Abstract
The incorporation and activation of arsenic from tris(dimethylamino)arsine in CdTe layers grown by metal-organic chemical vapor deposition from dimethylcadmium and diisopropyltellurium on GaAs substrates are investigated. The incorporation of arsenic into CdTe depends on the crystallographic orientation of the layers and increases in the series (111)B → (100) → (310). The arsenic concentration in the CdTe layers is proportional to the tris(dimethylamino)arsine flow rate at a power of 1.4 and increases with decreasing diisopropyltellurium/dimethylcadmium ratio from 1.4 to 0.5. After deposition, the CdTe:As layers have p-type conductivity with an arsenic concentration of 1 × 1017–7 × 1018 cm–3 and a hole concentration of 2.7 × 1014–4.6 × 1015 cm–3, respectively; the fraction of electrically active arsenic does not exceed ~0.3%. After annealing in argon (250–450°C), the highest hole concentration is 1 × 1017 cm–3, and the arsenic activation efficiency is ~4.5%. The ionization energy of arsenic determined from the temperature dependence of the hole concentration is in the range of 98–124 meV. The low-temperature photoluminescence spectra of the layers have an emission peak with an energy of ~1.51 eV, which can be attributed to donor–acceptor recombination, where AsTe is an acceptor with an ionization energy of ~90 meV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Cadmium telluride (CdTe) is a direct-gap semiconductor with a band gap of 1.48 eV at 300 K, which is close to ideal for solar-energy conversion. CdTe has a high absorption coefficient of optical radiation (~105 cm–1 at 700 nm), which makes it possible to absorb ~90% of incident radiation in layers 2–3 μm thick. This makes CdTe one of the most promising materials for creating efficient solar-energy converters. Thin-film solar cells based on p-CdTe/n-CdS polycrystalline structures are characterized by one of the highest efficiencies (22.1% [1]) among solar cells of this type. The main problem limiting their effectiveness is a low open-circuit voltage, the value of which depends on the holes concentration and the minority charge carriers lifetime in p-CdTe [2].
The achievement of a hole concentration of p > 1017 cm–3 in p-CdTe layers is one of the necessary conditions for manufacturing efficient solar cells. In CdTe, certain intrinsic defects (for example, VCd) as well as impurity elements of group I (Cu, Ag, Au) and group V (N, P, As, Sb) of the periodic table have acceptor properties. The elements of group V have low ionization energies (the ionization energies of N, P, and As are 56, 68, and 60–120 meV, respectively [3–6], and that of Sb is 280 meV [7]) and noticeably lower values of the diffusivity in comparison with the elements of group I, which makes them most promising for controlling the electrophysical properties of CdTe. In this case, the energies of incorporation for N and P atoms into the CdTe crystalline lattice are rather high, and doping with them in most deposition technologies presents certain difficulties. Therefore, the behavior of the As impurity in CdTe is currently being studied most actively. However, the production of p-type CdTe:As with a high concentration of holes remains a difficult scientific challenge for different growth methods. This is due to the amphoteric behavior of arsenic in CdTe (being incorporated into the anion AsTe or cation AsCd sublattice, arsenic exhibits acceptor or donor properties, respectively) and a high degree of compensation in the material induced by the formation of complexes of the intrinsic-defect–impurity type and AX centers [8, 9]. In the case of using a polycrystalline material, effects associated with an inhomogeneous impurity distribution in crystallites and at the grain boundaries appear. Investigation of the behavior of the arsenic impurity in CdTe single-crystal layers simplifies the task.
The production of CdTe epitaxial layers by metal-organic chemical vapor deposition (MOCVD) makes it possible to control the concentration of their intrinsic defects and change the conditions for embedding impurities into layers due to the use of various precursors and their ratios. Arsine and its derivatives are used as sources of arsenic impurity in the MOCVD method [10–12] and, recently, tris-dimethylaminoarsine (TDMAAs, As[N(CH3)2]3) is most widely applying [6, 13–16]. This is due to its low decomposition temperature (50% at 350°C [17]) as well as a low vapor pressure for better doping control.
