contaminated Artemia salina as food for goldfish (Carassius auratus) led to the accumulation of CuO and ZnO NPs in the intestines, gills, and liver [25]. Along with this, the negative biomagnification of TiO2 NPs was revealed in a simplified food chain due to the depuration of TiO2 NPs from contaminated feed [21]. In addition, the three-level trophic transfer of quantum dots along the aquatic food chain has been demonstrated [26]. The ingestion of food contaminated with NPs may represent their main route through the food web [21–26], which is a potential route for NPs to enter the human food chain.
Thus, NPs can accumulate in aquatic organisms and be transported to various trophic levels, including algae, fish, and benthic animals. However, there are still some conflicting results regarding the biomagnification of NPs in the aquatic environment that require further study.
EXPERIMENTAL
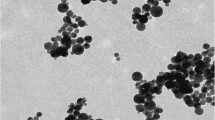
Pt NPs in the form of an aqueous colloidal solution with a concentration of 50 mg/dm3 were obtained at the Laboratory of New Materials and Advanced Technologies, Siberian Institute of Physics and Technology of Tomsk State University. The colloidal solutions of NPs were obtained by laser ablation in distilled water from metal ingots high purity [27]. To determine the average NP size, a Zetasizer Nano ZS (USA) analyzer and Phillips CM-12 (France) transmission electron microscope were used. The specific surface area was measured by the BET method (Brunauer-Emmett-Teller method) (TriStar 3000, USA). To determine the concentration of the ionic fraction in the suspension, a SOLAAR S2 atomic absorption spectroscope (USA) was used. The concentration of the Pt element in the samples of biological objects was determined by inductively coupled plasma mass spectrometry (Elan DRC-e spectrometer, USA). The optical density of the algal-cell suspension was determined with an IPS-3 suspension density meter (Europolitest, Russia).
The tested disperse systems (DS) of Pt NPs were created according to the method developed by us by diluting the initial colloidal solution with a concentration of 50.0-mg/dm3 cultivation medium and subsequent ultrasonic redispersion (ultrasonic power 30 W/L) for 5 min [2]. The cultivation medium for algae was 10% Tamiya medium; for Daphnia magna and Cyprinus carpio, it was drinking water, aerated, in accordance with regulatory documents [28]. Based on previous studies of the stability and toxicity of DS [29], a Pt NP concentration of 1.0 mg/dm3 was chosen for the work.
The test organisms Chlorella vulgaris Beijer and Daphnia magna Straus were purchased from LLC Europolitest (Russia). Cyprinus carpio (weight 3–5 g, one litter) juveniles were provided by LLC Tomsk scientific and production fish-breeding complex (Russia). The conditions of caring for the test organisms before and during the experiment met the requirements of standard bioassay procedures: photoperiod of 12/12, pH 7.0–8.2, and an O2 content of 26 mg/L [30]. 10% of the cultivation medium was replenished daily.
The accumulation studies included two phases: accumulation and purification. The samples for quantitative determination of the Pt concentration in the test organisms were taken in the accumulation phase for chlorella at the end of days 1, 2, 3, 4, 5, 10, and 20 of cultivation; for Daphnia, at the end of days 1, 5, 10, 20, and 28; and for the fish, at the end of 1, 5, 10, 20 days. In the purification phase, they were taken at the end of days 1 and 7 after transfer to a medium free of NPs.
The chlorella suspension was concentrated by threefold separation with washing with the cultivation medium and final centrifugation for 15 min at 1600 rpm. The precipitate was dried on filter paper. Samples with a fresh weight of ~1 g were submitted for analysis. A total of 33 samples.
Daphnia crustaceans were collected using a sieve, rinsed from above with cultivation water and dried on a gauze napkin for 5 min, after which they were collected in a test tube and weighed. Samples with a wet weight of ~1 g were submitted for analysis. There was a total of 42 samples.
After being caught, the juvenile fish were anesthetized with tricaine solution and decapitated. To assess the integral accumulation, homogenization was performed. To assess the accumulation in fish organs, a tissue homogenate was dissected and prepared. Samples with a fresh weight of ~1 g were submitted for analysis. There was a total of 55 samples.
At the same time, studies were carried out using the same scheme with a cultivation medium free of NPs.
Statistical processing of the obtained data was carried out in the programs Statistica 10 and Excel 2010.
RESULTS AND DISCUSSION
The results presented in the work refer to a part of the process of the translocation of nanomaterials in the environment, i.e., translocation in the food chain of aquatic organisms (Fig. 1).
