Abstract
To describe the viscosity of aqueous suspensions of carbon nanotubes, a structural (fractal) model was used, which was previously used for polymer solutions. This model adequately interprets the dependence of the suspension viscosity on the concentration of carbon nanotubes. When the percolation threshold for this nanofiller is reached, a sharp increase in the viscosity of aqueous suspensions is observed. The model also adequately reflects the dependence of viscosity on the geometry of carbon nanotubes. Knowledge of the nanofiller structure, characterized by its fractal dimension, allows predicting the degree of reinforcement of solid-phase polymer nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Carbon nanotubes (CNTs) have a specific highly anisotropic structure, which determines their properties: a high aspect ratio and a relatively low modulus of transversal elasticity [1]. Therefore, CNTs in various states (solution, suspension, melt, solid phase) form annular formations, which are a structural analog of macromolecular coils of branched polymer chains [2]. In addition, it was experimentally [2] and theoretically [3] demonstrated that these CNT formations are fractal objects with dimension Df varying in a fairly wide range: Df = 1–3. These circumstances make it possible to use well-developed methods of both classical [4] and fractal [5] physical chemistry of polymer solutions to describe the structure of CNTs in different states. For example, the authors of [6] studied aqueous suspensions of CNTs and found that the behavior of their viscosity is correctly described within the framework of the well-known Shultz–Blashke and Kuhn−Mark−Houwink equations. However, for practical purposes, it is necessary to predict the structure of CNTs when obtaining films of polymer nanocomposites from solution, i.e., when the environment surrounding the CNT changes from solvent to polymer, and further prediction of the properties of solid-phase nanocomposites on this basis. In this work, these problems are solved using the methods of fractal physical chemistry [5] and mechanics [7].
1. MATERIALS AND METHODS
In this work, we used the experimental data [6] for aqueous suspensions of multiwalled carbon nanotubes (MWCNTs) with two lengths (0.37 and 1.10 μm) and a diameter of 10 ± 2 nm. Stable aqueous suspensions of MWCNTs were formed by treating them with acid. The viscosity of these suspensions as a function of the MWCNT concentration in the range of 0.00005–0.012 by volume was determined on a semiautomatic Lauda Viscoboy 2 system using Ubbelohde Schott-Geräte capillary viscometers at a test temperature of 302 K maintained with a water bath [6].
2. RESULTS AND DISCUSSIONS
The Schulz–Blaschke and Kuhn−Mark−Houwink equations used by the authors of [6] do not contain structural characteristics for macromolecular coils of polymers and ring-shaped formations of CNTs. Therefore, in this work, to describe the viscosity of aqueous suspensions of CNTs ηCNT, we used the following fractal relationship [5, 8]:
where c(α) is a constant depending on the swelling coefficient α and is equal in the considered case to 0.049, MM is the molecular weight of CNTs, d is the dimension of the Euclidean space in which the fractal is considered (obviously, in our case, d = 3), Df is the fractal dimension of the ring-shaped formation of CNTs, and m0 is the molecular weight of the CNT “stiffness segment” (analog of the Kuhn segment for a macromolecular coil).
Let us consider the methods for determining parameters MM, Df, and m0 included in Eq. (1). Molecular weight MM is determined as follows [9]:
where VCNT is the volume of CNTs determined from geometric considerations; ρCNT is its density, which is equal to 1500 kg/m3 [6]; and NA is Avogadro’s number. MM is 5.4 × 104 g/mol for long CNTs, and 1.8 × 104 g/mol for short ones.
The value of dimension Df is determined using the equation [3]
where RCNT is the radius of the ring-shaped formations of CNTs, which can be calculated using the following ratio [3]:
where rCNT and LCNT are the radius and length of CNTs, respectively, and φn is the volumetric content of CNTs, which is given in [6] for the considered aqueous CNT suspensions.
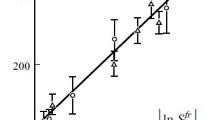
A comparison of dependences ηCNT(φn) in double logarithmic coordinates calculated according to the proposed method and given in [6] is shown in Fig. 1. Their comparison showed good agreement of both datasets for CNTs with two different lengths: 0.37 and 1.10 μm. It follows from the above graph that, at φn ≈ 0.0015, a sharp increase in the viscosity of the aqueous CNT suspension begins with an increase in their content. This transition can be associated with the achievement of the φn value of CNT percolation threshold φc, after which highly anisotropic nanotubes form a continuous percolation framework. The easiest way to estimate the value of φc is as follows [10]:
where DCNT is the outer diameter of the CNTs.
The obtained values of φc are shown in Fig. 1 with vertical dashed lines and are in good agreement with the onset of a sharp increase in the viscosity of the CNT aqueous suspension.
The process of obtaining nanocomposite films from solutions in organic solvents is characterized by the evaporation of the latter, which leads to a change in the environment surrounding the CNTs from solvent molecules to macromolecular coils of the polymer matrix. The degree of influence of such a change on the structure of CNTs in a polymer matrix, characterized by dimension Df, can be estimated within the framework of the model [11] by applying the following equation:
where \(D_{f}^{m}\) and \(D_{f}^{{{\text{nan}}}}\) are the fractal dimension of the macromolecular coil of the polymer matrix, taken equal to 1.80 [5], and the initial nanofiller (\(D_{f}^{{{\text{nan}}}}\) = 1.0), respectively.
Estimates according to Eqs. (3) and (6) had demonstrated that a change in the environment surrounding CNTs from a low-molecular solvent (in this case, water) to a high-molecular polymer matrix leads to a significant increase in the fractal dimension of their structure, which is especially pronounced at low concentrations of CNTs (Fig. 2).