The purpose of this study is investigation of the dependens of incorporation and activation of arsenic in epitaxial CdTe layers on MOCVD growth conditions—concentration of the doping precursor (TDMAAs), the crystallographic orientation of the substrate, and the ratio between the initial dimethylcadmium (DMCd) and diisopropyltellurium (DIPTe), as well as the post-growth annealing of the layers.
2 EXPERIMENTAL
Epitaxial CdTe layers were grown from DMCd and DIPTe vapors in a vertical quartz reactor with hot walls (~300°C) at a total pressure of 20 kPa and a substrate temperature of 330°C. Semi-insulating “epiready” GaAs substrates with the crystallographic orientation (100), the deviation was 4° → 110, (310) ± 0.5°, and (111)B ± 0.5° were used. The purity of metalorganic compounds (MOCs) was at least 99.999% (manufacturer LLC “ELMOS”). The carrier gas was hydrogen purified by diffusion through a Pd filter.
Before deposition of the layers, the substrates were annealed at 550°C for 15 min in a hydrogen flow. A buffer layer of undoped CdTe 2–3 μm thick was preliminarily deposited onto the substrates to prevent diffusion of the substrate components into the layer. The DMCd flow was fixed at 1 × 10–4 mol/min. The DIPTe flow was varied from 5 × 10–5 to 1.4 × 10–4 mol/min (the ratio was DIPTe/DMCd = 0.5–1.4), and the TDMAAs flow was varied from 2 × 10–7 to 1 × 10–6 mol/min. The total hydrogen flow into the reactor was 3 norm.L/min. Under similar conditions, we also grew undoped CdTe layers (without adding TDMAAs to the gas phase) and used them as reference samples for reliable interpretation of the properties of the doped layers. The crystallographic orientation of all the grown layers coincided with the orientation of the substrates used according to data of the double-crystal X-ray diffraction method.
The thickness of the grown layers was determined from the infrared (IR) transmission spectra obtained using a Nicolet 6700 Fourier spectrometer. The spectra were measured at 295 K in the range of wave numbers from 500 to 6000 cm–1 with a resolution of 0.5 cm–1. The arsenic concentration and its distribution over the thickness of the structures were determined by secondary ion-mass spectrometry (SIMS) using a TOF.SIMS-5 device. The concentration profiles were obtained by alternating pulsed ion beams: an analyzing beam of Bi+3 with an energy of 25 keV, and a current of 1 pA; and a sputtering beam of Cs+ with an energy of 1 keV. The absolute arsenic concentration was calculated by the quantitative analysis of SIMS spectra using reference samples obtained by the ion-implantation method.
The dark electrophysical parameters of the grown films were determined from measurements of the Hall effect in a constant magnetic field B = 0.5 T on samples with dimensions of 1.5 × 1.5 cm and with a doped-layer thickness no less than 3 μm in the temperature range of 240–340 K. Ohmic contacts with a diameter of ~1.5 mm to p-CdTe were made by the chemical deposition of gold onto the corners of the samples from an aqueous solution of HAuCl4 ⋅ 4H2O.
We studied the nature of the presence of arsenic atoms in the CdTe crystal lattice and the effect of production conditions and the post-growth annealing of the layers on the behavior of the impurity by the method of steady-state photoluminescence (PL). The PL was detected using an Acton SP2500i grating spectrometer with a base of 0.5 m equipped with a Spec-10:256ETE CCD camera (Princeton Research) with a resolution of 0.5 nm. The PL was excited by a femtosecond Ti:Sapphire laser “Tsunami” (Spectra-Physics) with the frequency doubled to 390 nm. The average excitation power was 10 mW at an illumination-spot diameter of ~100 μm. The measurements were carried out at a temperature of 4 K; to maintain the temperature, the sample was placed in a closed-cycle cryostat.
To increase the concentration of electrically active arsenic, we carried out the post-growth annealing of CdTe layers in a horizontal quartz tube in a flow of high-purity argon (0.3 norm.L/min) in the temperature range of 250–450°C followed by sharp cooling (30–50°C/min).
