Abstract
The big-scale sand smelt (Atherina boyeri) is an Atlanto-Mediterranean amphidromous fish species found within the Black Sea. Here, we assess differences in the parasite fauna of big-scale sand smelt populations from their natural range in the northwestern Black Sea and from their expansion range in the Lower and Middle River Dnipro. In addition, we undertook a microsatellite analysis to assess the genetic similarity of fish from the different locations. We found that the parasite community of fish in their natural range was wider than that from their expansion range. While the Gulf of Odesa was most distant from all other localities by parasite community composition and the Dnipro Reservoir was characterised by an absence of parasites (newest and most distant expansion locality), only fish from the Danube Delta showed a significant genetic difference. Our results suggest that the parasite community of big-scale sand smelt is primarily influenced by environmental factors, such as habitat type, water salinity and/or prey composition. Both microsatellite analysis and parasite community species composition (e.g. the presence of the marine Telosentis exiguus in the Kakhovka Reservoir and freshwater Raphidascaris sp. in the Gulf of Odesa) confirmed that populations in the River Dnipro reservoirs had, at some time, been connected with native marine populations, thus also confirming the species’ amphidromous nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ukraine is situated on the crossing of several transport corridors, making it a hub for the spread of alien species, with two important routes for aquatic invasive species, the so-called Southern and Central corridors, meeting here (Panov et al. 2009). These corridors have allowed both distant aliens, introduced from remote geographic regions, and neighbouring aliens, to increase their ranges within the same geographic region (Alexandrov et al. 2007). The terminology describing such invasions has received much attention in recent years (e.g. Copp et al. 2005); however, most of these studies have focused on the distribution of Ponto-Caspian gobiids (Gobiidae) in Northern and Southern Europe (see Kvach et al. 2021). It should be noted that there are many other Ponto-Caspian species increasing their ranges along these corridors. For example, in recent years, the riverine systems of Ukraine have been invaded by a group known as neolimnetics, i.e. species that originate in brackish waters but are able to increase their range by invading upstream stretches of freshwater rivers (Kvach and Kutsokon 2017).
One such neolimnetic species is the big-scale sand smelt (Atherina boyeri Risso, 1810; Actinopterygii: Atherinidae; Kvach and Kutsokon 2017), an Atlanto-Mediterranean species that naturally inhabits the eastern Atlantic from the British Isles and the Netherlands in the north to Mauritania and Madeira in the south, including the Mediterranean and Black Seas (Quignard and Pras 1986). Freshwater populations are known from the Santo André lagoon on the Iberian Peninsula, Lake Trichonis in Greece and Lake Trasimeno in Italy (Kottelat and Freyhof 2007). In Italy, the species’ range has expanded into many lakes, most likely through accidental introductions (Moretti et al. 1959; Bianco et al. 2013). In the Black Sea basin, the fish has been translocated into several natural lakes in Central Anatolia (Turkey), reservoirs in the Kizilirmak river basin and some parts of the Dardanelles Straight and Mediterranean drainage basins (Partal et al. 2019). In Ukraine, the species has penetrated as far as the River Dnipro (Dnieper) and has established populations in the Kakhovka, Dnipro and Kamianske reservoirs (Marenkov 2018; Zhukinsky et al. 2007).
Small founding populations, originating from a single introduction, have less variable genotype diversity than populations in their natural range, in part due to ‘bottleneck effects’ or gene drift (Bock et al. 2015). Conversely, multiple introductions significantly increase the success of invasive populations as each new introduction increases the genetic diversity of the introduced population (Roman and Darling 2007). In doing so, they reduce the negative effects of inbreeding caused by a lack of natural selection (Roman and Darling 2007), and, as a result, some alien populations can show higher genetic diversity than native populations of the same species (Kolbe et al. 2008).