The marker NPs used in the study were characterized by the following indicators: average LF size of Δ50 = 5 nm, specific surface area of Ssp ≈ 15 m2/g, and the concentration of the ionic fraction in the suspension did not exceed 0.2%.
Accumulation of Pt NPs by chlorella. The question of the dispersity of accumulated particles was not considered, since many publications show the accumulation of NPs, in particular, in various biological objects [23–25]. In the work performed, the accumulation of Pt in the form of NPs is evidenced by both its low solubility and, accordingly, the practical absence of the ionic form, and accumulation in muscle and skeletal tissues even when it enters through the gastrointestinal tract.
When cultivating unicellular algae Chlorella in a dispersed system of Pt NPs, the concentration of the Pt element increased to 570 ± 96 μg/g after 24 h (Fig. 2a). As the exposure time increased, the Pt concentration in chlorella decreased and after 20 days was 106 ± 18 μg/g.
One of the most important parameters for assessing the accumulation of NPs by aquatic organisms from the environment is the bioaccumulation factor (BAF), which reflects the ratio of the concentration of the substance in the test organism to the concentration of the substance in the cultivation medium of the test organism [31, 32] and is calculated by the formula: BAF = Cchl/Cw, where Cchl is the concentration of a chemical element in chlorella and Cw is the concentration of a chemical element in the cultivation medium.
Taking into account the initial concentration of Pt NPs equal to 1 mg/L (1 μg/mL), one would expect a BAC range from 100 to 600; however, in reality, a number of processes occur that significantly change this indicator.
The contact of NPs with the aquatic environment when they enter the ecosystem leads to a complex chain of physical-chemical rearrangements of the DS structure, one of the most important links of which is aggregation followed by the sedimentation or flotation elimination of NPs from the habitat of aquatic organisms into bottom sediments or onto the surface of water bodies. The processes of NP elimination, along with the concentration parameters, are determined by a number of factors: the electrolyte composition of the medium, temperature, pH of the medium, particle charge, degree of aeration, etc. [33].
In addition, the processes of the uptake of NPs by chlorella cells during the first 24 h of cultivation (the concentration in the medium decreases from 1.0 to 0.005 mg/L) make a significant contribution. The subsequent addition of NP suspensions to the test system cannot fully compensate for this change.
It is extremely difficult to estimate the stability of DS by the calculation method, especially when its polyelectrolyte composition is typical for natural aquatic environments. Due to the instability of the dispersed system of Pt NPs, according to the principles of the globally agreed system of hazard classification and labeling of chemicals [34], when determining the BAC, it is necessary to carry out calculations taking into account the effective concentration of the pollutant in the medium, which is calculated as the geometric mean of the initial and final concentrations of NPs for the exposure period.
However, studies [29] showed that calculation of the effective concentration by the formula C e =(C in × C fin)1/2, where C in and C fin are the initial and final concentrations, respectively, are possible only with the single introduction of NPs. In the case of the repeated introduction of NPs in the concentration C in (introduction at higher concentrations is unacceptable, since short-term effects of increased concentrations on test organisms are possible) in the first days \(C_{1}^{e}\) = (C inC in(e–24Ke))1/2 = C ine–12Ke. The elimination coefficient (Ke) is the coefficient of the exponential-approximation equation Ct = C0e–Ke×t of the graph of a decrease in the concentration of suspended NPs during settling, where C0 is the initial concentration of NPs, and t is the exposure time in hours. This indicator, which characterizes the stability of DS, the effective concentration of the effect of dispersed NPs, and the possible time of their effect on the test organism, must be determined experimentally. According to [29], this value for the used disperse systems of Pt NPs 1.0 mg/L is: in the cultivation medium for chlorella, 0.0144 h–1; in the cultivation medium for Daphnia, 0.0201 h–1; and in the cultivation medium for fish, 0.0261 h–1.
For subsequent i = (n – 1) day \(C_{i}^{m}\) = ((\(C_{{i - 1}}^{{{\text{fin}}}}\) + C in × Krep)\(C_{i}^{{{\text{fin}}}}\))1/2, where \(C_{{i - 1}}^{{{\text{fin}}}}\) is the final concentration in the previous days, Krep is the coefficient of replacement of the cultivation medium in fractions of the volume. Then the average concentration for the period n > 1 day can be calculated using the formula
An alternative is multiple (during each day) sampling of the culture, their purification from test organisms, measurement of the NP concentrations in the medium by the method of mass spectrometry and integration of the obtained values. However, this leads both to a violation of the cultivation conditions and to a significant increase in the cost of obtaining results.
