Abstract
The quaternary semiconductor compounds Cu2ZnSnSe4, Cu2ZnSiSe4, and Cu2ZnSn1 – xSixSe4 solid solutions on their basis have been synthesized from the elementary components Cu, Zn, Sn, Si, and Se by the single-temperature method. The room- temperature unit-cell parameters of the obtained compounds and solid solutions are determined by the X-ray method. It is established that two series of solid solutions are formed in the Cu2ZnSn1 – xSixSe4 system: one is based on the Cu2ZnSnSe4 compound with a tetragonal structure at x ≤ 0.5 and the other is based on the Cu2ZnSiSe4 compound with a orthorhombic structure at x ≥ 0.7.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The quaternary compounds Cu2ZnSnSe4 and Cu2ZnSiSe4 belong to the large group of chalcogenide semiconductors of the Cu2BIICIVX4 type (where B = Zn, Cd; C = Si, Ge, Sn; X = S, Se), which have been very intensively studied in recent years. The great interest in these compounds is related to their unique optical and electric properties and their application potential in optoelectronics, nonlinear optics, and photovoltaics (as absorbing layers in converters of solar radiation into electric current) [1–14]. In contrast to silicon, which is widely used in solar power engineering, compounds of this class are direct-gap semiconductors with a large absorption coefficient in the visible and near-IR spectral regions. However, despite the efforts of researchers and many works devoted to both obtainment of these compounds and study of their various physical properties, the maximum efficiency of solar radiation conversion of achieved to date for Cu2ZnSnSe4 is at a level of ∼11.1–12.6% [15]. Since Cu2ZnSnSe4 compounds are quaternary, it is very difficult to obtain (using modern technologies) thin Cu2ZnSnSe4 films with a high structural quality and good electrical properties (optimal for solar cells). Generally, one can observe a significant deviation of composition from stoichiometric, which leads to a high concentration of intrinsic structural defects of different nature and occurrence of impurities in the form of binary and ternary phases.
Note that most compounds of the Cu2BIICIVX4 family are crystallized into orthorhombic (sp. gr. Pmn21) or tetragonal (sp. gr. I\(\bar {4}\)2m) crystal structures. As was shown in [16], the Cu2ZnSiSe4 compound is crystallized into an orthorhombic structure of the wurzite‒stannite type (sp. gr. Pmn21) with the unit cell parameters a = 7.8208(2) Å, b = 6.73380(10) Å, and c = 6.45290(10) Å. The Cu2ZnSnSe4 compound has a tetragonal structure (sp. gr. I\(\bar {4}\)2m) with the unit cell parameters a = 5.6955(2) Å and c = 11.3847(3) Å [4].
Since Cu2ZnSnSe4 and Cu2ZnSiSe4 crystals have different crystal structures and are interesting not only from the scientific point of view but also promising for practical applications, much attention is paid to their solid solutions for the following reason: varying the composition, one can obtain novel materials with continuously changing physical properties.
The purpose of this study was to synthesize crystals of Cu2ZnSnSe4 and Cu2ZnSiSe4 compounds and Cu2ZnSn1 – xSixSe4 solid solutions on their basis and find out how their structural characteristics depend on composition.
SAMPLE PREPARATION TECHNIQUE
Quaternary compounds Cu2ZnSnSe4 and Cu2ZnSiSe4 and Cu2ZnSn1 – xSixSe4 solid solutions were synthesized the single-temperature method, which provides purity of prepared material and absence of component loss. The initial substances were elementary components: cooper, zinc, tin, and silicon of 99.999% purity and selenium of high purity. The synthesis was carried out in double quartz ampoules, which were subjected to preliminary thermochemical treatment. Double ampoules were used to protect the synthesized composition from oxidation in air in the case of inner ampoule cracking during crystallization. The ampoule was loaded with initial components in ratios corresponding to a certain formula composition in amount of ∼10–12 g, evacuated to a residual pressure of ∼10–3 Pa, and unsoldered from the vacuum system. Then this ampoule was placed in another ampoule, which was also evacuated and unsoldered, and inserted in a vertical single-zone furnace.