In invaded ecosystems, non-native species that have been freed from their natural enemies, e.g. parasites, may be competitively superior to native species, leading to increased population growth due to a lack of spatial regulation (Keane and Crawley 2002). Likewise, native species will have to deal with a full range of pathogens, while alien species, as a rule, tend to have a lower number of natural enemies and a lower proportion of infected individuals (Torchin et al. 2003). The effects of this ‘parasite release’, or more controversially, of ‘co-introduction of parasites with the host’, will depend on different factors, including the size of the founding population (single or multiple introductions), its origin (from source regions with rich/poor parasite fauna) and/or its developmental stage (eggs, larvae or adults). In the case of co-introduction, the parasites ability to spread will also reflect the size of the founding population. Furthermore, any successful introduction of parasites with complex life cycles will require the presence of all components of its life cycle in the recipient ecosystem (MacLeod et al. 2010). In many cases, alien species acquire local parasites and become new competent hosts (Tompkins et al. 2011). In doing so, these new hosts could amplify the transmission dynamics of native parasites, thereby increasing infection levels for native hosts (Kelly et al. 2009).
The aim of the present study was to investigate the parasite community of natural and neolimnetic populations of big-scale sand smelt in different Ukrainian habitats, also characterising the host genetic lineage.
Material and methods
Fish sampling
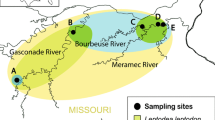
The fish in this study were sampled from the species’ natural range in the northwestern Black Sea and from within its expansion range in the Lower and Middle River Dnipro (see Fig. 1) from July to September 2021 (warm period). All specimens from each locality were caught on the same day and from the same geographical coordinates. Five sites were sampled in total, i.e.:
-
1)
Solonyi Kut Bay in the Danube Delta; 45.472490N, 29.650889E (natural range)
-
2)
Near Cape Malyi Fontan, Gulf of Odesa; 46.441210N, 30.772868E (natural range)
-
3)
Village of Rybakivka, near Cape Adzhiyask, Dnipro Estuary forefront; 46.616132N, 31.376388E (natural range)
-
4)
Village of Mylove, Kakhovka Reservoir, Lower Dnipro, 47.048524N, 33.651200E (expansion)
-
5)
Village of Stari Kodaky, Dnipro Reservoir, Middle Dnipro, 48.370007N, 35.148804E (expansion)
The fish were caught using a 10-m seine with a 6-mm bar mesh size. All fish were immediately transported to the laboratories alive in aerated water (taken from the place of sampling), where they were placed into aerated aquaria to await examination.
Parasitological analysis
In total, 76 fish were examined for parasites (Table 1). All fish were dissected within 3 days of sampling to ensure maximum parasite recovery (see Kvach et al. 2016). Prior to dissection, each fish was measured for standard (SL, mm) and total length (TL, mm). The dissection of larger number of fish was not possible, because the big-scale sand smelt is very sensitive to transportation, quickly dying because of stress. Because the distance between localities is quite high, the transportation and holding of alive specimens was problematic. The additional sampling is impossible now, because of Russian Invasion in Ukraine: several sampling localities are on occupied territory, the Black Sea and low Dnipro ecosystem is totally changed because of the Kakhovka disaster.
For each specimen, the fins, skin, gills, muscle tissue and internal organs were examined for presence of parasites. Unicellular parasites were studied live using light microscopy. Monogeneans were preserved in Glycerine-ammonium-picrate (GAP) and prepared as semi-permanent slides (Malmberg 1970), while digeneans, cestodes and nematodes were preserved in hot 4% formaldehyde solution and stained with iron acetic carmine, dehydrated in ethanol of increasing concentration and mounted in Canada balsam as permanent slides (Georgiev et al. 1986; Cribb and Bray 2010). Acanthocephalans were preserved in 70% ethanol, compressed between two glass slides and then mounted in glycerol as temporary slides for light microscopy. All parasites were identified to species or to genus if identification to species was not possible.
Identification keys were used to identify parasites to species level, or to the lowest possible taxa, based on specific morphological features. Microsporideans were identified alive based on morphology of xenomas and spores (Ovcharenko et al. 2017), while the morphological description provided by Kvach et al. (2019) was used to identify monogeneans and that of Moravec (2013) to identify nematodes. Other parasites were identified using the key provided in Gayevskaya et al. (1975). Scientific names and taxonomy were according to WoRMS (2023).