Another important source of error in determining the BAF is the growth of chlorella during exposure. At low amounts of NPs introduced into the medium, algal cells of subsequent generations are “diluters” that do not associate NPs in the same amounts as the cells of the first generation. Thus, it is necessary to take into account the growth rate of chlorella and correct both the determined concentration of NPs adsorbed by chlorella and the BAF value.
Figure 2b shows the growth dynamics of chlorella determined by the standard method for measuring the absorbance of a suspension [35].
It is clearly seen that the duration of the exponential growth phase is three days. In this phase of exponential growth of a culture in the control medium, the coefficient of average specific growth µ = lnCt/t amounted to 0.71. When cultivating Chlorella vulgaris Beijer in the dispersion medium of Pt NPs, the average specific-growth coefficient increases to µ = 1.04, which indicates the stimulating effect of Pt NPs in the exponential growth phase.
Based on the obtained data and by applying the specified correction, it is possible to calculate the main indicators of bioaccumulation: kcl is the coefficient of the rate of purification of test organisms after transfer to an environment free of NPs, and t1/2 is the “half-purification” time, i.e., the period during which the concentration of associated NPs decreases by 2 times:
The results obtained after 1 and 7 days of washing show that the purification process slows down over time. On the first day kcl = 0.16/day, t1/2 = 4.2 days, in the interval 1–7 days kcl = 0.10/day, t1/2 = 7.0 days. This may be due to the rapid initial purification of the culture from NPs that are weakly bound to the cell surface.
The coefficient of the NP accumulation rate (kup) is calculated by the formula
Since Cchl and Cw change during the accumulation phase, the values of the coefficient are also not constant (Table 1).
The kinetic bioaccumulation factor (BAFk) is calculated by the formula
The difference in the BAF values should be noted. The maximum value of BAF is 9400 ± 1800, while BAFk on the second day of exposure reaches a value of 31 000 ± 5800. This fact may reflect the potential ability of the chlorella culture to accumulate a significant amount of NPs. The implementation of this possibility is limited by a decrease in the passive sorption of NPs due to the saturation of binding loci, a slowdown in the growth of chlorella beyond the exponential growth phase, and, possibly, a limited number of NPs that can exist in the free state for a long time under natural conditions. At the same time, the high level of accumulation of Pt NPs already on the first day of exposure indicates the great significance of this process in the possible transfer of NPs along the food chain.
Accumulation of Pt NPs by Daphnia from the cultivation medium. In this and subsequent series of experiments, the Pt content was measured under control conditions without the introduction of NPs. In all cases, the concentration of Pt recorded in the test organisms did not exceed the value 0.0002 ± 0.0004 µg/g of weight.
Upon incubation in a medium containing Pt NPs, the concentration of the Pt element in Daphnia crustaceans increased to the maximum after 5 days to 1680 ± 96 μg/g. On subsequent days, the concentration decreased to 860 ± 45 µg/g after 28 days (Fig. 3a). The decrease in the Pt concentration in Daphnia crustaceans from days 10 to 28 of exposure is partly due to its loss during two or three molts that occurred during this time, with excretion through the digestive tract, and with the birth of juveniles, which were not sent for analysis.
The results obtained after one day of washing show that the purification of Daphnia from accumulated Pt NPs is very fast: kcl = 5.2/day (0.2/h), t1/2 = 0.13 days (3.2 h).
This indicates the accumulation of NPs on the surface of the body and in the digestive tract of Daphnia and their fairly rapid washing out upon the transition to a medium free of NPs. In addition, there is the factor of the adhesion of NPs to the exoskeleton, described in [36] for TiO2 NPs. This can be confirmed by the overall balance of accumulated Pt per day of incubation: with a Daphnia weight of ~50 mg and filtration up to 10 dm3 liquids per day of 1 g of Daphnia from a medium with a concentration of 1 mg/dm3 can absorb no more than 200 µg of Pt. Exceeding this value indicates the process of adhesion.
The main indicators of bioaccumulation are also not constant and depend on the duration of exposure (Table 2).