3 RESULTS AND DISCUSSION
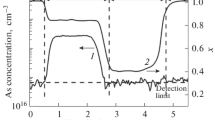
In Fig. 1, we show the arsenic-concentration distribution over the thickness of CdTe layers with the crystallographic orientations (100), (310), and (111)B grown in a single process at the flow ratio DIPTe/DMCd = 1 and a stepwise increase in the TDMAAs flow from 2 × 10–7 to 1 × 10–6 mol/min. An increase in the TDMAAs flow leads to increasing arsenic content in the CdTe layers for all crystallographic orientations. With a change in the TDMAAs flow, a sharp profile of changes in the arsenic concentration is observed for the CdTe (100) and (310) layers, while we observed a smooth change in its content for CdTe (111)B. This is explained by the large surface roughness of CdTe (111)B under our deposition conditions, which leads to averaging of the results of measuring the arsenic concentration over depth and to the lack of sharp boundaries between areas with different levels of doping.
The dependence of the arsenic content in the CdTe layers (100) and (310) on the TDMAAs flow (Fig. 1) is described by the expression
where a ≈ 1.4.
The exponent in the power-law dependence of the arsenic concentration in CdTe on the TDMAAs flow is fractional. Due to the complexity of the chemical system, it seems to be a difficult task separating a particular limiting stage of the incorporation of As into CdTe. From [17], it is known that the limiting stage of the gas-phase thermal decomposition of TDMAAs in hydrogen is homolytic cleavage of the As–N bond. The dimethylaminyl and hydrogen radicals formed in the decay process interact with the initial TDMAAs molecule, which increases the rate of its decomposition. In the process of CdTe growth, reactions involving precursors can take place both in the gas phase and on the surface. The deviation of a from 1 may be due to the incorporation of arsenic into layers also because of the supply of arsenic through other channels of the joint decomposition of TDMAAs, DMCd, and DIPTe, which is complicated by the effect of the deposition surface instead of only as a result of the complex kinetics of TDMAAs decomposition. In this case, we can rule out the formation of an adduct between DMCd, TDMAAs, and its decomposition products [14], since an increase in the TDMAAs flow from 2 × 10–7 to 1 × 10–6 mol/min did not lead to an increase in the CdTe deposition rate for the growth orientations under study. For TDMAAs-doped CdTe(100) layers grown from diethyltellurium (DETe) and DMCd vapors, we previously obtained a similar power-law dependence with the coefficient a = 1.5, which is close in value [13].
The results of the experiment on growing the CdTe layers (100), (310) and (111)B in one process at a varying DIPTe/DMCd ratio and fixed TDMAAs flow of 5 × 10–7 mol/min are shown in Fig. 2. For all crystallographic orientations, three doping steps are distinguishable. As expected [6, 13], the incorporation of arsenic into CdTe layers increases with decreasing DIPTe/DMCd ratio from 1.4 to 0.5. The change in the DIPTe/DMCd ratio in the gas phase enables us to control the effective Te/Cd ratio on the growth surface and pass from Te to Cd saturation according to the deposition conditions. Under Cd-rich conditions, the formation energy of tellurium vacancies VTe decreases, which increases the incorporation of arsenic into the anion sublattice [14].
From Figs. 1 and 2, it follows that the incorporation of arsenic in CdTe has a pronounced dependence on the crystallographic orientation of growth and increases in the series (111)B → (100) → (310). This is indicative of the limiting role of the surface stages in the doping process. The smallest incorporation of arsenic is observed for the Te-enriched (111)B surface, which contains atoms with one dangling bond available for attaching a Cd atom. The arsenic content for the orientation (100) is higher than for (111)B, since the Cd or Te atoms on its surface contain two dangling bonds [10]. The unreconstructed surface (310) of crystals such as diamond and sphalerite represents a system of terraces (100) of equal width separated by steps extending along the directions [100] [18]. Therefore, the probability of arsenic incorporation on it should be higher than for the orientation (100).