Indices of prevalence (P, %), intensity range (IR), mean intensity (MI) and mean abundance (A) were calculated for each parasite species and each locality (Bush et al. 1997), with the Czekanowski-Sørensen Index (CSI), presented as a percentage, used to compare the parasite fauna (CSI parameters > 50% considered significant; Sørensen 1948). The Shapiro-Wilk test was used to assess normality of fish size, and the t-test used to assess differences in fish from different localities. Finally, discriminant analysis was used to distinguish fish from different localities by parasite abundance. All statistical analyses were undertaken using StatSoft Statistica for Windows 10.
Molecular study
Material for the molecular study was obtained from 50 big-scale sand smelt sampled from the River Dnipro drainage, i.e. the Dnipro Reservoir (10 ind.) and the Kakhovka Reservoir (10 ind.), and from the Black Sea drainage, i.e. the Gulf of Odesa (10 ind.), Cape Adzhiyask near-shore (10 ind.) and the Danube Delta (10 ind.) (Table 1).
Genomic DNA was extracted from fin clips (preserved in 96% ethanol), based on the method of Sambrook and Russell (2001), using cetyl trimethylammonium bromide (CTAB). Each population was then analysed via PCR using three species-specific microsatellite markers (AthB5, AthF1 and AthD6), as recommended by Milana et al. (2009). The PCR amplification products were then analysed by 7% polyacrylamide gel electrophoresis (Sambrook and Russell 2001). Silver nitrate (AgNO3) staining was then used to visualise the amplification fragments in polyacrylamide gel, according to the Promega Technical Manual (1999).
Genetic diversity between fish populations from different locations was assessed using microsatellite markers and allele frequencies, number of effective and different alleles, Shannon’s information index, observed and expected heterozygosity, fixation index and Nei genetic distance and identity, all calculated using GenAlEx v.6.5 (Nei 1978, 1987; Nei and Li 1979; Peakall and Smouse 2012). A dendrogram of the study populations was then constructed using the paleontological statistics software package for education and data analysis (PAST) (Hammer et al. 2001).
Results
The t-test showed that both SL and TL of fish from the Dnipro Reservoir differed significantly from that at all other localities (p < 0.05). While fish from Cape Adzhiyask had a significantly higher SL than those from the Danube Delta (t = 2.48, df = 33, p < 0.05), there was no significant difference in TL (t = 1.99, df = 33, p < 0.05). In all other cases, no significant differences were observed in fish size between localities (Table 1).
Parasite community
The total parasite fauna comprised 11 species, including one microsporidian, one ciliate, one monogenean, one cestode, four digeneans, one acanthocephalan and two nematode species (Table 2). Highest parasite richness was recorded from the Gulf of Odesa (eight species) and the Danube Delta (seven species), and lowest richness in the Kakhovka Reservoir (two species), with levels near cape Adzhiyask being intermediate (five species). No parasites were found on fish from the Dnipro Reservoir; consequently, these data are not included in Table 2. The highest difference in parasite fauna occurred between the Danube Delta, Gulf of Odesa and Cape Adzhiyask (Table 3), differences between Kakhovka Reservoir and the other study sites being < 50%.
Parasite abundance was non-normally distributed in all cases (Shapiro-Wilk test). While the parasite community from the Gulf of Odesa was significantly different compared to all other study localities (p < 0.05; Table 3), no significant differences were observed between communities from Cape Adzhiyask and the Danube Delta and between Cape Adzhiyask and Kakhovka Reservoir.
Microsatellite analysis
Microsatellite analysis revealed a total of 77 different alleles, with high genetic variability between populations. For the AthF1, AthB5 and AthD6 locі, 26, 30 and 21 alleles were detected, respectively. The number of alleles per locus in each population ranged from 7 to 14, with an average of 10.4 alleles/locus (Table 4). The average number of effective alleles per locus was 7.2 in each population, while the allele size range ranged from 142 to 287 bp at the AthF1 locus, 195 to 372 bp at the AthB5 locus and 110 to 226 bp at the AthD6 locus. Mean level of observed heterozygosity was similar between populations, ranging from 0.67 to 0.93. Overall, the observed heterozygosity was lower than expected for all loci in all populations, with the average value per locus being 0.77 and 0.84, respectively (Table 4). Shannon’s information index and the effective number of alleles were highest for the Cape Adzhiyask population, being 2.23 and 8.73, respectively, indicating a higher genetic diversity in this population.
