Abstract—
The quaternary compounds Cu2ZnGeSe4, Cu2ZnSiSe4 and their solid solutions are synthesized from the elementary components Cu, Zn, Ge, Si, and Se by the single-temperature synthesis method. The unit-cell parameters of the synthesized compounds and Cu2ZnGe1 –xSixSe4 solid solutions are determined at room temperature by X-ray diffraction (XRD). It is shown that the unit-cell parameters a, b, and c decrease as x increases. It is found that two series of solid solutions are formed in the Cu2ZnGe1– xSixSe4 system: one is based on the Cu2ZnGeSe4 compound and the other is based on Cu2ZnSiSe4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The quaternary compounds Cu2ZnGeSe4 and Cu2ZnSiSe4 belong to a large family of chalcogenide semiconductors of the Cu2BIIC IVX4 type (where B = Zn and Cd; C = Si, Ge, and Sn; X = S, Se, and Te), which are of not only scientific but also practical interest. A number of crystals of this family have nonlinear optical characteristics and can be of interest to optoelectronics. Representatives of this family are promising materials for solar energy—they can be used as converters of solar radiation into electric current [1–9]. In addition, some crystals of this group are good high-temperature thermoelectric materials [10, 11]. Quaternary chalcogenide semiconductors of the Cu2BIICIVX4 family have been studied for several decades. However, their physical properties are still being studied and the nature of their crystal structure is being clarified [12–18].
It is known that some compounds of this family, in particular Cu2ZnSiSe4 and Cu2ZnGeSe4, can have the structure of kesterite, stannite, and wurzite–stannite types depending on the ordering of zinc and copper cations. The authors of [15] calculated the structures that were most stable from an energetic point of view, investigating the phase stability of Cu2ZnSiSe4 and Cu2ZnGeSe4 compounds and the condition of the electronic structure of the kesterite, stannite, and wurtzite–stannite types. It was concluded that the phase of kesterite is more stable than the stannite and wurzite–stannite phases in these compounds contrary to experimental results [17]. One of the reasons for this discrepancy between theoretical and experimental data can be that the atomic scattering factors of copper and zinc X-rays are very close and this makes it difficult to accurately determine the positions of these atoms in the structure.
Precision radiographic studies of the structural characteristics of the Cu2ZnSiSe4 compound on both single crystals and polycrystals were carried out in [17]. It was shown that the Cu2ZnSiSe4 compound has a rhombic structure of the wurzite-stannite type (Space Group Pmn21) with the unit-cell parameters a = 7.8208(2), b = 6.73380(10), and c = 6.45290(10) Å.
The Cu2ZnGeSe4 compound has a tetragonal structure with the unit-cell parameters a = 5.61043(8) and c = 11.0457 (3) Å (Space Group I\(\overline 4 \)2m) [18]; a = 5.6112(1) and c = 11.0473(3) Å [19].
Since the crystals of the Cu2ZnSiSe4 and Cu2ZnGeSe4 compounds have unique physical properties from both scientific and practical points of view and different structures, solid solutions that are based on Cu2ZnGe1 –xSixSe4 are of great interest.
Solid solutions are attractive because it is possible to obtain materials with continuously changing physical properties by varying their composition, which makes it possible to synthesize materials with predetermined characteristics that are practically important.
In this regard, the aim of this work is the synthesis of Cu2ZnSiSe4 and Cu2ZnGeSe4 compounds, as well as solid solutions based on them and determination of the crystallographic characteristics of the obtained materials depending on the composition.
METHODS OF SAMPLE SYNTHESIS
A single-temperature method that ensures purity of the substances being obtained and no loss of components was used for synthesizing the quaternary compounds Cu2ZnSiSe4 and Cu2ZnGeSe4 and Cu2ZnGe1– xSixSe4 solid solutions. The elementary components: copper, zinc, germanium, silicon of 99.999% purity, and selenium of high purity served as elementary components. The initial elementary components were loaded into double quartz cells in ratios corresponding to a certain formula composition in the amount of 15 g. After the internal cell had been evacuated, it was placed in a second quartz cell of larger diameter, which was also evacuated. Double cells were used in order to protect the synthesized composition from oxidation in air if the inner cell cracked upon heating.