To study the electrophysical properties, we used doped CdTe (100) and (310) samples grown at DIPTe/DMCd = 0.5, 1, 1.4 and a TDMAAs flow of 5 × 10–7 mol/min. According to the results of SIMS analysis, the concentration of arsenic in the obtained layers was in the range of (1–7) × 1018 cm–3, which coincides within the measurement error with the data for the samples with a stepped doping profile (Fig. 2). As the comparison samples, undoped CdTe layers obtained under the same conditions without a TDMAAs flow were used. All undoped samples were highly resistive, their resistivity exceeded 106 Ω cm, and the resistivity of the doped layers was in the range of (0.8–5) × 102 Ω cm. The dependences of the concentration and mobility of holes in these layers on the DIPTe/DMCd ratio are presented in Fig. 3. For doped CdTe (100) layers, the concentration of holes increases from 3.8 × 1014 to 4.6 × 1015 cm–3, and their mobility decreases from 40 to 17 cm2/(V s) with an increase in the DIPTe/DMCd ratio from 0.5 to 1.4. At the same time for CdTe (310) layers at DIPTe/DMCd = 1, the minimum concentration and maximum hole mobility are observed at the level of 2.7 × 1014 cm–3 and 42 cm2/(V s), respectively. Thus, the fraction of electrically active arsenic in the obtained layers slightly increased with the DIPTe/DMCd ratio but did not exceed 0.3%.
The electrophysical parameters of CdTe:As(100) layers and their dependence on the DIPTe/DMCd ratio is in a good agreement with the results of [16] in which CdTe(100) was doped with arsenic using the same initial compounds at a higher temperature of 400°C. The resulting layers had p-type conductivity with a hole concentration in the range of 8 × 1014–3 × 1015 cm–3, which increased with the DIPTe/DMCd ratio. This dependence was explained by a decrease in the degree of self-compensation of arsenic due to a decrease its total concentration in the CdTe layers as a result of a growing DIPTe excess. In the model of the compensation of As in CdTe proposed in [19], several types of compensating defects are considered: AsCd and AX centers exhibiting donor properties, and the most probable VCd–AsCd complex, which gives a deep acceptor level (~293 meV), and the VCd–AsTe complex creating a deep donor level (~377 meV). An increase in the DIPTe excess in our case can lead to an increase in the concentration of cadmium vacancies VCd and their complexes VCd–AsCd with arsenic and a simultaneous decrease in the content of compensating donors VCd–AsTe. An increase in the concentration of ionized defects is indirectly indicates by a noticeable decrease in the mobility of charge carriers in the layers obtained at a DIPTe excess.
In the case of the CdTe:As(310) layers, the dependence has a more complex nature, which may be induced by the equilibrium concentration of compensating arsenic intrinsic defects and their complexes different from CdTe:As(100) under these deposition conditions.
To increase the fraction of electrically active arsenic, we carried out a series of annealings of the CdTe(100) doped layers in argon flow. At the same time, a change in the equilibrium concentrations of intrinsic defects, arsenic–defect complexes, and AX centers took place. Undoped CdTe(100) layers, the resistivity of which after treatment exceeded 106 Ω cm, were also annealed. In Fig. 4, we also show the dependences of the concentration and mobility of holes in the CdTe:As(100) layers grown at DIPTe/DMCd = 0.5, 1, 1.4 and the temperature of annealing for 15 min. It can be seen from the figure that the change in the hole concentration in the CdTe:As(100) grown at different DIPTe/DMCd ratios exhibits a similar behavior after annealing at temperatures up to 350°C. After annealing at the temperature Tann = 250°C, the concentration of holes in the layers increases to p = (7–10) × 1015 cm–3. For the layers grown at DIPTe/DMCd = 1 and 1.4, the hole concentration increases further with increasing annealing temperature and reaches a peak of p = (5–6) × 1016 cm–3 at Tann = 400–450°C. The change in the electrophysical parameters noticeably differ after annealing of the samples grown at DIPTe/DMCd = 0.5. With increasing annealing temperature from 250 to 350°C, the hole concentration in the layers increases reaching the values of p = (2–2.5) × 1016 cm–3 and, then, sharply decreases to the level of p = 1 × 1015 cm–3 with increasing annealing temperature. The mobility of holes in the layers increases with rising of annealing temperature, passes through a peak of 33–42 cm2/(V s) at a temperatures of 300–350°C and, then, decreases. The largest value of the fraction of electrically active arsenic after such annealing in the CdTe(100) layers is at the level of ~4.5%. The values of the hole concentration and the fractions of electrically active arsenic obtained by us are close to the data of [11, 16] in which CdTe:As layers with a hole concentration up to p = 6 × 1016 cm–3 were obtained from DIPTe and DMCd vapors. The fraction of electrically active arsenic in this case was ~3% in [11] and did not exceed 1% in [16].