Allele frequencies for each microsatellite locus indicated that the alleles of 211 bp, 221 bp and 237 bp occurred with a maximum frequency of 0.2 in the AthF1 locus, while the most common alleles were 112 bp and 218 bp at the AthD6 and AthB5 loci, with a frequency 0.55 and 0.45, respectively. While it was possible to observe alleles that distinguished each population, these only occurred at low frequency (Figs. 2 and 3).
A dendrogram constructed based on genetic distances calculated according to allele frequencies at the AthF1, AthB5 and AthD6 locі was able to differentiate all the study populations. The population from the Danube Delta showed highest genetic distance, creating a separate branch with a bootstrap index of 100. In comparison, the population from the Kakhovka Reservoir was differentiated from the other three populations with a bootstrap index of 51, while the other three populations were differentiated from each other with genetic distances of 0.5 and 0.6, though the bootstrap indices were low.
Discussion
The results of this study indicate that the big-scale sand smelt from the Gulf of Odesa were most ‘distant’ from all other study localities, being characterised by their high parasite diversity. Thus, this population (native range) is likely to be the oldest and most stable of those studied.
Compared with other native populations, several common species were absent. For example, the parasitic isopod Mothocya epimerica Costa, 1851, is a common parasite of this species in waters of Greece (Leonardos and Trilles 2003), Italy (Bello et al. 1997) and Turkey (Öktener and Sezgin 2000) and has also been recoded from the Ukrainian coastline (Kvach and Drobiniak 2017); however, it was not recorded at all in the current survey (see Table 2). In all places, it has been found in Ukraine; however, it has only been recorded at low abundance (Kvach and Drobiniak 2017). Likewise, the digenean Bacciger bacciger, which has been recorded as common in the Black Sea (Kvach and Drobiniak 2017) and Mediterranean Sea (Culurgioni et al. 2014), occurred only very rarely in the Gulf of Odesa.
Published data on the parasites of translocated sand smelt populations in freshwaters are very rare. In Lakes Massaciuccoli and Trasimeno (Branciari et al. 2016; Guardone et al. 2021) in Italy and Lake Iznik in Turkey (Çolak 2013), translocated sand smelt are known to host larvae of Eustrongylides spp. (including Eustrongylides excisus). Here, we also report the presence of this parasite in sand smelt from the Danube delta (see Table 2). A second parasite found in introduced sand smelt in the Mediterranean basin, the parasitic copepod Lernaea cyprinacea L., 1758, found in the Draa and Ghir basins, Morocco (Clavero et al. 2015), was also not confirmed in the Black Sea basin. In addition to E. excisus, three other parasite species were recorded on sand smelt in Lake Iznik, Turkey, i.e. Bothriocephalus cf. acheilognathi, Diplostomum sp. and Tylodelphys clavata (Çolak 2013), none of which was confirmed in our study. Note, however, that B. acheilognathi and Diplostomum sp. are both already known from sand smelt in the northwestern Black Sea (Kvach and Drobiniak 2017).
The parasite community of big-scale sand smelt in its natural range (Danube Delta, Gulf of Odesa, Cape Adzhiyask) was higher than that in its expansion range, which was relatively poor in comparison, being absent (Dnipro Reservoir) or represented by just two sporadic species (Kakhovka Reservoir). These results are in line with the ‘enemy release hypothesis’, where non-native species escape from parasites in their new habitats, allowing them to establish stable populations (Torchin et al. 2003; Roy et al. 2011). This trend is commonly observed in non-native fish species after invading both freshwater (Sheath et al. 2015; Ondračková et al. 2021) and marine (Merella et al. 2016; Tuttle et al. 2017) ecosystems.
On the other hand, the development of parasite communities in native and expansion ranges does not depend on host population genetics. While parasite community composition in the Gulf of Odesa was most distant from all other localities, that for the Dnipro Reservoir was characterised by an absence of parasites (most distant and newest expansion locality), while only fish from the Danube Delta differed genetically.