A quartz rod was welded to the outer cell from below, the second end of which was attached to a mechanical vibrator. The cell that had been prepared in this way was placed in a vertical single-zone furnace. Vibration mixing, which greatly accelerates synthesis of the compound and prevents explosion of the cell, was used in the process of heating the cell in the furnace. The temperature in the furnace was increased in stages with holding for ~2 h, including vibration. Isothermal annealing of the obtained ingots of compounds and solid solutions was carried out in vacuum at ~720°C for 800 h for their homogenization.
EXPERIMENTAL
The crystallographic parameters of the Cu2ZnSiSe4 and Cu2ZnGeSe4 compounds and Cu2ZnGe1– xSixSe4 solid solutions were measured by a DRONE-3 X-ray diffractometer using monochromatic CuKα-radiation. A single-crystal graphite plate, which was installed along the path of the reflected beam, was used as a monochromator. Powders of the studied compounds, which were pressed into plastic cuvettes, served as samples. The diffraction reflections were recorded automatically with a step of 0.03° by 2θ. The unit-cell parameters were determined by Rietveld refinement using the Fullprof software package [20].
RESEARCH RESULTS AND DISCUSSION
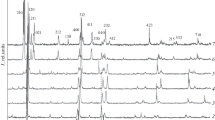
The diffraction patterns of Cu2ZnGe1– xSixSe4 solid solutions of different composition with x = 0, 0.3, 0.5, 0.7, and 1 at room temperature are presented in Fig. 1. The figure shows that two series of solid solutions are formed in the Cu2ZnGe1 – xSixSe4 system: Cu2ZnSiSe4−Cu2ZnGe0.5Si0.5Se4, which is based on Cu2ZnSiSe4 (rhombic structure and space group Pmn21) and Cu2ZnGeSe4−Cu2ZnGe0.7Si0.3Se4, which is based on Cu2ZnGeSe4 (tetragonal structure and space group I\(\overline 4 \)2m) with a boundary between them at x ~ 0.4. It can be seen from the above diffraction patterns that the reflections of the rhombic and tetragonal phases shift to the region of large angles, i.e., the interplanar distances decrease, upon a change in the composition (increase in x).
The parameters of the unit cell of the investigated Cu2ZnGe1– xSixSe4 system were calculated from the obtained diffraction patterns of the samples of different compositions using the Fullprof software package. The parameters of the unit cell and its volume are shown in Table 1.
The concentration dependences of the parameters of the a and b unit cell of the Cu2ZnGe1– xSixSe4 system are presented in Fig. 2. It can be seen from the figure that two series of solid solutions with tetragonal and rhombic structures are observed in this system and the parameters of their elementary cells decrease as x grows.
The dependences of the c parameter and volume V of the unit cell of the Cu2ZnGe1 –xSixSe4 system on the concentration of x are presented in Fig. 3. It is seen that the c parameter and volume V of the unit cell of this system, as well as the a and b parameters, decrease with an increase in the concentration of x. The replacement of Ge atoms with Si atoms leads to a decrease in the crystallographic parameters. This is due to the fact that the atomic radius of silicon is slightly smaller than that of germanium. We note that the investigated crystallographic parameters of the Cu2ZnGe1 –xSixSe4 system decrease linearly when the composition changes.
The values of the unit-cell parameters of Cu2ZnSiSe4 (a = 7.830, b = 6.737, and c = 6.449 Å) and Cu2ZnGeSe4 (a = 5.611and c = 11.051 Å) crystals at room temperature that were obtained in the present work are in good agreement with the data in [17, 18].
Thus, it follows from the obtained experimental data that the a, b, and c parameters of the unit cell and volume V of the crystals of the Cu2ZnGe1– xSixSe4 system gradually decrease with increasing concentration x. The monotonicity and linear character of the change in the parameters indicate that two series of solid solutions with the boundary x ~ 0.4 are formed in the Cu2ZnGe1 –xSixSe4 system according to Vegard’s law.