Dependences of fractal dimension Df of CNTs on their volumetric content φn calculated according to (1) Eqs. (3) and (2) (6). Dashed line 3 shows calculation of the hypothetical dependence Df(φn) according to Eq. (6) provided \(D_{f}^{m}\) = 0.
The estimate of the Df value, according to Eq. (3), can be checked using formula (6) with the replacement of the dimension by dimension of the low-molecular solvent (water) \(D_{f}^{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}\), assuming the latter to be zero. This means that H2O molecules are treated as point objects. In Fig. 2, dependence Df(φn), calculated under the condition \(D_{f}^{m}\) = \(D_{f}^{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}\) = 0, is shown by a dashed line, and a fairly good correspondence between them follows from comparison of curves 1 and 3: the average discrepancy is ~10%. One of the reasons for this discrepancy may be the assumption of the condition \(D_{f}^{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}\) = 0, although it is currently known that water molecules can form clusters with dimension \(D_{f}^{{{{{\text{H}}}_{{\text{2}}}}{\text{O}}}}\) > 0 [12].
Using the values of fractal dimension Df of CNTs in the polymer matrix determined according to Eq. (6), one can predict the degree of reinforcement of polymer/CNT film samples within the framework of the fractal model of reinforcement of polymer nanocomposites, the main relation of which has the following form [13]:
where En and Em are the elastic moduli of the nanocomposite and the matrix polymer, respectively (the ratio En/Em is usually called the “degree of reinforcement of the nanocomposite”).
The results of [14] for polyvinyl alcohol (PVA)/carbon nanotube (CNT) composites prepared from solutions of these nanocomposites in deionized water were used as experimental data to check the correctness of prediction, according to the proposed technique. These experimental results are closest to those obtained in this work by the theoretical method. Figure 3 shows a comparison of the experimentally obtained for PVA/CNT nanocomposites and dependences of degree of reinforcement En/Em on volumetric nanofiller content φn calculated according to Eq. (7), which showed their good agreement (the average discrepancy between the theory and experiment is less than 7%). The indicated correspondence presupposes the correctness of the theoretical analysis performed in this work.
In conclusion, let us note different trends in the viscosity of CNT aqueous suspensions and the degree of reinforcement of polymer nanocomposites: if, after the value of φn reaches CNT percolation threshold φc, a sharp increase in the viscosity of the CNT suspension is observed (Fig. 1), then the degree of reinforcement of nanocomposites slows down its increase under the same conditions, tending to an asymptotic value (Fig. 3).
CONCLUSIONS
Thus, the results of this work have demonstrated that the proposed fractal model correctly describes the experimentally obtained dependences of the viscosity of an aqueous suspension of CNTs on their content. At the same time, the specified model includes only geometric and structural parameters of this type of nanofiller. The environment surrounding nanotubes significantly affects their structure. Achievement of the percolation threshold by the nanofiller content determines a sharp increase in the viscosity of CNT aqueous slurry. The most important result is the ability to predict the characteristics of the final nanocomposites based on the structural parameters of aqueous suspensions of nanofillers (CNTs).
REFERENCES
M. Moniruzzaman and K. I. Winey, Macromolecules 39 (16), 5194 (2006). https://doi.org/10.1021/ma060733p
D. W. Schaefer and R. S. Justice, Macromolecules 40 (24), 8501 (2007).
A. K. Mikitaev and G. V. Kozlov, Dokl. Phys. 60 (5), 203 (2015). https://doi.org/10.1134/S102833581505002X
V. P. Budtov, Physical Chemistry of Polymer Solutions (Khimiya, St. Petersburg, 1992) [in Russian].
G. V. Kozlov, I. V. Dolbin, and G. E. Zaikov, The Fractal Physical Chemistry of Polymer Solutions and Melts (Apple Academic, Toronto, 2014).
M. S. P. Shaffer and A. H. Windle, Macromolecules 32 (20), 6864 (1999). https://doi.org/10.1021/ma990095t
L. B. Atlukhanova and G. V. Kozlov, Physicochemistry of Nanocomposites Polymer-Carbon Nanotubes (Sputnik+, Moscow, 2020) [in Russian].
G. V. Kozlov and I. V. Dolbin, Polym. Sci., Ser. B 44 (1–2), 4 (2002). https://elibrary.ru/item.asp?id=13393065
G. V. Kozlov and D. S. Sanditov, Anharmonic Effects and Physico-Mechanical Properties of Polymers (Nauka, Novosibirsk, 1994) [in Russian].
L. Berhan and A. M. Sastry, Phys. Rev. E 75 (4), 041120 (2007). https://doi.org/10.1103/PhysRevE.75.041120
H. G. E. Hentschel and J. M. Deutch, Phys. Rev. A 29 (3), 1609 (1984). https://doi.org/org/10.1103/PhysRevA.29.1609
R. K. Chakraborti, K. H. Gardner, J. E. Atkinson, and J. E. V. Benschoten, Water Res. 37 (4), 873 (2003). https://doi.org/10.1016/s0043-1354(02)00379-2
L. B. Atlukhanova, G. V. Kozlov, and I. V. Dolbin, Inorg. Mater.: Appl. Res. 11, 188 (2020). https://doi.org/10.1134/S2075113320010049
M. Cadek, J. N. Coleman, V. Barron, K. Hedicke, and W. J. Blau, Appl. Phys. Lett. 81 (27), 5123 (2002). https://doi.org/10.1063/1.1609255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Selikhanovich
Rights and permissions
About this article
Cite this article
Kozlov, G.V., Dolbin, I.V. Physicochemical Analysis of the Structure and Properties of Polymer/Carbon Nanotube Nanocomposites Obtained from Solution. Tech. Phys. 66, 1131–1134 (2021). https://doi.org/10.1134/S1063784221080119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784221080119