Two freshwater parasites were recorded; these being represented by larvae of the nematodes Eustrongylides sp. and Raphidascaris sp. Both species have already been registered for big-scale sand smelt from the northwestern Black Sea (Kvach and Drobiniak 2017). Interestingly, the presence of freshwater parasites confirms migration of the fish into riverine deltas and then back to the sea. It is most likely that migration/introduction of the species into the Kakhovka Reservoir is responsible for presence of the Mediterranean acanthocephalan T. exiguus (see Table 2). In addition to T. exiguus, the digenean T. imbutiformis, another Atlanto-Mediterranean species, could be used to discriminate fish populations. As in the case of the freshwater nematodes, the species acts as a host for the larvae, the presence of which depends on the density of both definitive and intermediate hosts at the locality. For example, T. imbutiformis is a typical marine parasite, which requires Hydrobia sp. mud snails as its first intermediate host (see Kvach et al. 2018 and references therein). As their name implies, these mud snails prefer muddy habitats, which dominate the brackish Solonyi Kut Bay in the Danube Delta. The two other localities in the northwestern Black Sea, the Gulf of Odesa and Cape Adzhiyask, are mainly characterised by sandy bottoms, and hence, mud snails are not abundant. Likewise, this marine parasite is absent in the host’s expansion range (freshwater reservoirs) due to the lack of a first intermediate host.
The monogenean Gyrodactylus ginestrae also acts as a marker-parasite discriminating sand smelt populations as the species, which is specific to the sand smelt, has been described from the Gulf of Odesa and is also known from adjacent open seawaters (Kvach and Drobiniak 2017; Kvach et al. 2019). Recently, it has only been recorded in the Gulf of Odesa, being absent from localities close to the riverine deltas and in host’s expansion range (see Table 2). The species’ low tolerance to salinity change is typical of the monogeneans, and especially of Gyrodactylus, the systematic of which is based on the shape of their osmoregulatory organ (Malmberg 1970; Lebedeva et al. 2021). This is the most likely reason for the smelt’s escape from monogeneans in desalinated environments.
Overall, the parasite community of the big-scale sand smelt appears to depend mainly on environmental factors, such as habitat type, salinity and prey composition. Consequently, microsatellite analysis indicated only slight differences between native sand smelt populations and their neolimnetic form in the River Dnipro, with bootstrap data indicating that the population from the Danube Delta was the only population differing genetically from populations at all other localities, presumably as this was oldest and most geographically distant from all other sites. On the other hand, parasite infestation in Danube Delta fish only differed from that in the Gulf of Odesa and at Cape Adzhiyask, showing similarity with the neolimnetics from the River Dnipro. This was most likely due to the influence of freshwater on parasite community formation. Nevertheless, both microsatellite analysis and parasite community species composition both suggest that populations in the River Dnipro reservoirs are not isolated from native populations, as confirmed by the presence of the marine species T. exiguus in the Kakhovka Reservoir and the freshwater Raphidascaris sp. in the Gulf of Odesa.
Data availability
Not applicable.