Thus, NPs entering the hydrosphere accumulate in the bodies of Daphnia magna in large quantities already on the first day after entering the habitat and reach a maximum concentration within one week. The amount of accumulated NPs (approximately 2000 times concentrated) may pose a danger to consumers of a higher trophic level, and such accumulation should be taken into account when assessing the possible impact of objects of the nanoindustry on the adjacent area.
Accumulation of Pt NPs by Daphnia feeding on contaminated algae. In this experiment, a suspension of chlorella grown over 24-h cultivation in a dispersed system of Pt NPs with a concentration of 1.0 mg/dm3 was used as food for Daphnia. After washing off Tamiya medium and free NPs (as indicated above), the chlorella concentrate was diluted with cultivation water to an optical density of 0.300 units, which corresponds to 1.7 × 107 cells/mL suspension. The exposure of Daphnia was carried out in 2-L containers at a rate of 1 L of cultivation medium per 25 Daphnia. 20 mL of feed suspension was added per liter.
Based on the average chlorella-cell diameter of 2.5 μm, the indicated number of cells per milliliter of suspension, and the Pt concentration of 600 μg/g of chlorella, ~1.6 μg of Pt per liter of cultivation medium was introduced into the test system at a single feeding (1.6 × 10–9 g/g medium).
24 h after the intake of NPs in the form of food with contaminated algae, the Pt concentration in the body of Daphnia was 5.44 ± 0.91 μg/g and increased to 12.83 ± 2.16 μg/g after 5 days of feeding (Fig. 3b). Thus, on the first day, the BAF amounted to 3400, which exceeds bioconcentration from the environment.
The purification-rate coefficient is kcl = 0.7/day (0.03/h), t1/2 = 1 day, which is significantly less than when entering directly from the environment. This indicates the possible transition of NPs from the gastrointestinal tract (GI tract) to tissue and difficult cleansing after transfer to an intact environment.
The main indicators of bioaccumulation depending on the duration of exposure are given in Table 3.
The data obtained are consistent with the bioconcentration data presented in [22]. Thus, for Au NPs with a diameter of 4 and 18 nm, accumulation was shown for four days with a BAF of 6641 and 10 207 [37]. However, the calculations given here for bioaccumulation from contaminated algae Selenastrum capricornutum do not stand up to criticism without taking into account the amount of NPs introduced with the algae.
We note the complexity of modeling these processes as applied to NPs. Under incubation conditions, several processes take place simultaneously:
—the consumption of NP-contaminated Chlorella cells by Daphnia, determined by the coefficient of trophic activity;
—transition of a part of the NPs into Daphnia tissues (active and passive);
—ejection from the body of Daphnia of a part of NPs with waste products (kcl);
—decrease in the specific concentration of NPs in the biomass of Daphnia, associated with dilution of the growing biomass (kg).
In addition, the processes of the purification of chlorella cells from NPs, determined by kcl chlorella, and the transition of NPs into the cultivation medium with subsequent aggregation-sedimentation elimination proceed. This creates significant difficulties for modeling and suggests (as the optimal one) the way of direct measurement of these quantities to assess the potential risks of NPs entering the environment.
Accumulation of Pt NPs by fish from the cultivation medium. The experiment on BAF estimation in juveniles of Carpio was carried out according to the standard procedure [31]. After 24 h of incubation in the experimental medium containing Pt 5 nm in size at a concentration of 1 mg/L, the concentration of the Pt element in the fish body increased insignificantly, up to 0.7 ± 0.1 µg/g. The maximum increase in the concentration was observed after 10 days: up to 51.7 ± 10.0 µg/g (Fig. 4a). The subsequent decrease in the concentration to 18.8 ± 3.2 µg/g may be associated with the activation of protective mechanisms that prevent the accumulation of heavy metals.
The effective average daily concentration was calculated for each period, taking into account daily replenishment of the solution by 10% and a complete change in the solution once every 10 days. Simultaneously with each sampling of Carpio the analysis of samples of the cultivation water was carried out. Based on the obtained data, the purification rate was kcl = 5/day (0.1/h), t1/2 = 0.3 days (6.73 h).
The purification of Carpio from the accumulated LF passes very quickly. Possibly, the predominant accumulation of NPs occurs on the skin and in the digestive tract of Carpio, and in the phase of purification, they are rather quickly washed away upon transition to a medium free from NPs. This assumption is confirmed by the results of analysis of accumulation in the organs and tissues of Carpio (Fig. 5).
The main indicators of bioaccumulation depending on the duration of exposure are given in Table 4.