It is known that high-temperature annealing leads to a change in the equilibrium concentration of defects both intrinsic and associated with impurity atoms in doped layers; subsequent rapid cooling results in “hardening” of this state. An increase in the annealing temperature of the CdTe samples obtained at DIPTe/DMCd = 1.0 and 1.4, apparently, leads to a decrease in the concentration of arsenic-induced AX centers (\({\text{As}}_{{{\text{Te}}}}^{ + }\)), the state of which in the crystal lattice is metastable, in favor of the ground acceptor state \({\text{As}}_{{{\text{Te}}}}^{ - }\). It decreases the degree of compensation of acceptors in the layers and increases the concentration of holes. A more complex change in the electrophysical parameters during the annealing of CdTe:As samples obtained at DIPTe/DMCd = 0.5, likely, is explained by the presence of a significant concentration of interstitial cadmium in the layers, which independently forms a deep donor level with an ionization energy of 330 meV [20] and can interact with defects associated with arsenic at higher annealing temperatures.
The hole concentration in the layers grown at DIPTe/DMCd = 0.5 and 1.4 is practically independent of the annealing time and varies in the intervals of p = (0.8–2) × 1016 and (5–6) × 1016 cm–3, respectively. A similar dependence was already observed by us previously during the annealing of CdTe:As(100) layers grown using diethyltellurium (DET), a more thermally stable MOC of tellurium, at 355°C and the ratio DETe/DMCd = 0.5, i.e., at an excess of DMCd [6]. The hole concentration in the layers grown at the ratio DIPTe/DMCd = 1 increases with the duration of annealing and reaches a peak value of p = 1 × 1017 cm–3 for 60 min. This result is slightly higher than the values obtained in [11, 12, 15, 16] and is close to the record values of the hole concentration p = (1–2) × 1017 cm–3 in the arsenic-doped epitaxial CdTe layers obtained by MOCVD [6, 13, 21, 22]. Nevertheless, the fraction of electrically active arsenic in CdTe layers obtained using DIPTe (~4.5%) is significantly lower than the fraction in the CdTe layers obtained previously with DETe (10–85%) [6]. This may be due primarily to a decrease in the concentration of tellurium vacancies (VTe) in the CdTe lattice as a result of the use of less thermally stable MOC tellurium and, therefore, a higher probability of As incorporation into the cation sublattice.
In Fig. 5, we show the temperature dependences of the hole concentration in CdTe:As(100) layers grown at DIPTe/DMCd = 1 and annealed in various modes. From these dependences, we determined the ionization energy of the acceptor level from a model assuming the presence of one discrete acceptor level near the parabolic valence band and a similar donor level near the conduction band [23]. The values of the ionization energy of arsenic in the CdTe:As layers determined from the temperature hole-concentration dependences lie in the range of 98–124 meV. The data obtained agree well with the values of the ionization energy of arsenic in the CdTe layers presented in publications (60–120 meV in [5, 6, 12, 16, 21]).