References
Alexandrov B, Boltachev A, Kharchenko T, Lyashenko A, Son M, Tsarenko P, Zhukinsky V (2007) Trends of aquatic alien species invasions in Ukraine. Aquat Invasions 2(3):215–242. https://doi.org/10.3391/ai.2007.2.3.8
Bello G, Vaglio A, Piscitelli G (1997) The reproductive cycle of Mothocya epimerica (Isopoda: Cymothoidae) a parasite of the sand smelt, Atherina boyeri (Osteichthyes: Atherinidae), in the Lesina Lagoon, Italy. J Nat Hist 31(7):1055–1066. https://doi.org/10.1080/00222939700770551
Bianco PG, Caputo V, Ferrito V, Lorenzoni M, Nonnis Marzano F, Stefani F, Sabatini A, Tancioni L (2013) Atherina boyeri Risso, 1810. In: Rondinini C et al (eds) Liste Rosse italiane. Available at http://www.iucn.it [Accessed 10 Sep 2014]
Bock DG, Caseys C, Cusens RD, Hahn MA, Heredia SM, Hübner S, Turner KG, Whitney KD, Rieseberg LH (2015) What we still don’t know about invasion genetics. Mol Ecol 24:2277–2297. https://doi.org/10.1111/mec.13032
Branciari R, Ranucci D, Miraglia D, Valiani A, Veronesi F, Urbani E, Vaglio GL, Pascucci L, Franceschini R (2016) Occurrence of parasites of the genus Eustrongylides spp. (Nematoda: Dioctophymatidae) in fish caught in Trasimeno lake, Italy. Italian J Food Safety 5:6130. https://doi.org/10.4081/ijfs.2016.6130
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Clavero M, Esquivias J, Qninba A, Riesco M, Calzada J, Ribeiro F, Fernández F, Delibes M (2015) Fish invading deserts: non-native species in arid Moroccan rivers. Aquatic Conserv: Mar Freshw Ecosyst 25(1):49–60. https://doi.org/10.1002/aqc.2487
Çolak SÖ (2013) The helminth community of the sand smelt (Atherina boyeri Risso, 1810) from Lake Iznik, Turkey. J Helminth 87(2):129–134. https://doi.org/10.1017/S0022149X11000770
Copp GH, Bianco PG, Bogutskaya NG, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakėnas S et al (2005) To be, or not to be, a non-native freshwater fish? J Appl Ichthyol 21:242–262. https://doi.org/10.1111/j.1439-0426.2005.00690.x
Cribb TH, Bray RA (2010) Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Syst Parasitol 76:1–7. https://doi.org/10.1007/s11230-010-9229-z
Culurgioni J, Sabatini A, De Murtas R, Mattiucci S, Figus V (2014) Helminth parasites of fish and shellfish from the Santa Gilla Lagoon in southern Sardinia, Italy. J Helminthol 88:489–498. https://doi.org/10.1017/S0022149X13000461
Gayevskaya AV, Gusev AV, Delamure SL, Donets ZS, Iskova NI, Kornushin VV, Kovaleva AA, Margaritov NM, Markevich AP, Mordvinova TN, Najdenova NN, Nikolayeva VM, Parukhin AM, Pogoreltseva TP, Smogorzhevskaya LA, Solonchenko LA, Shtein GA, Shulman SS (1975) Opredelitel parazitov pozvonochnyh Chernogo i Azovskogo morey. Nauka Dumka, Kiev
Georgiev B, Biserkov V, Genov T (1986) In toto staining method for cestodes with iron acetocarmine. Helminthologia 23:279–281
Guardone L, Ricci E, Susini F, Polsinelli E, Guglielmone G, Armani A (2021) First detection of Eustrongylides excisus (Nematoda: Dioctophymatidae) in big-scale sand smelt (Atherina boyeri) from the lake Massaciuccoli (Northwest Tuscany, Italy): implications for public health and seafood quality. Food Control 120:107517. https://doi.org/10.1016/j.foodcont.2020.107517
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4:4
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17(4):164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: a neglected concept in invasion ecology? Ecol 90(8):2047–2056. https://doi.org/10.1890/08-1085.1
Kolbe JJ, Larson A, Losos JB, de Queiroz K (2008) Admixture determines genetic diversity and population differentiation in the biological invasion of a lizard species. Biol Lett 4(4):434–437. https://doi.org/10.1098/rsbl.2008.0205
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Publications Kottelat, Cornol and Freyhof, Berlin
Kvach Y, Bryjová A, Sasal P, Winkler HM (2018) The taxonomic and phylogenetic status of digeneans from the genus Timoniella (Digenea: Cryptogonimidae) in the Black and Baltic Seas. J Helminthol 92(5):596–603. https://doi.org/10.1017/S0022149X1700075X
Kvach Y, Drobiniak O (2017) The parasites of the big-scale sand-smelt, Atherina boyeri Risso, 1810 (Actinopterygii: Atherinidae), in the North-Western Black Sea. Sci Bull Uzhgorod Univ (Ser Biol) 42:38–43 [in Ukrainian with English summary]