CONCLUSIONS
The presence of two series of solid solutions in the Cu2ZnGe1 –x SixSe4 system: on the basis of the Cu2ZnSiSe4 compound with a rhombic structure in the Cu2ZnSiSe4−Cu2ZnGe0.5Si0.5Se4 region and on the basis of Cu2ZnGeSe4 with a tetragonal structure in the Cu2ZnGeSe4−Cu2ZnGe0.7Si0.3Se4 region has been determined by the X-ray method for the first time.
The parameters of the unit cell of crystals of the Cu2ZnGe1– xSixSe4 system depending on the concentration of x. It was shown that the parameters and volume of the unit cell of the crystals of the Cu2ZnGe1– xSixSe4 system gradually decrease on both sides of the x ~ 0.4 interface between two rows of solid solutions with the growth of x.
REFERENCES
M. Grossberg, J. Krustok, J. Raudoja, et al., Appl. Phys. Lett. 101 (10), 102102 (2012).
R. Lydia and R. P. Sreedhara, J. Nano-Electronic Physics 5 (3), 03017 (2013).
F. Luckert, D. I. Hamilton, M. V. Yakushev, et al., Appl. Phys. Lett. 99 (6), 062104 (2011).
M. León, S. Levcenko, R. Serna, et al., Mater. Chem. Phys. 141 (1), 58 (2013).
A. Singh, S. Singh, S. Levcenko, et al., Angew. Chem., Int. Ed. Engl. 52 (35), 9120 (2013).
T. K. Todorov, J. Tang, S. Bag, et al., Adv. Energy Mater 3 (1), 34 (2013).
A. U. Sheleg, V. G. Hurtavy, A. V. Mudryi, et al., J. Applied Spectroscopy 81 (5), 776 (2014).
A. U. Sheleg, V. G. Hurtavy, A. V. Mudryi, et al., Semiconductors 48 (10), 1296 (2014).
A. U. Sheleg, V. G. Hurtavy, and V. A. Chumak, Crystallogr. Rep. 60 (5), 758 (2015).
X. Y. Shi, F. Q. Huang, M. L. Liu, et al., Appl. Phys. Lett. 94 (12), 122103 (2009).
M. Ibáñez, R. Zamani, A. LaLonde, et al., J. Am. Chem. Soc. 134 (9), 4060 (2012).
D. B. Mitzi, O. Gunawan, T. K. Todorov, et al., Solar Energy Mater. Solar Cells 95 (6), 1421 (2011).
S. Chen, A. Walsh, Y. Luo, et al., Phys. Rev. 82 (19), 195203.
Q. Shu, J.-H. Yang, S. Chen, et al., Phys. Rev. B 87 (11), 115208.
S. Nakamura, T. Maeda, and T. Wada, Jpn. J. Appl. Phys. 49 (12R), 121203 (2010).
G. Q. Yao, H. S. Shen, E. D. Honig, et al., Solid State Ionics 24 (3), 249 (1987).
G. Gurieva, S. Levcenko, V. C. Kravtsov, et al., Z. Kristallogr. 230 (8), 507 (2015).
O. V. Parasyuk, L. D. Gulay, Y. E. Romanyuk, et al., J. Alloys Compd. 329 (1–2), 202 (2001).
O. Y. Khyzhun, V. L. Bekenev, V. A. Ocheretova, et al., Physica B 461, 75 (2015).
J. Rodriguez-Carvajal, Commission on powder diffraction (IUCr). Newsletter 26, 12 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Petrov
Rights and permissions
About this article
Cite this article
Sheleg, A.U., Hurtavy, V.G. & Chumak, V.A. Synthesis and X-Ray Studies of Cu2ZnGe1 –xSixSe4 Solid Solutions. J. Surf. Investig. 13, 378–381 (2019). https://doi.org/10.1134/S1027451019030182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451019030182