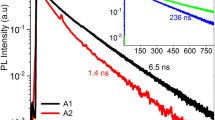
The arsenic ionization energy was estimated from measurements of the low-temperature PL. In Fig. 6, we show the low-temperature PL spectra of CdTe:As(100) layers with a hole concentration of 1 × 1016, 4.1 × 1016, and 5.2 × 1016 cm–3 grown at a ratio of DIPTe/DMCd = 1 and annealed at 300°C, 400°C, and 450°C, respectively. In the PL spectrum of the layer with a hole concentration of 1 × 1016 cm–3, the line with an energy of 1.516 eV is associated with donor–acceptor recombination and has phonon replicas in the low-energy spectral region at 1.488 and 1.453 eV [24]. As the hole concentration increases to 5.2 × 1016 cm–3, the band at 1.516 eV becomes more intense and acquires a more developed phonon structure. The peak with an energy of 1.591 eV is attributed in publications to the recombination of an exciton bound at the AsTe acceptor with an ionization energy of ~90 meV [24]. Using Haynes’ rule [25], we obtain an ionization energy for this acceptor of 92 meV.
Changes in the spectra of low-temperature PL (4 K) of the CdTe:As(100) layers depending on the annealing conditions. The ratio DIPTe/DMCd = 1, the arsenic concentration in the layers is 2.2 × 1018 cm–3, the annealing time is 15 min. The annealing temperature and the hole concentration: 1—300°C, 1.1 × 1016 cm–3; 2—400°C, 4.1 × 1016 cm–3; and 3—450°C, 5.2 × 1016 cm–3.
4 CONCLUSIONS
In a vertical quartz reactor, we grew CdTe epitaxial layers doped with arsenic from TDMAAs using the MOCVD method on GaAs(100), (310), and (111)B substrates at 330°C and a pressure of 20 kPa. According to SIMS data, the arsenic content was in the range of 2 × 1017–7 × 1018 cm–3. It is established that the dependence of the arsenic content in the CdTe(100) and (310) layers is described by the equation CAs ∝ [TDMAAs]1.4 in the range of TDMAAs flows of 2 × 10–7–1 × 10–6 mol/min. With a decrease in the DIPTe/DMCd ratio from 1.4 to 0.5, the arsenic concentration in CdTe increases, which is induced by a change in the effective Te/Cd ratio for atoms on the growth surface. The incorporation of arsenic in CdTe depends on the crystallographic orientation of growth and increases in the series (111)B → (100) → (310). This indicates the limiting role of surface stages in the process. It is shown that the layers of CdTe:As(100) and (310) after deposition had the p-type conductivity with a hole concentration in the range of 2.7 × 1014–4.6 × 1015 cm–3 depending on the DIPTe/DMCd ratio. The post-growth annealing of CdTe:As(100) layers in argon resulted in the partial activation of arsenic as an acceptor. The peak concentration of holes and the fraction of electrically active arsenic were 1 × 1017 cm–3 and ~4.5%, respectively, after annealing of the CdTe:As(100) layers at 400°C for 60 min. From the temperature dependences of the hole concentration in the CdTe:As(100) layers, we determined the ionization energy of arsenic, whose values lie in the range of 98–124 meV. Arsenic in the anion sublattice AsTe acts as an acceptor with ionization energy of ~90 meV and demonstrates a peak in the low temperature photoluminescence spectra due to donor-acceptor recombination with an energy of ~1.51 eV.
REFERENCES
M. A. Green, E. D. Dunlop, D. H. Levi, J. Hohl-Ebinger, M. Yoshita, and A. W. Y. Ho-Baillie, Progr. Photovolt.: Res. Appl. 27, 565 (2019).
Kanevce, M. O. Reese, T. M. Barnes, S. A. Jensen, and W. K. Metzger, J. Appl. Phys. 121, 214506 (2017).
E. Molva, K. Saminadayar, and J. L. Pautrat, Solid State Commun. 48, 955 (1983).
M. Soltani, M. Certier, R. Evrard, and E. Kartheuser, J. Appl. Phys. 78, 5626 (1995).
J. M. Arias, S. H. Shin, D. E. Cooper, M. Zandian, J. G. Pasko, E. R. Gertner, R. E. DeWames, and J. Singh, J. Vac. Sci. Technol. A 8, 1025 (1990).