Kvach Y, Kutsokon Y (2017) The non-indigenous fishes in the fauna of Ukraine: a potentia ad actum. BioInvasions Rec 6(3):269–279. https://doi.org/10.3391/bir.2017.6.3.13
Kvach Y, Ondračková M, Janáč M, Jurajda P (2016) Methodological issues affecting the study of fish parasites. I. Duration of live fish storage prior to dissection. Dis Aquat Organ 119(2):107–115. https://doi.org/10.3354/dao02990
Kvach Y, Ondračková M, Seifertová M, Hulak B (2019) Gyrodactylus ginestrae n. sp. (Monogenea: Gyrodactylidae), a parasite of the big-scale sand smelt, Atherina boyeri Risso, 1810 (Actinopterygii: Atherinidae) from the Black Sea. Parasitol Res 118(12):3315–3325. https://doi.org/10.1007/s00436-019-06483-8
Kvach Y, Zamorov V, Pupins M (2021) Review of invasive Ponto-Caspian gobiids: current range and history of expansion. Saule, Daugavpils
Lebedeva D, Muñoz G, Lumme J (2021) New salinity tolerant species of Gyrodactylus (Platyhelminthes, Monogenea) on intertidal and supratidal fish species from the Chilean coast. Acta Parasitol 66:1021–1030. https://doi.org/10.1007/s11686-021-00347-x
Leonardos I, Trilles J-P (2003) Host-parasite relationships: occurrence and effect of the parasitic isopod Mothocya epimerica on sand smelt Atherina boyeri in the Mesolongi and Etolikon Lagoons (W. Greece). Dis Aquat Org 54:243–251. https://doi.org/10.3354/dao054243
MacLeod CJ, Paterson AM, Tompkins DM, Duncan RP (2010) Parasites lost – do invaders miss the boat or drown on arrival? Ecol Lett 13:516–527. https://doi.org/10.1111/j.1461-0248.2010.01446.x
Malmberg G (1970) The excretory systems and the marginal hooks as a basis for the systematics of Gyrodactylus (Trematoda, Monogenea). Arkiv för Zoologi 23:1–235
Marenkov ON (2018) Abundance and biomass estimation of this summer individuals of alien fish species in Zaporizke Reservoir. Ukr J Ecol 8(1):92–96. https://doi.org/10.15421/2018_192
Merella P, Pais A, Follesa MC, Farjallah S, Mele S, Piras MC, Garippa G (2016) Parasites and Lessepsian migration of Fistularia commersonii (Osteichthyes, Fistulariidae): shadows and light on the enemy release hypothesis. Mar Biol 163:97. https://doi.org/10.1007/s00227-016-2865-3
Milana V, Sola L, Rossi AR, Barbisan F, Congiu L (2009) Isolation, characterization and cross-species testing of microsatellites obtained from a sand smelt (Atherina boyeri) genomic library. Mol Ecol Resour 9:889–892. https://doi.org/10.1111/j.1755-0998.2008.02366.x
Moravec F (2013) Parasitic nematodes of freshwater fishes of Europe. Revised 2nd edn. Academia, Prague
Moretti GP, Gianotti FS, Giganti A (1959) Il latterino (Atherina mochon Cuv.) nel Trasimeno (biometria, regime dietetico, pesca e parassitismo). Rivista d’Idrobiologia 11:3–38
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3):583–590. https://doi.org/10.1093/genetics/89.3.583
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76(10):5269–5273. https://doi.org/10.1073/pnas.76.10.5269
Öktener A, Sezgin M (2000) Mothocya epimerica Costa,l851 (Flabellifera: Cymothoidae), an isopod parasite in the branchial cavities of the Black Sea silverfish Atherina boyeri Risso,1810 (Perciformes, Atherinidae). Turk J Mar Sci 6(1):23–29
Ondračková M, Janáč M, Borcherding J, Grabowska J, Bartáková V, Jurajda P (2021) Non-native gobies share predominantly immature parasites with local fish hosts. J Vertebrate Biol 70(4):21050. https://doi.org/10.25225/jvb.21050
Ovcharenko M, Wróblewski P, Kvach Y, Drobiniak O (2017) Study of Loma acerinae (Microsporidia) detected from three Ponto-Caspian gobies (Gobiidae) in Ukraine. Parasitol Res 116(5):1453–1462. https://doi.org/10.1007/s00436-017-5422-1
Panov VE, Alexandrov B, Arbačiauskas K, Binimelis R, Copp GH, Grabowski M, Lucy F, Leuven RSEW, Nehring S, Paunović M, Semenchenko V, Son MO (2009) Assessing the risks of aquatic species invasions via European inland waterways: from concepts to environmental indicators. Integr Environ Assess Manag 5:110–126. https://doi.org/10.1897/IEAM_2008-034.1