At the initial stage, the furnace temperature was increased with a rate of ∼200°С/h to 600°С and kept at this level for 2 h. Then the temperature was increased with a rate of ∼50°С/h to 960°С. Upon reaching this temperature, vibration mixing was switched on, and the furnace was kept under these conditions for 24 h. Then the vibration was switched off, and the temperature was decreased with a rate of ∼5°С/h to ∼700°С. To homogenize the prepared ingots of compounds and solid solutions, they were isothermally annealed in vacuum at ∼700°С for 300 h. After the annealing the prepared ingots were cooled down to room temperature with a rate of ∼5°С/h.
EXPERIMENTAL
A structural study of the synthesized Cu2ZnSnSe4, Cu2ZnSiSe4 compounds and Cu2ZnSn1 – xSixSe4 solid solutions was carried out on a DRON-3 X-ray diffractometer using monochromatic CuKα radiation. A single-crystal graphite plate, installed in the path of reflected X-ray beam, was used as a monochromator. The diffraction patterns of powder samples of the compounds and solid solutions under study were automatically recorded with a step of 0.03° on the 2θ scale with an exposure of 3 s. The X-ray samples were powders of the prepared compounds and solid solutions, pressed into plastic cuvettes. The crystal structure and unit-cell parameters of the samples were determined from the recorded diffraction patterns by the Rietveld method using the Fullprof software package [17].
RESULTS AND DISCUSSION
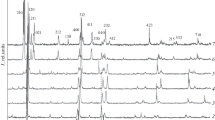
Figure 1 presents room-temperature diffraction patterns of Cu2ZnSn1 – xSixSe4 solid solutions of different compositions with x = 0, 0.3, 0.5, 0.7, and 1. It can be seen that the diffraction patterns of the samples with x = 0, 0.3, and 0.5 contain reflections of only the tetragonal structure, which indicates the single-phase character of these samples. However, at x ≈ 0.6, a structural phase transition from the tetragonal phase into the orthorhombic one occurs. The diffraction patterns of the samples with x = 0.7 and 1 contain only reflections of the orthorhombic structure.
The presented diffraction patterns for the Cu2ZnSn1 – xSixSe4 system indicate that the replacement of tin atoms with silicon atoms of smaller atomic radius leads to a shift of all reflections towards larger angles; i.e., the crystal lattice undergoes compression.
The unit-cell parameters of the synthesized Cu2ZnSn1 – xSixSe4 solid solutions are determined from the obtained diffraction patterns. Figure 2 presents dependences of these parameters on the composition. As can be seen in the figure, the unit-cell parameters of the tetragonal and orthorhombic phases decrease according to a linear law with an increase of x, which corresponds to the Vegard law and indicates the formation of two series of solid solutions in the Cu2ZnSn1 – xSixSe4 system: one is based on the Cu2ZnSnSe4 compound with a tetragonal structure at x ≤ 0.5 and the other is based on the Cu2ZnSiSe4 compound with an orthorhombic structure at x ≥ 0.7.
When analyzing the quality of samples with a tetragonal structure, which is important for solar radiation conversion efficiency, one often estimates the degree of imperfection, which is related to the parameter of lattice tetragonal distortions. This parameter is determined as a deviation of the ratio η = c/2a (a and c are the unit cell parameters) from unity [18].
Table 1 presents the unit-cell parameters of the Cu2ZnSn1 – xSixSe4 solid solutions for both phases. The η values are close to unity for the solid solutions with a tetragonal structure, which indicates small lattice distortions for the Cu2ZnSnSe4 compound and solid solutions on its basis, obtained by the single-temperature method.