Accumulation of Pt NPs by fish feeding on contaminated Daphnia. In the used contamination model of Carpio according to the food chain, the source of NPs was Daphnia aged 4–6 days after 24-h cultivation in a dispersed system of Pt NPs. The concentration of Daphnia-associated NPs was 3063 ± 582 μg/g.
Carpio. The NPs were kept in cultivation water at a rate of 3 L of water per 1 g of wet weight of fish. Every day, 10% of the cultivation water was replaced with fresh water. Feeding was carried out in the volume of 1–2% of the wet weight of the fish. Such a volume of feed allows a constant weight of the fish to be maintained [31]. Based on the concentration of NPs accumulated in Daphnia, the mass of Daphnia used for feeding, and the volume of water, the concentration of Pt NPs in the model was 0.02 mg/L (0.02 μg/g of medium).
24 h after the intake of NPs in the form of food, the Pt concentration in the fish homogenate increased to 6.8 ± 1.4 μg/g followed by a decrease and re-growth up to 5.9 ± 1.2 µg/g on the 20th day (Fig. 4).
Despite a significant difference in the concentrations of NPs introduced into the medium by the indicated methods (1.0 mg/L in DS and 0.02 mg/L in Daphnia), the accumulated concentration in fish differs not so significantly: the maximum recorded values are 51.7 and 6.8 μg/L, respectively.
Purification from NPs in this experiment was much slower than in the series with the accumulation of NPs from the medium. The purification-rate coefficient (kcl) is 0.8/day (0.03/h), t1/2 = 0.9 days (21.7 h), which is 3 times slower than in the experiment on the accumulation of NPs from the medium. The main indicators of bioaccumulation depending on the duration of exposure are given in Table 5.
Distribution of Pt NPs in the tissues and organs of Carpio upon accumulation from the cultivation medium. Both after the first day of exposure and in subsequent periods, the distribution of accumulated Pt over tissues was uneven (Fig. 5). If we consider accumulation on the first day as a model of the emergency release of nanomaterials, then the danger of using and manufacturing livestock feed decreases in the following order: gastrointestinal tissues–gills–skin–muscles–skeleton. With prolonged exposure, the danger decreases in the following order: gastrointestinal tissues–gills–muscles–skeleton–skin.
CONCLUSIONS
It has been established that the introduction of Pt NPs into the habitat of aquatic organisms leads to accumulation with chlorella with a coefficient close to 10 000 (9400 ± 1800).
The accumulation of Pt from the environment in crustaceans and fish is approximately the same (PBAmax = 2000 ± 380 and 2400 ± 440, respectively). But when transferred through the food chain, there is a higher level of accumulation of NPs in Daphnia (BAFmax = 8000 ± 1300), than in Carpio (BAFmax = 340 ± 70), which is apparently associated with the accumulation of NPs in their food, chlorella.
After the accidental release of nanomaterials into the environment, to cleanse fish from NPs, exposure to clean water for at least three days is necessary.
REFERENCES
T. Morgaleva, Y. Morgalev, I. Gosteva, et al., AIP Conf. Proc. 1899, 050004 (2017). https://doi.org/10.1063/1.5009867
S. Morgalev, T. Morgaleva, I. Gosteva, and Y. Morgalev, IOP Conf. Ser.: Mater. Sci. Eng. 98 (2015). https://doi.org/10.1088/1757-899X/98/1/012006
C. Coll, D. Notter, F. Gottschalk, et al., Nanotoxico-logy 10, 436 (2016).
I. V. Lushchaeva, Y. N. Morgalyev, and S. V. Loiko, Nano Hybrids Composites 13, 108 (2017).
Y. Li, W. Zhang, J. Niu, and Y. Chen, Environ. Sci. Technol. 47, 10293 (2013).
W. Zhang, Y. Li, J. Niu, and Y. Chen, Langmuir 29, 4647 (2013).
T. Astafurova, A. Zotikova, Y. Morgalev, et al., IOP Conf. Ser.: Mater. Sci. Eng. 98 (2015). https://doi.org/10.1088/1757-899X/98/1/012004