Partal N, Ozdilek SY, Ekmekçi FG (2019) The introduction of a marine species Atherina boyeri into Bayramiç Reservoir, Çanakkale. NESciences 4(2):141–152. https://doi.org/10.28978/nesciences.567088
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research - an update. Bioinformatics 28(19):2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Promega Corporation (1999) Gene Print™ STR Systems Technical Manual, Vol. 7. Madison, WI
Quignard J-P, Pras A (1986) Atherinidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (eds) Fishes of the North-eastern Atlantic and the Mediterranean, vol 3. UNESCO, Paris, pp 1207–1210
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464. https://doi.org/10.1016/j.tree.2007.07.002
Roy HE, Lawson Handley L-J, Schönrogge K, Poland RL, Purse BV (2011) Can the enemy release hypothesis explain the success of invasive alien predators and parasitoids? BioControl 56:451–468. https://doi.org/10.1007/s10526-011-9349-7
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Sheath DJ, Williams CF, Reading AJ, Britton JR (2015) Parasites of non-native freshwater fishes introduced into England and Wales suggest enemy release and parasite acquisition. Biol Invasions 17:2235–2246. https://doi.org/10.1007/s10530-015-0857-8
Sørensen TA (1948) A new method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analysis of vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs (Biologiske Skrifter) 5:1–34
Tompkins DM, Dunn AM, Smith MJ, Telfer S (2011) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80:19–38. https://doi.org/10.1111/j.1365-2656.2010.01742.x
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–629. https://doi.org/10.1038/nature01346
Tuttle LJ, Sikkel PC, Cure K, Hixon MA (2017) Parasite-mediated enemy release and low biotic resistance may facilitate invasion of Atlantic coral reefs by Pacific red lionfish (Pterois volitans). Biol Invasions 19:563–575. https://doi.org/10.1007/s10530-016-1342-8
WoRMS (2023) World Register of Marine Species. Available from https://www.marinespecies.org (Accessed 2023-11-01). https://doi.org/10.14284/170
Zhukinsky VN, Kharchenko TA, Lyashenko AV (2007) Adventitious species and changes in natural habitats of native aquatic organisms in the surface water bodies of Ukraine. Report 2. Actinopterygian fishes. Hydrobiol J 43(6):3–22. https://doi.org/10.1615/HydrobJ.v43.i6.10
Acknowledgements
We thank Dr. Oleh Marenkov (Dnipro National University, Dnipro, Ukraine) for his help with sampling of fish from the Dnipro Reservoir. We also would like to thank Dr. Kevin Roche for proof-reading the English text.
Funding
National Research Foundation of Ukraine: project ‘Development of scientific backgrounds of comprehensive monitoring and threats of distribution of invasive fish species by riverine systems and transitional waters of Ukraine (based on parasite, population and genetic markers)’ (#2020.02/0171).
Author information
Authors and Affiliations
Contributions
YKv wrote the main manuscript, sampled the fish and provided the parasitological analysis and analysed the data and conceptualisation of the study. YKut sampled the fish, organised the field trips and general conceptualisation of the study and participated in the discussion preparation. AB provided the PCR analysis, analysed the microsatellite data and wrote the genetic part of the manuscript. SC analysed the microsatellite data and wrote the genetic part of the manuscript. VD sampled the fish and organised the field trips. AD sampled the fish, organised the field trips and participated in the discussion preparation. SS sampled the fish and organised the field trips. VY sampled the fish, organised the field trips and general conceptualisation of the study and participated in the discussion preparation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Julia Walochnik
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kvach, Y., Kutsokon, Y., Bakuma, A. et al. Parasite and genetic diversity of big-scale sand smelt (Atherina boyeri Risso, 1810) populations in their natural and expansion ranges in Ukraine. Parasitol Res 123, 154 (2024). https://doi.org/10.1007/s00436-024-08174-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-024-08174-5