Note that the unit-cell parameters of the synthesized Cu2ZnSnSe4 compounds (a = 5.688 Å, c = 11.401 Å) and Cu2ZnSiSe4 (a = 7.825 Å, b = 6.733 Å, c = 6.452 Å) agree well with data of [4, 16].
CONCLUSIONS
The quaternary compounds Cu2ZnSnSe4 with a tetragonal crystal structure, the Cu2ZnSiSe4 compounds with an orthorhombic crystal structure, and Cu2ZnSn1 – xSixSe4 solid solutions were synthesized by the single-temperature method. The unit-cell parameters of the obtained system were determined by the X-ray diffraction method. It was established that a structural phase transition from the tetragonal phase to the orthorhombic phase occurs in the Cu2ZnSn1 – xSixSe4 system at x ≈ 0.6. It is shown that the unit-cell parameters and volume for the Cu2ZnSn1 – xSixSe4 system smoothly decrease according to a linear law with an increase in concentration x, which indicates the formation of two series of solid solutions in the system: one at x ≤ 0.5 on the basis of the Cu2ZnSnSe4 compound with a tetragonal structure and the other at x ≥ 0.7 on the basis of the Cu2ZnSiSe4 compound with an orthorhombic structure.
REFERENCES
D. B. Mitzi, O. Gunawan, T. K. Todorov, et al., Sol. Energy Mater. Sol. Cells 95 (6), 1421 (2011).
A. Walsh, S. Chen, S.-H. Wei, et al., Adv. Energy Mater. 2 (4), 400 (2012).
M. Grossberg, J. Krustok, J. Raudoja, et al., Appl. Phys. Lett. 101 (10), 102102 (2012).
J. He, L. Sun, S. Chen, et al., J. Alloys Compd. 511 (1), 129 (2012).
T. K. Todorov, J. Tang, S. Bag, et al., Adv. Energy Mater. 3 (1), 34 (2013).
N. N. Syrbu, V. Zalamai, M. Guc, et al., J. Alloys Compd. 635, 188 (2015).
A. P. Litvinchuk, V. M. Dzhagan, V. O. Yukhymchuk, et al., Phys. Status Solidi B 253 (9), 1808 (2016).
F.-I. Lai, J.-F. Yang, Y.-L. Wei, et al., Green Chem. 19 (3), 795 (2017).
G. Rey, F. Babbe, T. P. Weiss, et al., Thin Solid Films 633, 162 (2017).
L. T. Schelhas, K. H. Stone, S. P. Harvey, et al., Phys. Status Solidi B 254 (9), 1770247 (2017).
A. U. Sheleg, V. G. Gurtovoi, A. V. Mudryi, et al., Zh. Prikl. Spektrosk. 81 (5), 704 (2014).
A. U. Sheleg, V. G. Gurtovoi, A. V. Mudryi, et al., Semiconductors 48 (10), 1296 (2014).
A. U. Sheleg, V. G. Gurtovoi, and V. A. Chumak, Crystallogr. Rep. 60 (5), 758 (2015).
V. G. Gurtovoi and A. U. Sheleg, Phys. Solid State 59 (2), 242 (2017).
W. Wang, M. T. Winkler, O. Gunawan, et al., Adv. Energy Mater. 4 (7), 36 (2014).
G. Gurieva, S. Levcenko, Victor. C. Kravtsov, et al., Z. Kristallogr.–Cryst. Mater. 230 (8), 507 (2015).
J. Rodríguez-Carvajal, Comm. Powder Diffr. (IUCr), Newslett. 26, 12 (2001).
S. Siebentritt and S. Schorr, Prog. Photovoltaics 20 (5), 512 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Churochkin
Rights and permissions
About this article
Cite this article
Sheleg, A.U., Hurtavy, V.G. & Chumak, V.A. Synthesis and Structural Study of the Cu2ZnSn1 – xSixSe4 System. Crystallogr. Rep. 64, 879–882 (2019). https://doi.org/10.1134/S106377451906018X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106377451906018X