Z. Y. Wang, J. Li, J. Zhao, and B. S. Xing, Environ. Sci. Technol. 45, 6032 (2011).
J. Geisler-Lee, Q. Wang, Y. Yao, et al., Nanotoxicology 7, 323 (2013).
W. Zhang, B. Rittmann, and Y. Chen, Environ. Sci. Technol. 45, 2172 (2011).
S. Patil, A. Sberg, and E. Heckert, et al., Biomaterials 28, 4600 (2007).
H. Schwegmann, A. J. Feitz, and F. H. Frimmel, J. Colloid Interface Sci. 347, 43 (2010).
S. Lekamge, A. F. Mira, A. S. Ball, et al., PLoS One 14 (4) (2019). https://doi.org/10.1371/1
Y. Wang, X. Zhu, Y. Lao, et al., Sci. Total Environ. 565, 818 (2016).
B. Zhu, S. Zhu, J. Li, et al., Toxicol. Res. 7, 897 (2018). https://doi.org/10.1039/c8tx00084k
X. Zhu, L. Zhu, Y. Chen, and S. Tian, J. Nanopart. Res. 11, 67 (2009).
L. K. Adams, D. Y. Lyon, A. McIntosh, and P. J. Alvarez, Water Sci. Technol. 54, 327 (2006).
X. Zhu, Y. Chang, and Y. Chen, Chemosphere 78, 209 (2010).
C. M. Zhao and W. X. Wang, Environ. Sci. Technol. 44, 7699 (2010).
J. Hu, D. Wang, and J. Wang, Environ. Pollut. 162, 216 (2012).
X. Zhu, J. Wang, X. Zhang, et al., Chemosphere 79, 928 (2010).
X. Zhang, H. Sun, Z. Zhang, et al., Chemosphere 67, 160 (2007).
J. L. Bouldin, T. M. Ingle, A. Sengupta, et al., Environ. Toxicol. Chem. 27, 1958 (2008).
R. Werlin, J. H. Priester, R. E. Mielke, et al., Nat. Nanotechnol. 6, 65 (2011).
M. Ates, Z. Arslan, V. Demir, et al., Environ Toxicol. 30, 119 (2015). https://doi.org/10.1002/tox.22002
W. M. Lee, S. J. Yoon, Y. J. Shin, and Y. J. An, Environ. Pollut. 201, 10 (2015).
V. Svetlichnyi, A. Shabalina, I. Lapin, et al., Appl. Surf. Sci. 372, 20 (2016).
ISO Water Quality Sampling, “Guidance on biotesting of samples,” ISO 5667, Pt. 16 (Wiley-VCH, Weinheim, 1997). http://www.iso.org.
S. Morgalev, T. Morgaleva, Y. Morgalev, and I. Gosteva, Adv. Mater. Res. 1085, 424 (2015). https://doi.org/10.4028/www.scientific.net/amr.1085.424
OECD Test No. 236, “Fish Embryo Acute Toxicity (FET),” Guideline for Testing of Chemicals (2013).
OECD Test No. 305, “Bioaccumulation in fish: Aqueous and dietary exposure,” OECD Guidelines for the Testing of Chemicals, Sect. 3 (OECD, Paris, 2012). https://doi.org/10.1787/9789264185296-en
OECD Test No. 317, “Bioaccumulation in terrestrial oligochaetes,” OECD Guidelines for the Testing of Chemicals, Sect. 3 (OECD, Paris, 2010). https://doi.org/10.1787/9789264090934-en
S. J. Klaine, P. J. Alvarez, G. E. Batley, et al., Environ. Toxicol. Chem. 27, 1825 (2008).
Globally Harmonised System of Classification and Labelling of Chemicals ST/SG/AC.10/30/Rev.6 (2015), p. 536. https://doi.org/10.18356/4e868e57-ru
“Method for determining the toxicity of water and water extracts from soils, sewage sludge and waste, drinking, waste and natural water by changing the optical density of the Chlorella vulgaris Beijer algae culture,” PND F T 14.1:2:4.10-2004 (Moscow, 2014).
X. Zhu, J. Wang, X. Zhang, et al., Chemosphere 79, 928 (2010).
M. Osborne-Koch, “Uptake of gold nanoparticles in an algae–daphnid food chain,” Master’s Thesis (Clemson Univ., Clemson, SC, 2009).
Funding
This work was carried out with the financial support of the Russian Science Foundation (grant no. 20-17-00185).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgalev, Y.N., Morgaleva, T.G. & Morgalev, S.Y. Transfer of Pt Marker Nanoparticles in a Three-Link Trophic Chain Chlorella Beijer–Daphnia magna Straus–Cyprinus carpio. Nanotechnol Russia 17, 202–210 (2022). https://doi.org/10.1134/S2635167622020148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2635167622020148