V. S. Evstigneev, A. V. Chilyasov, A. N. Moiseev, and M. V. Kostunin, Thin Solid Films 689, 137514 (2019).
E. S. Nikonyuk, Z. I. Zakharuk, V. L. Shlyakhovy, P. M. Fochuk, and A. I. Rarenko, Semiconductors 35, 405 (2001).
D. J. Chadi and C. H. Park, Mater. Sci. Forum 196, 285 (1995).
S. H. Wei and S. B. Zhang, Phys. Rev. B 66, 155211 (2002).
L. Svob, I. Cheze, A. Lusson, D. Ballutaud, J. F. Rommeluere, and Y. Marfaing, J. Cryst. Growth 184, 459 (1998).
P. Y. Su, R. Dahal, G. C. Wang, S. Zhang, T. M. Lu, and I. B. Bhat, J. Electron. Mater. 44, 3118 (2015).
M. Ekawa, K. Yasuda, T. Ferid, and M. Saji, J. Appl. Phys. 71, 2669 (1992).
A. V. Chilyasov, A. N. Moiseev, V. S. Evstigneev, B. S. Stepanov, and M. N. Drozdov, Inorg. Mater. 52, 1210 (2016).
V. S. Evstigneev, A. V. Chilyasov, A. N. Moiseev, and M. V. Kostyunin, Inorg. Mater. 55, 984 (2019).
G. Kartopu, O. Oklobia, D. Turkay, D. R. Diercks, B. P. Gorman, V. Barrioz, S. Campbell, J. D. Major, M. K. Al Turkestani, S. Yerci, T. M. Barnes, N. S. Beattie, G. Zoppi, S. Jones, and S. J. C. Irvine, Sol. Energy Mater. Sol. Cells 194, 259 (2019).
P. Y. Su, C. Lee, G. C. Wang, T. M. Lu, and I. B. Bhat, J. Electron. Mater. 43, 2895 (2014).
S. Salim, C. K. Lim, and K. F. Jensen, Chem. Mater. 7, 507 (1995).
M. V. Yakushev, D. V. Brunev, and Yu. G. Sidorov, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 4, 64 (2010).
T. Ablekim, S. K. Swain, W. J. Yin, K. Zaunbrecher, J. Burst, T. M. Barnes, D. Kuciauskas, S. H. Wei, and K. G. Lynn, Sci. Rep. 7, 4563 (2017).
B. E. McCandless, W. A. Buchanan, C. P. Thompson, G. Sriramagiri, R. J. Lovelett, J. Duenow, D. Albin, S. Jensen, E. Colegrove, J. Moseley, H. Moutinho, S. Harvey, M. Al-Jassim, and W. K. Metzger, Sci. Rep. 8, 1 (2018).
S. K. Ghandhi, N. R. Taskar, and I. B. Bhat, Appl. Phys. Lett. 50, 900 (1987).
L. Svob, Y. Marfaing, B. Clerjaud, D. Côte, A. Lebkiri, and R. Druilhe, J. Cryst. Growth 159, 72 (1996).
W. Scott, E. L. Stelzer, and R. J. Hager, J. Appl. Phys. 47, 1408 (1976).
G. L. Burton, D. R. Diercks, O. S. Ogedengbe, P. A. R. D. Jayathilaka, M. Edirisooriya, T. H. Myers, K. N. Zaunbrecher, J. Moseley, T. M. Barnes, and B. P. Gorman, Sol. Energy Mater. Sol. Cells 182, 68 (2018).
J. R. Haynes, Phys. Rev. Lett. 4, 361 (1960).
ACKNOWLEDGMENTS
Analysis of the structures by the SIMS method was carried out using equipment of the Center for Collective Use “Diagnostics of microstructures and nanostructures” of Yaroslavl State University.
Funding
The study was fulfilled on the state order of the Ministry of Science and Education of the Russian Federation (topic no. 0095-2019-0004) and partially supported by the Russian Scientific Foundation (project no. 17-12-01360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Bukhanov
Rights and permissions
About this article
Cite this article
Evstigneev, V.S., Chilyasov, A.V., Moiseev, A.N. et al. Arsenic Doping Upon the Deposition of CdTe Layers from Dimethylcadmium and Diisopropyltellurium. Semiconductors 55, 7–13 (2021). https://doi.org/10.1134/S1063782621010061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063782621010061